The anti-endotoxic effect of o-aminobenzoic acid from Radix Isatidis1
Introduction
The pharmacological action of antipyretic and detoxicant materials is mainly related to their antibiotic and anti-endotoxic effects. It has been reported that Radix Isatidis (Banlan-gen, BLG) has antagonistic effects on endotoxin (ET) produced by E Coli O111B4[1–6]. In all chemical constituents, the o-aminobenzoic acid (OABA) represented 70% of the total five organic acids[7]. The Tachypleus Amebocyte Lysale (TAL) test in vitro showed that OABA had the strongest anti-endotoxin action[8]. In the present study, the anti-endotoxic effect of the OABA was studied.
Materials and methods
Extraction and isolation of OABA BLG, which was grown in Xingtai, Hebei, China and identified as the root of Isatis indigotica Fort belonging to Cruciferae, was infused in ethanol for 72 h and percolated by ethanol after being powdered. Being concentrated in depression, the extract formed was extracted repeatedly by petroleum ether. When the petroleum ether was removed, the remaining was extracted by chloromethane so the F02 part was obtained (0.8%). To get a purer active ingredient, the F02 part was isolated on silica gel column chromatography and the mobile phase was a mixture of CHCl3-CH3OH with different proportions. Four different polar fractions were obtained. By doing tests in vitro and in vivo, the F022 part was found to have the strongest anti-endotoxic activity with a productivity of 0.31%[9,10]. By further isolation with other proportions of CHCl3-CH3OH, we got another 14 components. Using the same method, we found that the part of F02209 whose productivity was 0.093% had the strongest activity among the 14 parts. The component was identified as OABA by Shanghai Institute of Materia Medica, Chinese Academy of Sciences (purity 99.7%).
Preparation of OABA solution With some flux being added, 0.5 g OABA was heated in a water bath until melted, then diluted in distilled water to 100 mL and adjusted to pH 6–7 with NaOH solution kept for later use after disinfection.
Reagents and instruments Lipopolysaccharides (LPS, E Coli O26B6, 5 mg each unit) were purchased from Sigma (St Louis, MO, USA). Working standard materials of Bacterial Endotoxin (Endotoxin, ET, E Coli O111B4, 120 EU each unit, batch N
EDS98-Bacterial ET Detector was provided by Beijing Jinshan Science Development Co Ltd (Beijing, China). DG3022A type of enzyme-linked immunodetection instrument (Huangdong Radio Tube Company, Nanjing); 1815 TC type of the CO2 cultivated box (Shel-Lab Company, USA); and XW-Vortex mixer from Instrumental Factory of Shanghai Medical University (Shanghai, China).
Animals Japanese big-ear rabbits of both sexes (weigh-ing 2.0–2.5 kg) and Kunming strain mice of both sexes (weigh-ing 16–18 g) were provided by the Experimental Animal Center of Tongji Medical College, Huazhong University of Science and Technology.
Quantitative determination of ET after being destroyed by o-aminobenzoic acid (OABA)
Preparation of ET solution One unit of ET (120 EU each unit) was dissolved in BET water to 1 mL, and spun homogeneously on XW-Vortex mixer for 30 s with concentration of 120 EU/mL.
Calibration curve A series of ET solution of 5.0, 2.5, 1.0, 0.5, 0.25, and 0.1 EU/mL were prepared. For every concentra-tion, two tubes of TAL were used (two-tube method) and 0.2 mL solution was moved into either of them. When air bubbles disappeared by vibrating, the two tubes were rapidly inserted into the access holes of quantitative detector for bacterial endotoxins and the formation time of gel (Tg) was recorded. Correlation between Tg and lgC was analyzed with linear regression. The regression equation was: Tg=2.80049–0.23326 lgC, and the regression coefficient (r) was 0.9905. If the concentration of ET was in the range of 5.0–0.1 EU/mL, the linearity was fine and the lowest detecting limit was 0.05 EU/mL.
Recovery rate Tg of 4 EU/mL ET was determined in the same way as described above. When the data were put into the regression equation, the result was 4.218±0.243 EU/mL and the recovery ratio was (105.45±10.52)%.
