Inhibitory effects of idoxifene on hepatic fibrosis in rats1
Introduction
Chronic injury leading to liver fibrosis occurs in response to a variety of insults, including viral hepatitis, alcohol abuse, drugs, metabolic diseases due to overload of iron, etc. Hepatic fibrosis is reversible, whereas cirrhosis, the end-stage consequence of fibrosis, is generally irreversible. Suppression of hepatic fibrogenesis and prevention of cirrhosis has attracted the attention of researchers from a therapeutic perspective. Hepatic fibrosis induced by dimethylnitrosamine (DMN) in rats is generally used as a model of hepatic fibrosis[1,2]. Female hormone 17β-estradiol possesses inhibitory effect on DMN-induced hepatic fibrosis in rats[1]. However, administration of estrogen leads to some potential risks including breast cancer.
Idoxifene (E-1-[2-[4-[1-(4-iodophenyl)-2-phenyl-1-butenyl]-phenoxy] ethyl] pyrrolidine) is a novel tissue-specific selective estrogen receptor modulator (SERM). Idoxifene was originally developed for the treatment of advanced breast cancer[3] and had no side effects of estrogen[4]. Idoxifene possessed the protective roles in vascular smooth muscle cells by its blunting the angiotensin II-induced production of reactive oxygen species (ROS)[5]. Chronic liver injury causes overproduction of ROS which result in oxidative stress and oxidative stress is a link between chronic liver injury and hepatic fibrosis. Moreover, idoxifene could also protect hepatocytes from inflammatory cell injury[6].
Based on the information, the aim of present study is to ascertain the effect of idoxifene on DMN-induced hepatic fibrosis in rats.
ROS are by-products of the inflammatory response, and contribute to both onset and progession of hepatic fibrosis[7]. Copper,zinc-dependent superoxide dismutase (CuZn-SOD) and cellular glutathione peroxidase (GSHPx) are important enzymatic antioxidants in cells. Hepatic stellate cells (HSCs) play a central role in the development and resolution of hepatic fibrosis[8–10]. In response to liver damage, HSCs “activate” to a myofibroblast-like (α-SMA-expressing) phenotype. Activation of HSCs is a critical step in hepatic fibrogenesis. Culturing quiescent HSC on plastic plates causes spontaneous activation leading to a myofibroblast-like phenotype, mimicking the process seen in vivo. This provides a simple and useful model for studying HSC activa-tion. Therefore, in this report the impacts of idoxifene on enzymatic antioxidant and on cultured HSCs were explored.
Materials and methods
DMN model Thirty male Wistar rats (200±6 g, provided by the Animal Center of Nan Tong Medical College) were used for the DMN model of hepatic fibrosis (five groups of six each). The animals, were administered a single intraperitoneal injection of DMN (Sigma, St Louis, MO) (diluted with saline) at a dose of 40 mg/kg body weight[1]. After the DMN treatments, the rats received daily oral gavage of idoxifene (synthesized at SmithKline Beecham Pharmaceuticals, King of Prussia, PA) in a dosing vehicle (methylcellulose) at a dose of 0.02 mg·kg-1·d-1, 0.1 mg·kg-1·d-1, or 0.4 mg·kg-1·d-1 respectively for two weeks[1]. The controls received vehicle. After two weeks, the livers were removed and the protein levels of collagen were measured; hepatic fibrosis was shown by immunohitochemistry using an antibody against type I collagen. The protein levels of CuZn-SOD and the activities of cellular GSHPx in the liver were also detected.
Cell culture Hepatocytes were isolated from the livers of male Wistar rats (500–550 g) as described previously[11]. Approximately 5×105 cells were introduced into 20-mm diameter plastic dishes. Cells were cultured in 1 mL Williams medium E supplemented with 5% fetal bovine serum (FBS), penicillin 100 kU/L, streptomycin 0.1 g/L, and L-glutamine 2 mmol/L at 37 oC in 5% CO2 atmosphere and 100% humidity. Overnight, the cell medium was removed, and the serum-free medium with 1×10-9, 1×10-8, or 1×10-7 mol/L of idoxifene was added to the cells respectively. After incubation for 1 h, FeNTA was added into the wells at a final concentration of 100 µmol/L which is generally used to cause oxidative stress in cultured hepatocytes[6,12,13]. After 24 h[12], the cells were collected and the protein levels of CuZn-SOD and the activities of cellular GSHPx were measured.
