Phase II metabolites of etofesalamide in filamentous fungi1
Introduction
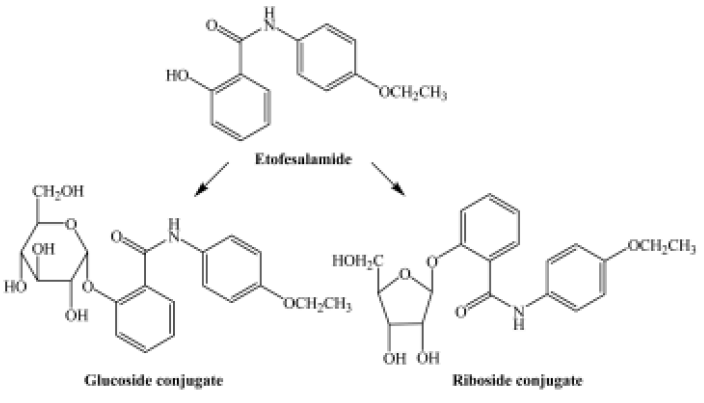
Etofesalamide [N-(4-ethoxyphenyl)-2-hydroxylbenza-mide] is a new drug that has been developed by Shenyang Pharmaceutical University[1]. Etofesalamide is applied to acne, psoriasis, sensitization dermatitis, chronic eczema, and neurodermatitis in clinical use. The metabolism of etofesala-mide in rabbits has been well studied[2,3], and etofesalamide-2-glucuronide is the major metabolite.
Biotransformation of drugs by microbes has been examined extensively[4]. In recent years, a number of studies have shown that some fungi, particularly Cunninghamella spp, possess cytochrome P-450 monooxygenase systems analogous to those in mammals and phase II drug metabolism enzymes[5–7]. The present study uses etofesalamide as a substrate to explore the ability of fungi to convert etofesala-mide into phase II metabolites, and compares these results with the results of etofesalamide metabolism in mammals to further investigate special characteristics of filamentous fungi in drug metabolism.
Materials and methods
Chemicals Etofesalamide was provided by the School of Pharmaceutical Engineering, Shenyang Pharmaceutical University (Shenyang, China) (purity >99%). β-D-glucosidase (EC 3.2.1.21) was purchased from Sigma (St Louis, MO, USA). All solvents used for assay were high performance liquid chromatography (HPLC) grade; the remaining chemicals were analytical grade or biochemical reagents.
Microorganisms Aspergillus niger and Penicillium were supplied by the Department of Microbiology, Shenyang Pharmaceutical University (Shenyang, China). Mucor circinel-loides (AS 3.3421) was purchased from the Institute of Applied Ecology, Chinese Academy of Sciences (Shenyang, China). Other filamentous fungi, Cunninghamella elegans AS 3.156, Cunninghamella elegans AS 3.2028, Cunninghamella echinulata AS 3.2004, and Cunninghamella blacksleana AS 3.153, were purchased from the Institute of Microbiology, Chinese Academy of Sciences (Beijing, China).
Cultures Microbial cultures were maintained on potato dextrose agar slants at 4 °C and transferred every 6 months to maintain viability.
The broth consisted of glucose 20 g, peptone 5 g, yeast extract 5 g, K2HPO4 5 g, and NaCl 5 g. These ingredients were mixed in 1 000 mL of distilled water, and the pH was adjusted to 6.0 with HCl (6.0 mol/L). The broth was autoclaved in individual Erlenmeyer flasks at 115 °C for 30 min and cooled before incubation.
Microbial transformation Special strains kept at 4 °C were transferred to respective solid cultures and incubated at 28 °C for 7 d to obtain well-grown mycelium with spores. In first-stage fermentation, a loop of fresh spores was inoculated with a 250 mL Erlenmeyer flask containing 50 mL broth. The cultures were incubated at 28 °C for 24 h on a rotary shaker operating at 220 rpm to receive seed culture. A 1.0 mL portion from the first-stage flask was inoculated with a second-stage 100 mL flask containing 20 mL broth. After 24 h incubation, etofesalamide dissolved in acetone was added to yield a final concentration of 0.25 g/L and incubated for an additional 48 h. After microbial transformation, the flask contents were centrifuged at 1 500×g for 20 min, and the supernatant was transferred to tubes and kept at -20 °C until analysis.