Measurement of samples OABA solution 0.5 mL (0.5%)was homogenized with 0.1 mL ET (4 EU/mL), spun for 30 s, incubated in water bath at 37±1 ℃ for 60±2 min. Then 0.1 mL of the mixture was diluted in 0.4 mL fresh BET water. The final concentrations of OABA and ET were adjusted to 0.833 g/L and 4 EU/mL, respectively, serving as sample groups. We used 0.833 g/L OABA as a negative control and 4 EU/mL ET as a positive control. Tg of each group was determined and put into the regression equation, so concentrations of ET in each group and the destroy rate of OABA against ET could be obtained. The basal destroying rate was calculated according to the formula: r=[1-(Sample group-Negative group)/(Positive group-Negative group)]×100%[11].
Effects of OABA on ET-induced fever in rabbits
Preparation of reagent One unit of ET (120 EU) was diluted to 6 mL with sodium chloride injection and the concentration was 20 EU/mL.
Operation Before the experiment, rabbits were placed in the experimental environment and fed for a week. Three days before the experiment, anal temperatures of the rabbits were measured twice a day. The rabbits were fed only water as of the afternoon before the experiment day. Before administration, anal temperature was measured every 30 min. Fifteen rabbits whose anal-temperature fluctuations were below 0.2 ℃ were divided into three groups at random. Each group had five rabbits of both sex. Rabbits in the sample group and the negative group were given OABA (0.5%) 5 mL/kg via the marginal ear-vein. At the same time, the rabbits in the positive group were given a sodium chloride injection 5 mL/kg. Ten minutes later, the rabbits in the sample group and the positive group were injected with ET (20 EU/mL) at a dose of 2 mL/kg. Half an hour after the injection, the anal temperature of each rabbit was measured every 0.5 h for 4 h[12].
Protective effect of OABA on LPS-induced toxicity in mice
BCG-induced enhancement of endotoxin sensitivity BCG (50 mg) was dissolved with sodium chloride injection and diluted to 5 mL (10 g/L). According to the method previously reported[13], each mouse was intraperitoneally injected with BCG at a dose of 0.4 mL, then reared in the conventional way. Nine days after the injection the mice were given water, but no food. The experiment began on the tenth day.
Operation After fasting for 16 h, the mice were randomly divided into three groups (n=20; in each group, either sex). The mice in the OABA group and the negative control group were given OABA (0.5%) at a dose of 0.4 mL/20 g. The mice in the LPS model group were all intraperitoneally injected with the same dose of sodium chloride injection at the start of the experiment. An hour and a half later, they were injected with the same dosage of injection again. Half an hour after this injection, the OABA and the LPS groups were injected with LPS at a dose of 0.2 mL/20 g. Time of death for all mice was observed over the next 72 h .
Effect of OABA on the LPS-induced release of TNF-α and NO in the serum of mice
Preparation of sample solution OABA solution was diluted with BET water from 0.5% to 0.25% and 0.125%.
Preparation of LPS solution One unit of LPS (5 mg) was dissolved in BET water to 10 mL (500 mg/L), spun for 30 s; then 0.8 mL was diluted in fresh BET water to 100 mL (4 mg/L).
Preparation of serum samples Thirty mice, each ip BCG 3 mg, were fasted without water for 12 h before the experiment. They were randomly divided into five groups, six mice per group in each experiment. The three experiment groups were ig 0.5%, 0.25%, 0.125% of sample solution at dose of 0.4 mL respectively. The control group and model group were administered NS. After 0.5 h the experiment group and the model group were iv LPS 0.2 mL/20 g from tail vein, 9 h later, they were anaesthetized with ether and blood was taken from the eye sockets. The serum were kept in -20 ℃.
TNF-α examination Using the ELISA method, we proceeded examination according to the instruction in reagent box. On the enzyme-marked single quilted plank with anti-human cell factor, we added standard solution with which we acquired a series of concentration and 100 μL serum sample. In the meantime, we established the blank group (double tube method), and added TMB substrate to display the color for 15 min after function for 60 min at 37 ℃. The absorbance at 450 nm was checked, and the standard curve was drawn. As a result, the curve was in linearity at 10–1000 ng/L. We also tested the serum sample by using the same method, and calculated the concentration of TNF-α according to the standard curve.