Isolation of HSCs from the liver of male Wistar rats (500–550 g) and the assay of purity and viability of isolated HSCs were performed as described previously[14]. Both cell purity and viability was in excess of 90%. After HSCs were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS on uncoated plastic culture dishes for 5 d, the activation of HSCs occurred[2]. In present experiment, HSCs were initially cultured in DMEM supplemented with 10% FBS on uncoated plastic culture dishes for several days, then the culture medium was removed and the same medium with or without idoxifene was added to the cells which were cultured for an additional time. After the time, examination of activation, proliferation, and apoptosis of HSCs were performed.
Collagen determination Protein levels of collagen were determinated as described previously[2]. A portion of each liver was homogenized in 35 volumes (mL/g) of 0.5 mol/L acetic acid at 4 oC; homogenates were disrupted by freeze-thawing and sonicated for 2 min for collagen determination. The fraction of insoluble collagen after the acid extraction was then heated at 80 oC for 60 min and then converted into soluble gelatin. The collagen and gelatin contents of the acid extract were assayed using the Sircol collagen assay kit (Biocolor, Belfast, Northern Ireland) according to the manufacturer’s directions.
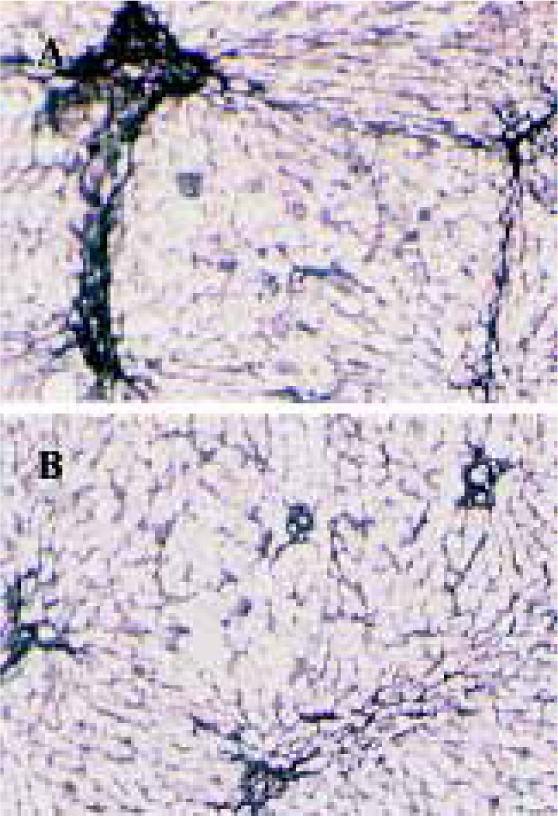
Immunohistochemistry Immunohistochemical examination of the liver fibrosis was performed using polyclonal antibody against type I collagen (Chemicon International, Temecula, CA, diluted 1:100) as described previously[2]. Briefly, after incubation with the antibody against type I collagen, liver samples were washed and were incubated with the biotin-conjugated IgG F(ab’)2 (DAKO, diluted 1:200), and finally with the avidin-biotin complex (Vectastain ABC reagent, Vector Laboratories, Burlingame, CA). Reaction products were visualized with diaminobenzidine and photographed.
For immunohistochemical examination of α-SMA, HSCs were initially cultured for 2 d in DMEM supplemented with 10% FBS. The culture medium was then removed and the same medium with or without 1×10-7 mol/L of idoxifene was added to the cells and the cells were cultured for an additional 4 d. During this period, the medium was replaced every other day. An immunohistochemical examination of α-SMA was performed as described previously[14]. Briefly, after incubation with the a monoclonal antibody against α-SMA (DAKO, diluted 1:50), cells were washed and were incubated with the biotin-conjugated IgG F(ab’)2 (DAKO, diluted 1:200), and finally with the avidin-biotin complex (Vectastain ABC reagent, Vector Laboratories, Burlingame, CA). Reaction products were visualized with diaminobenzi-dine and were photographed.