Two types of controls were run synchronously with the fermentation and worked-up using the same method. One was a blank fungi control that was used to define and exclude the indigenous secondary metabolites generated by the fungi. The other was a blank substrate control (ie the culture with the etofesalamide but without the fungi) that was used to test whether etofesalamide would be chemically decomposed or spontaneously transformed under broth and microbial transformation conditions.
Enzymatic hydrolysis A 1.0 mL portion of each sample transformed by fungi was screened and incubated with β-D-glucosidase (100 kU/L) at 37 °C for 24 h after adjusting the pH to 5.0 using 50 mmol/L NH4H2PO4 buffer (pH 3.0). Control experiments were carried out concurrently in the absence of α-D-glucosidase. Incubations were stopped by using solid-phase extraction (SPE) and analyzed using the liquid chromatography coupled to multistage mass spectrometry (LC/MSn) method.
Extraction of metabolites and LC/MSn assay After thawing, a 1.0 mL portion of each sample was applied to a Bond Elute C18 SPE column (Fuji Company, Tianjin, China) preconditioned with 2 mL methanol and 2 mL water. After loading the sample, the column was washed with 1 mL water and eluted with 2 mL methanol. The sample was evaporated to dryness at 35 °C under a gentle stream of nitrogen, and the residue was reconstituted with the addition of 100 µL of the mobile phase. A 20 µL aliquot of the solution was injected into the LC/MSn system.
LC/MSn analysis was carried out using a Finnigan LCQ ion trap mass spectrometer (San Jose, CA, USA) equipped with an electrospray ionization (ESI) source system, a Shimadzu LC-10AD pump (Kyoto, Japan) and a data system (Version 1.2, Finnigan). The interface was adjusted to the following conditions: ion mode, positive; spray voltage, 4.5 kV; capillary temperature, 200 °C; sheath gas (nitrogen), 0.75 L/min; auxiliary gas (nitrogen), 0.15 L/min. MS/MS spectra were obtained by collision-induced dissociation (CID) using helium (He) as the collision gas. The HPLC conditions were as follows: the column was a Diamonsil C18 column (particle size 5 µm, 4.6 mm × 200 mm ID, Dikma Company, Beijing, China). The mobile phase consisted of methanol-water (80:20, v/v) at a flow rate of 0.5 mL/min.
Results
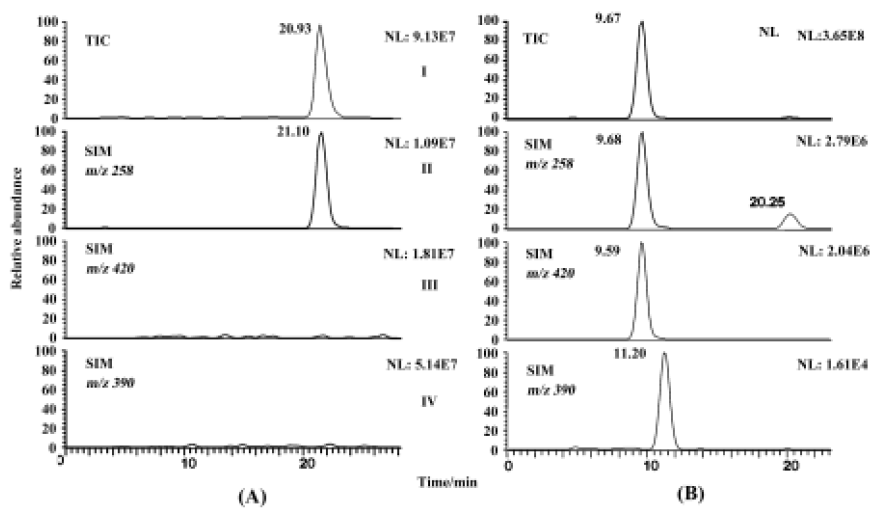
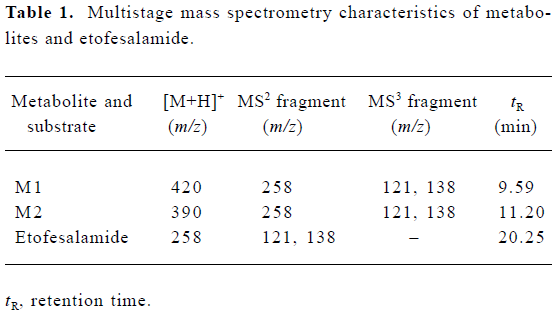
Fungal metabolite profile of etofesalamide C blacks-leana AS 3.153 supported significant metabolism and was chosen to illustrate the identification of metabolites. The microbial transformation and control samples were extracted and analyzed as described above. The chromatograms of the blank fungi controls showed no metabolites or etofe-salamide and the blank substrate controls revealed only the presence of etofesalamide and no metabolites of etofesa-lamide. Authentic etofesalamide could generate a pseudo-molecular ion [M+H]+ at m/z 258. Compared with the standards, two [M+H]+ ions correlated with the metabolism of etofesalamide were observed in the total ion current (TIC) of the sample transformed by C blacksleana AS 3.153, including ions at m/z 420 (M1) and m/z 390 (M2) (Figure 1). The LC/MSn data of etofesalamide and its two metabolites are shown in Table 1.