No examination Using the Griess reagent method, we took NaNO2 1 g precisely in a 100 mL volumetric flask and dissolved it with water. Then we took precisely 1 mL of this, and added water to 100 mL (100 mg/L) and diluted it to obtain a series of solutions. Each solution was 50 μL and the Griess liquid was added (containing 1% Sulfanilic amine, 0.1% N-1 Naphthalene ethylenediamine, 2.5% phosphoric acid) 50 μL, respectively, placed at room temperature (20 ℃) for 10 min, and the absorbance value was tested at 550 nm. On the enzyme-linked immunodetection instrument, a standard curve was drawn. As a result, the curve had a line behavior at 1–100 mg/L. We added Griess liquid at the same volume to 50 μL serum of mice, tested in the same way, and calculated the concentration of NO according to the standard curve.
Results
Destroying rate of OABA against ET The concentrations of ET were 0.668 EU/mL in the sample group, 4.036 EU/mL in the positive group, and 0.045 EU/mL in the negative group. We concluded that 0.833 g/L OABA could destroy ET directly and the destroy rate was [1-(0.668-0.045)/(4.036-0.045)]×100% = 84.4%.
The ability to induce fever by ET after pretreatment with OABA If the average body temperature before injection was taken as basal body-temperature and the difference between the maximum body-temperature and basal body-temperature was taken as maximum rising temperature, the average DTmax of each group could be obtained. The temperature reaction index in 4 h (TRI4) and DTmax are listed in Table 1.
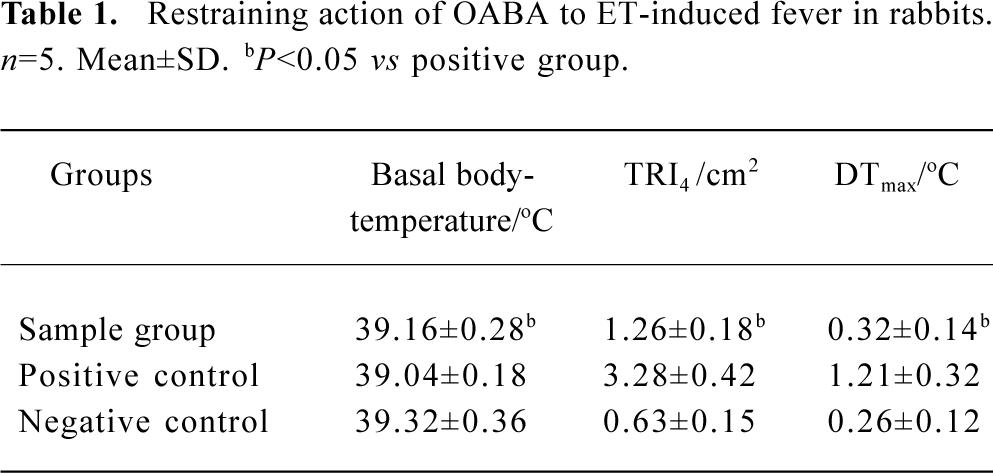
Full table
Table 1 shows that typical fever reaction occurred in rabbits given ET (40 EU/kg), while the TRI4 and DTmax dropped when the rabbits were given the OABA solution (5 mL/kg) before the same dosage of ET was administered. The difference between the two groups was significant and OABA did not have the activity to induce fever.
The mortality rate of mice treated with OABA Four of the 20 mice died within 10 h in the OABA group with a mortality rate of 20%. Fourteen of 20 mice died within 5 h in the ET model group with a mortality rate of 70%. In the negative control group, all of the 20 mice survived after 72 h. There was a significant difference in mortality between the OABA group and the LPS group.