Antioxidant enzyme assays Liver tissues were washed with a 0.5% heparin sodium solution in PBS, and were then homogenized on ice in 6 volumes (mL/g) of Tris buffer (50 mmol/L Tris-HCl, pH 7.5, 5 mmol/L ethylenediaminete-traacetic acid and 1 mmol/L diethiothreitol). Cultured hepatocytes were washed twice with ice-cold PBS, and lysed directly in 150 µL of Tris buffer. The resulting homogenate and cells were disrupted by sonication for 1 min. The suspension was then centrifuged at 14 000×g at 4 oC for 30 min. Aliquot samples of the supernatants were analyzed for antioxidant enzymes. Protein level of CuZn-SOD was detected using an enzyme-linked immunosorbent assay (ELISA) system kit (Amersham, Little Chalfont, UK). GSHPx activities were determined using a cellular glutathione peroxidase assay kit (Calbiochem, San Diego, CA). Enzyme assays were performed according to each manufacturer’s recommendation protocol.
Cell proliferation assays DNA synthesis in cultured HSCs was measured using a Cell Proliferation Biotrack ELISA system (Amersham, Little Chalfont, UK). HSCs were cultured in DMEM supplemented with 10% FBS in 96-well plate for 4 d. In the period, the medium was replaced every other day. After 4 d, the culture medium was removed and the same medium with or without 1×10-9, 1×10-8, or 1×10-7 mol/L of idoxifene was added to the cells respectively. After the cells were cultured for an additional 24 h, bromodeoxyuri-dine (BrdU) was added into each well at a final concentration of 10 µmol/L and the cells were incubated with BrdU for 24 h. The incorporated BrdU was detected according to the manufacturer’s recommended protocol.
Early apoptosis detection A combination of FITC-conjugated annexin V and propidium iodide (PI) is a powerful and selective tool for measuring early apoptosis by flow cytometry. HSCs cultured in DMEM supplemented with 10% FBS in 6-well plates for 5 d. In the period, the medium was replaced every other day. After 5 d, the cultured medium was removed and the same medium with or without 1×10-7 mol/L of idoxifene was added to the cells. The apop-totic cells were detected using an ANNEXIN V FITC kit (Immunotech, Marseille, France) according to the manufacturer’s recommended protocol. Flow cytometric analysis was performed on an ELICS XL flow cytometer (Coulter, Hialeah, FL).
Statistical analysis Data were expressed as mean±SD. Comparisons among groups were performed by an analysis of variance and Scheffe’s test. P<0.05 was considered statistically significant.
Results
Effects of idoxifene on hepatic fibrosis The protein level of collagen increased in rat liver treated with DMN; 0.1 mg·kg-1·d-1 or 0.4 mg·kg-1·d-1 of idoxifene could inhibit the production of collagen induced by DMN in liver and 0.4 mg·kg-1·d-1of idoxifene reduced the protein level of collagen induced by DMN in liver by 41.19% (P<0.05, Table 1). Immunohistochemistry study indicated that in DMN model, 0.4 mg·kg-1·d-1of idoxifene markedly suppressed the expression of type I collagen (Figure 1) which is the most excessive extracellular matrix protein in hepatic fibrosis.