Full table
The pseudomolecular ion of M1 was at m/z 420, 162 higher than that of etofesalamide, which suggests that the corresponding metabolite might be a glucoside conjugate. The MS/MS spectrum of the ion at m/z 420 yielded a single ion at m/z 258 similar to the [M + H]+ ion of etofesalamide. In addition, the MS3 spectrum of the M1 ion at m/z 258 yielded the same fragment ions as the MS/MS spectrum of etofesalamide, indicating that M1 could be the glucoside conjugate of etofesalamide.
According to the process described above, M2 could be a riboside conjugate of etofesalamide. Proposed metabolic pathways of etofesalamide in filamentous fungi are shown in Figure 2.
Enzymatic hydrolysis To further investigate the structure of M1, the sample of etofesalamide transformed by C blacksleana AS 3.153 was subjected to enzymatic hydro-lysis. The results are expressed as the percentage of metabolite that was hydrolyzed with β-D-glucosidase compared to the control, which was hydrolyzed spontaneously. Treatment with β-D-glucosidase led to a greater than 50% reduction in M1. This result provides further evidence that M1 is the metabolite conjugated with glucoside because β-D-glucosidase is substrate selective.
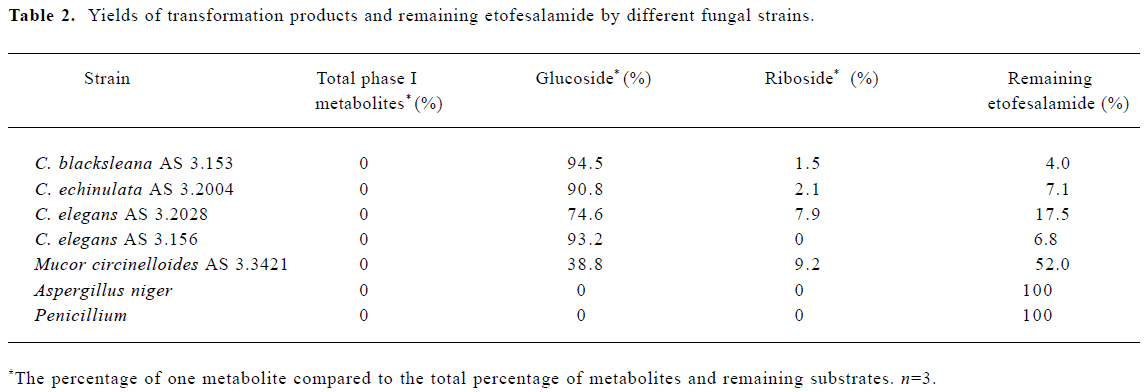
Microbial transformation of etofesalamide by the screened fungi Table 2 shows the percentages of etofesala-mide metabolized by different strains of fungi. In the seven fungi screened, Aspergillus niger and Penicillium had no ability to transform etofesalamide; four strains of Cunning-hamella spp resulted in almost complete meta-bolism, particularly the C blacksleana AS 3.153 strain. Mucor circinel-loides AS 3.3421 had a similar ability to Cunninghamella spp to transform etofesalamide. Etofesalamide was converted into two phase II metabolites by these fungi, glucoside and riboside conjugates, in which glucoside conjugate was the major product with the highest yield (94.5%). In addition, no phase I metabolites were detected, and C blacksleana AS 3.153 supported significant metabolism, which may be useful in the study of phase II metabolism of etofesalamide in vitro.