The inhibitory function of OABA on the excessive release of TNF-α and NO in the serum of mice induced by LPS Administering different concentrations of F022 part to mice, then also giving LPS at equal dosage, the TNF-α and NO in the serum and the percentage of inhibition were shown in Table 2. The formula of inhibitory percentage follows[11]: IP%=[1-(specimen group-blank control group)/(model group-blank control group)]×100%
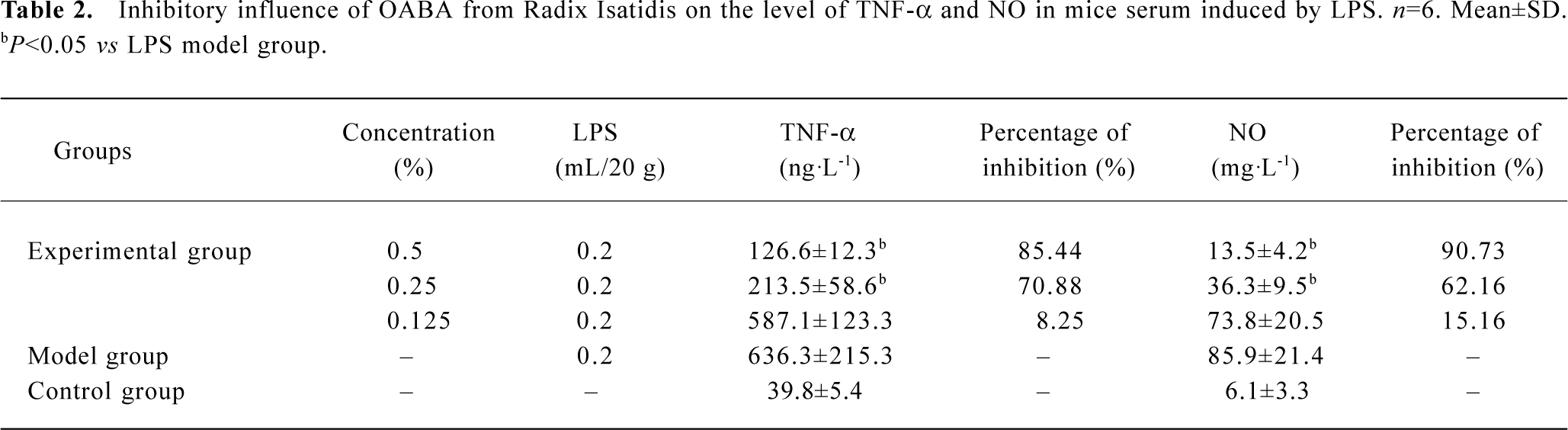
Full table
Table 2 shows OABA from Radix Isatidis had inhibitory function on the release of TNF-α and NO induced by LPS in mice, the percentage of inhibition was dependent on the dosage when the concentration was between 0.125% and 0.5%.
Discussion
The chemical components of traditional Chinese medicine (TCM) were the substance basis of its pharmacology. Studying the Radix Isatidis’s traditional function of reducing heat and detoxification was to study TCM with modernization research. Further study showed that organic acids of Radix Isatidis (quinazolinone acid, OABA, syringic acid, salicylic acid, and benzoic acid) had anti-endotoxic effects in vitro[14–19]. The study showed that OABA had anti-endotoxic effect in vivo and in vitro. The OABA content in the Radix Isatidis was higher than other organic acids and had strong anti-endotoxic activity. It could be taken as a single active anti-endotoxic ingredient. The OABA could be used in the quality control production of Radix Isatidis medicinal materials, technology of preparation, and manufacture.
The dynamic color matrix method has many merits such as simple procedures, economy, high sensitivity, and wide detectable area. Normally, ET content between 0.05–300 EU/mL can be quantitatively measured. The content of ET was decreased to 0.668 g/L with the destroying rate being up to 84.4% when 4 EU/mL ET reacted with 0.833 g/L OABA.
One of the features of ET is its ability to induce fever. Rabbits are often used to screen antipyretic drugs because they are sensitive to ET. The dosages reported to induce fever in rabbits were not consistent, and we found that the results between the positive control group given E Coli O111B4 endotoxin at 40 EU/kg and the negative control group given 0.5% OABA solution was comparable well.
The sensitivity of different kinds of experimental animals to LPS varies greatly. The lethal dose of 50% (LD50) in mice was 25 mg/kg[13]. After being sensitized by BCG, 2.42 mg/kg LPS could induce fatalities in 70% of mice. As BCG could stimulate T-cells to activate macrophages, the mitosis and metabolism in macrophages were strengthened substantially and the recognition ability of macrophages increased, so the quantity of LPS decreased. We also found that if OABA was given before LPS, OABA could exert a protective action on mice, while if OABA given after LPS, the protective action of OABA disappeared. The results showed that the action of OABA on LPS happened before the immune system was activated.