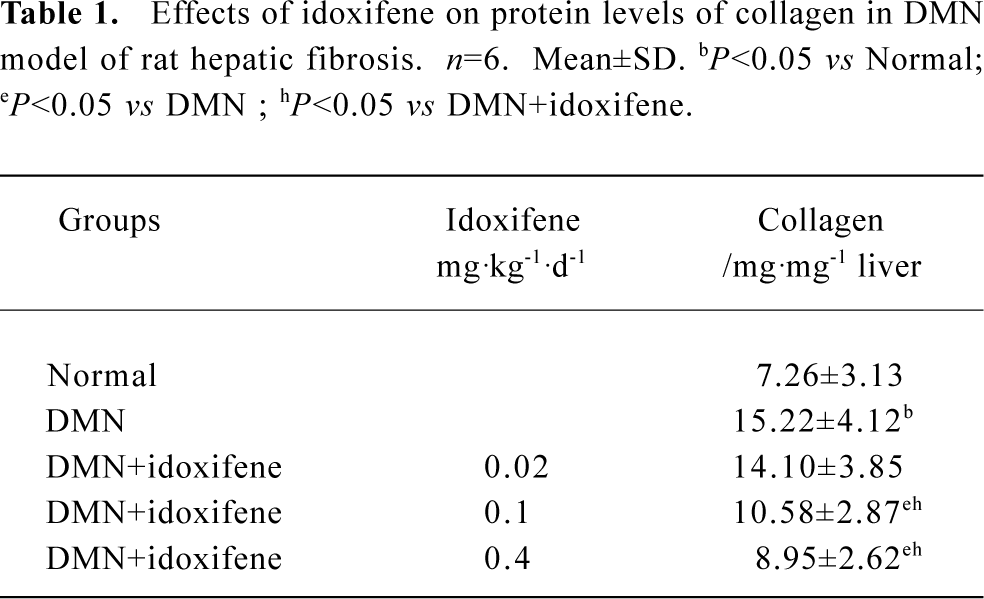
Full table
Effects of idoxifene on antioxidant enzymes Compared with the normal (control), the protein levels of CuZn-SOD and the activities of GSHPx both in liver treated with DMN alone and in hepatocytes cultured with FeNTA alone evidently declined, whereas all the decline were inhibited by idoxifene (Table 2, 3). The protein level of CuZn-SOD and the activity of GSHPx in the liver treated with DMN plus 0.4 mg·kg-1·d-1 of idoxifene were 2.65 (P<0.05) and 2.08 (P<0.05) times greater than that in liver treated with DMN alone (Table 2). The protein level of CuZn-SOD and the activity of GSHPx in hepatocytes cultured with FeNTA plus 1×10-7 mol/L of idoxifene were 3.43 (P<0.05) and 2.52 (P<0.05) times greater than that in hepatocytes cultured with FeNTA alone (Table 3).
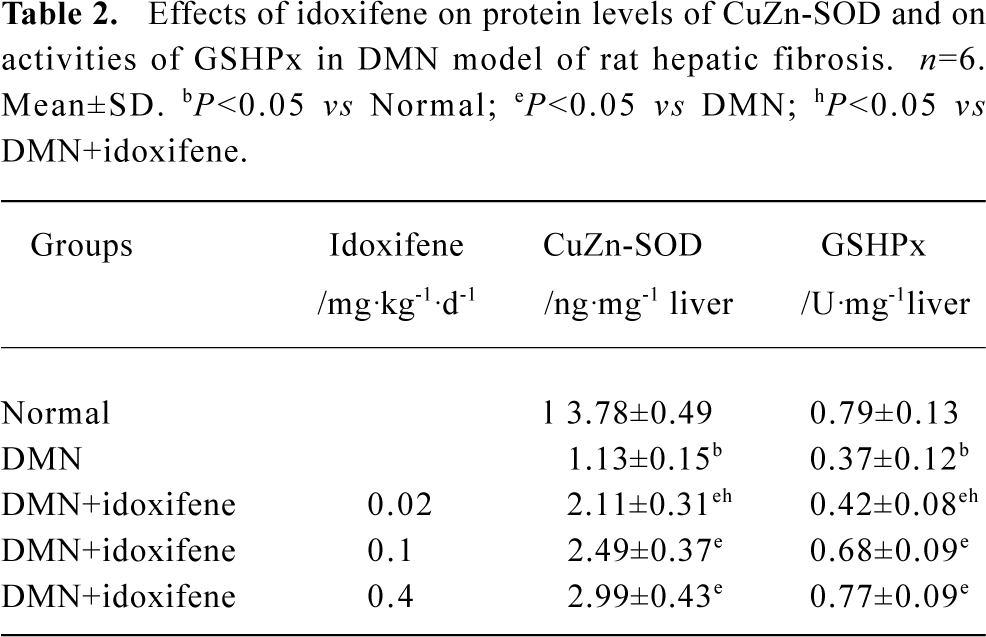
Full table
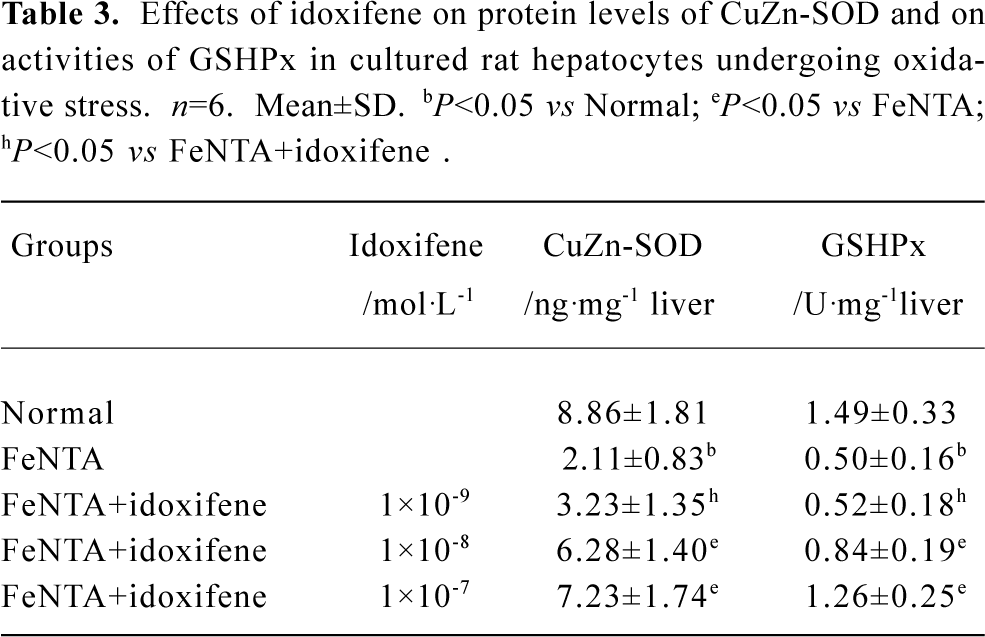
Full table
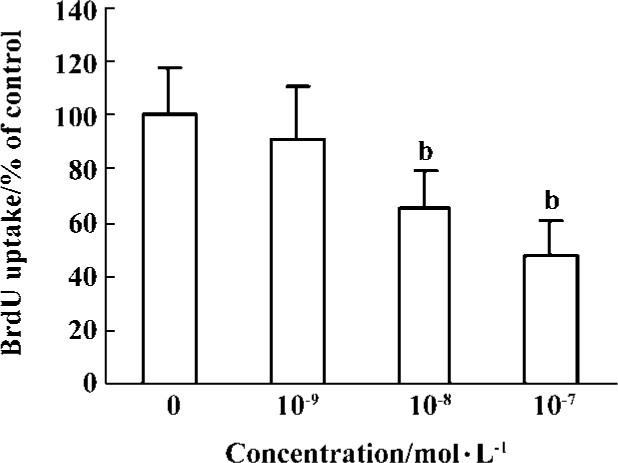
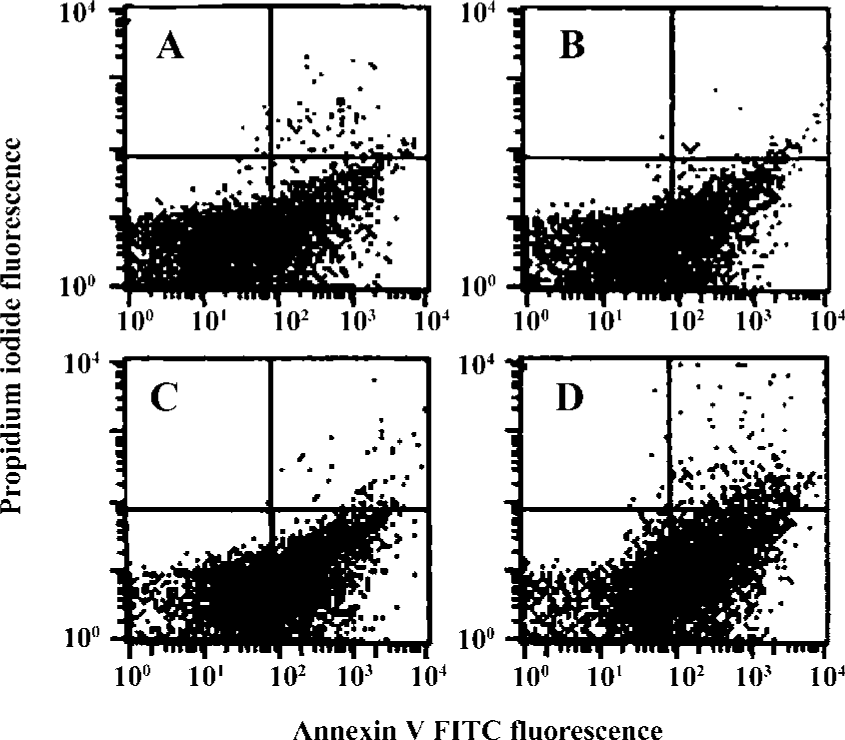
Effects of idoxifene on culture-activated HSCs Idoxifene evidently suppressed HSC activation (Figure 2), inhibited culture-activated HSC proliferation in a dose-dependent manner (Figure 3), and induced culture-activated HSC apoptosis in a time-dependent manner (Figure 4). Compared with the control, the uptake of BrdU in HSCs cultured with 1×10-7 mol/L of idoxifene was reduced by 51.87% (P<0.05) (Figure 3) and the number of apoptotic HSCs cultured with 1×10-7 mol/L of idoxifene increased by 94.52% (P<0.05) (Figure 4D).

Discussion