Full table
Discussion
In this study, seven filamentous fungi were screened for their abilities to metabolize etofesalamide. Four strains of Cunning-hamella species showed a strong ability to convert etofesalamide into the phase II metabolites, glucoside and riboside conjugates. No phase I metabolites were observed. The high potential of certain fungal strains to produce phase II metabolites was illustrated by these results.
Our results show that a number of filamentous fungi transform etofesalamide by phase II reactions to excretable sugar conjugates. Glucoside and riboside conjugations differ from the phase II metabolism observed in mammals. In mammalian species, such as rabbits, glucuronide conjugation rather than glucoside conjugation is the major metabolic pathway for etofesalamide[2,3]. Conjugations with glucuronic acid are considered to be a dominant metabolic pathway in humans for detoxifying and eliminating lipophilic chemicals from the body[8]. In comparison with glucuronide conjugation, there is only limited information in the literature regarding the glucoside conjugation process in humans. At present, apart from N-linked glucoside drug metabolites, only two O-linked glucosides of mycophenolic acid and morphine have been identified in humans[9,10]. In addition, glucuronide conjuga-tion, which is a characteristic conjugation in humans, is rarely found in microorganisms, and only two examples of glucuronide conjugation have been reported[11]. Compared with the phase II reactions in humans, glucoside conjugation is the major metabolic pathway in microorganisms, and it is used extensively to study the detoxification of cytotoxins and carcinogens[12-14]. Riboside conjugation is obviously another potential phase II reaction in biotransformation in filamentous fungi, but only one such conjugate has been reported recently[15].
References
- Liu BL, Zhang XY, Wang SZ. Synthesis of etofesalamide. Acta Pharm Sin 1987;22:312-5.
- Zhong DF, Chen XY, Chen RD, Luo X. The major metabolite of etofesalamide in rabbits. J Shenyang Pharm Univ 1996;13:240-4.
- Chu DF, Gu JK, Zhong DF, Si DY, Zhou H. Isolation and identification of etofesalamide-2-glucuronide. Chem J Chin Univ 2002;23:376-8.
- Srisilam K, Veeresham C. Biotransformation of drugs by microbial cultures for predicting mammalian drug metabolism. Biotechnol Adv 2003;21:3-39.
- Zhang DL, Yang YF, Leakey JE, Cerniglia CE. Phase I and phase II enzymes produced by Cunninghamella elegans for the metabolism of xenobiotics. FEMS Microbiol Lett 1996;138:221-6.
- Zhong DF, Sun L, Liu L, Huang HH. Microbial transformation of naproxen by Cunninghamella species. Acta Pharmacol Sin 2003;24:442-7.
- Sun L, Huang HH, Liu L, Zhong DF. Transformation of verapamil by Cunninghamella balkesleeama. Appl Environ Microbiol 2004;70:2722-7.
- Ritter JK. Roles of glucuronidation and UDP-glucuronosyl transferases in xenobiotic bioactivation reactions. Chem Biol Interact 2000;129:171-93.
- Shipkova M, Strassburg CP, Braun F, Streit F, Grone HT, Armstrong VW. Glucuronide and glucoside conjugation of mycophenolic acid by human liver, kidney and intestinal microsomes. Br J Pharmacol 2001;132:1027-34.
- Chen XY, Zhao LM, Zhong DF. A novel metabolic pathway of morphine: formation of morphine glucosides in cancer patients. Br J Clin Pharmacol 2003;55:570-8.
- Azerad R. Microbial models for drug metabolism. Adv Biochem Eng Biotech 1999;63:169-218.
- Kamimura H. Conversion of zearalenone to zearalenone glycoside by Rhizopus sp. Appl Environ Microbiol 1986;52:515-9.
- Pothuluri JV, Evans FE, Heinze TM, Cerniglia CE. Formation of sulfate and glucoside conjugates of benzo[e]pyrene by Cunninghamella elegans. Appl Microbiol Biotechnol 1996;45:677-83.
- Ye M, Qu G, Guo H, Guo D. Specific 12 beta-hydroxylation of cinobufagin by filamentous fungi. Appl Environ Microbiol 2004;70:3521-7.
- Gesell M, Hammer E, Mikolasch A, Schauer F. Oxidation and ring cleavage of dibenzofuran by the filamentous fungus Paecilomyces lilacinus. Arch Microbiol 2004;182:51-9.