There is more and more evidence about the function of excessive release of TNF-α and NO in the disease process of shock induced by LPS. Some measures of anti-TNF-α will become important pathways of prevention and cure for LPS-induced shock. The release of a large quantity of NO is the main factor of endotoxin shock, low blood pressure, and exhausted function of many organs. It could cause tissues and organs to be scathed when NO was combined with anion of oxidated subnitryl. In the shock and exhaustion of many visceras, inhibiting the release of a large quantity of NO could prevent low blood pressure and alleviate the oxidized harm of tissues. Radix Isatidis was able to inhibit the function of the excessive release of TNF α and NO induced by endotoxin in mice macrophages.
References
- Liu YH. Experimental study on the antiendotoxin of dyers woad (Isatis tinctoria) injection. Chin J Herb Med 1993;24:413-4.
- Li YW, Zhang SM. Influence of γ ray radiation on the anti-endotoxin effect of Radix Isatidis. China Pharm 1995;6:9-10.
- Li BH, Zhang HM, Wang Y, Ding RX, Xu TF. Antiendotoxic action of hairy roots of autotetraploid Isatis indigotica Fort. Acad J Sec Milit Med Univ 2000;21:201-3.
- Li BH, Zhang HM, Fan GR, Yin C, Ding RX, Xu TF, Qiao CZ. Studies on culture of hairy roots of autotetraploid Isatis indigotoca and analysis of its antiendotoxic active components. Chin Pharm J 2000;35:728-31.
- Wang Y, Qiao CZ, Yin C, Zhang HM. Determination of organic acids of Tetraploid Isatis indigotica with high performance capillary electrophoresis. J Chin Med Meter 2000;23:204-6.
- Liu ZF, Li GS, Fu FH, Liu K. Studies of anti-endotoxin effect of eight Chinese herb injections in vitro. Chin Trad Herb Drugs 2002;33:58-9.
- Wang Y, Qiao CZ. Determination of anthranilic acid in the leaves of Isatidis indigotica by first order derivative UV spectrophotometry. Chin J Herb Med 2000;31:664-5.
- Zhang HM, Zhang G, Qiao ZZ. Detection of indigo and indirubin in different part of Radix Isatidis and Folium Isatidis and anti-endotoxic action comparison of some ingredients J Pharm Pract 2000;18:347. (abstract).
- Liu YH, Lin AH, Ding SP, Fang JG, Li J, Chen X. Study on anti-endotoxin of chloroform extract from Radix Isatidis. Chin J Hosp Pharm 2001;21:326-8.
- Liu YH, Ding SP, Lin AH, Fang JG, Shi SP. The protective effects of different polar fractions from Radix Isatidis on endotoxin challenged mice. Acta Univ Med Tongji 2001;30:272-3.
- Du GH, Li XJ, Zhang YX. Guide of pharmacological experiments — drug discovery and evaluation pharmacological assays. Beijing: Beijing Science and Technology Publishing House; 2001.p 528–9
- The National Pharmacopoeia Commission. The Chinese Pharmacopoeia (part II). Beijing: Chemical Industry Press; 2000. Supplement, p 85–6.
- Vogel SN, Moore RN, Sipe JD. BCG-induced enhancement of endotoxin sensitivity in C3H/ HeJ mice. J Immunol 1980;124:2004.
- Wu XY, Liu YH, Sheng WY, Sun J, Qin GW. Chemical constituents of Isatis indigotica. Planta Med 1997;63:55-7.
- Liu YH, Liu YF, Guo XX. Current studes on anti-endotoxic chemical components of traditional Chinese medicine in China. Acta Pharmacol Sin 2001;22:1071-7.
- Liu YH, Du G, Han HG, Tan LX. Study on the anti-endotoxic effect of Radix Isatidis. Herald Med 2001;29:547-8.
- Liu YH, Qin GW, Fang JG, Wu XY. Screening of chemical constituents with antiendotoxin activity from Radix Isatidis. Heral Med 2002;21:74-5.
- Liu YH, Wu XY, Fang JG, Xie W. Chemical constituents from Radix Isatidis. Central South Pharm 2003;1:302-5.
- Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep 1998;15:595-606.