Hepatic fibrogenesis is a process where production of extracellular matrix surpasses degradation. Collagen is the main component of extracellular matrix. Abnormal accumulation of collagen in chronic liver injury is a direct index that indicates the hepatic fibrogenesis. Data in this report showed that idoxifene could reduce the protein level of collagen in DMN model of hepatic fibrosis in a dose-dependent manner. Immunohistochemical studies directly showed that idoxifene markedly suppressed hepatic fibrosis induced by DMN. Together, these data demonstrated the inhibitory effect of idoxifene on hepatic fibrosis in rats.
ROS mainly include superoxide anion and its metabolin such as hydrogen peroxide and lipid peroxide. Since overproduction of ROS results in oxidative stress which is a link between chronic liver injury and hepatic fibrosis, reduction of oxidative stress by antioxidants can prevent hepatic fibrogenesis. Idoxifene evidently inhibited the decline in enzymatic antioxidant levels of CuZn-SOD and GSHPx, which can neutralize the effects of ROS, both in DMN model of hepatic fibrosis and in hepatocytes undergoing oxidative stress. CuZn-SOD can eliminate superoxide anion and GSHPx can eliminate hydrogen peroxide and lipid peroxide. The data partly suggested the inhibitory mechanisms of idoxifene on rat hepatic fibrosis.
Activated HSCs are proliferative and are responsible for the majority of extracellular matrix protein deposition that forms scar tissue during liver fibrogenesis. Idoxifene clearly suppressed HSC activation and culture-activated HSC proliferation. The data were consistent with the inhibitory effect of idoxifene on rat hepatic fibrosis.
Accumulating evidence has indicated that oxidative stress plays critical roles in activation of HSC[8,15,16]. Hepatocytes and Kupffer cells after chronic liver injury are a potent source of reactive oxygen intermediates[17]. These compounds exert paracrine stimulation of stellate cells. In cultured stellate cells, conditioned medium from hepatocytes undergoing oxidative stress increases proliferation and collagen synthesis[18]. Thereby the effects of idoxifene on the antoxidant enzymes (CuZn-SOD, GSHPx) in vivo and in vitro as shown in results suggested that idoxifene could inhibit HSCs activation at least through indirectly blunting the roles of ROS.
Recovery from established experimental fibrosis can occur through apoptosis of activated HSCs and is associated with reductions in liver collagen[10,19]. The result that idoxifene effectively induced culture-activated HSC apoptosis further supported the inhibitory effect of idoxifene on hepatic fibrosis in rats.
Oxidative stress in liver causes hepatocyte apoptosis[20] which promotes the activation of HSCs and hepatic fibrogenesis[21–23]. Our recent results showed that idoxifene at 1×10-7 mol/L could markedly inhibit oxidative stress-induced hepatocyte apoptosis[24]. Those results demonstrated the protective effect of idoxifene on hepatocytes and implied another inhibitory mechanism of idoxifene on hepatic fibrosis in rats.
Collectively, our data provides evidence that idoxifene possesses the inhibitory effect on hepatic fibrosis in rats. The inhibitory effect of idoxifene on hepatic fibrosis in rats was, at least in part, through maintaining antioxidant enzyme levels (CuZn-SOD and GSHPx).
SERMs are a diverse group of compounds that bind with specific, high-affinity binding to the estrogen receptor (ER) and can act as either ER agonists or antagonists. Tamoxifen, one of SERMs, increases fibrogenesis in CCl4-induced liver fibrosis, showing estrogen antagonist activity[25]. The data presented in the present study demonstrates the inhibitory effect of idoxifene on DMN-induced liver fibrosis in rats, showing the potential of idoxifene as an estrogen agonist in rat liver. The different biologic actions can be related to the ER subtype involved, different conformational changes of the ER according to the ligand, and steroil receptor coac-tivators and corepressors that modulate the cellular response to the ER-ligand complex[26]. It is known that the alterations in the conformation of receptors influence their abilities to interact with coactivators and corepressors[27].
Estrogen can suppress the hepatic fibrosis[1], but it leads to serious side effects which restricts its application. The side effects of idoxifene is presently unknown. Idoxifene has been used for the treatment of advanced breast cancer and also has no side effects of estrogen[4], the findings in this report suggest that idoxifene might be useful in developing new therapeutic strategies for the treatment and prevention of hepatic fibrogenesis.
References
- Yasuda M, Shimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology 1999;29:719-27.
- Shimizu I, Ma YR, Mizobuchi Y, Liu F, Miura T, Nakai Y, et al. Effects of Sho-saiko-to, a japanese herbal madicine, on hepatic fibrosis in rats. Hepatology 1999;29:149-60.
- Johnston SR, Boeddinghaus IM, Riddler S, Haynes BP, Hardcastle IR, Rowlands M, et al. Idoxifene antagonizes estradiol-dependent MCF-7 breast cancer xenograft growth through sustained induction of apoptosis. Cancer Res 1999;59:3646-51.
- Hirsimaki P, Aaltonen A, Mantyla E. Toxicity of antiestrogens. Breast J 2002;8:92-6.
- Baumer AT, Wassmann S, Ahlbory K, Strehlow K, Muller C, Sauer H, et al. Reduction of oxidative stress and AT1 receptor expression by the selective oestrogen receptor modulator idoxifene. Br J Pharmacol 2001;134:579-84.
- Omoya T, Shimizu I, Zhou Y, Okamura Y, Inoue H, Lu G, et al. Effects of idoxifene and estradiol on NF-kappaB activation in cultured rat hepatocytes undergoing oxidative stress. Liver 2001;21:183-91.
- Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med 2000;21:49-98.
- Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem 2000;275:2247-50.
- Alcolado R, Arthur MJ, Iredale JP. Pathogenesis of liver fibrosis. Clin Sci (Lond) 1997;92:103-12.
- Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest 1998;102:538-49.
- Shimizu I, Ichihara A, Nakamura T. Hepatocyte growth factor in ascites from patiens with cirrhosis. J Biochem 1991;109:14-8.
- Morel I, Lescoat G, Cillard J, Pasdeloup N, Brissot P, Cillard P. Kinetic evaluation of free malondialdehyde and enzyme leakage as indices of iron damage in rat hepatocyte cultures. Biochem Pharmacol 1990;39:1647-55.
- Liu Y, Shimizu I, Omoya T, Ito S, Gu XS, Zuo J. Protective effects of estradiol on hepatocytic oxidative damage. World J Gastroenterol 2002;8:363-6.
- Zhou YJ, Yin DM, Chen HS, Zhu HX, Wan X. Effects of blocking transforming growth factor signaling on culture-activated rat hepatic stellate cells. Chin J Hepatol 2003;11:282-4.
- Lee KS, Buck M, Houglum K, Chojkier M. Activation of hepatic stellate cells by TGF α and collagen type I mediated by oxidative stress through c-myb expression. J Clin Invest 1995;96:2461-6.
- Fernandez-Checa JC, Kaplowitz N, Garcia-Ruiz C, Colell A. Mitochondrial glutathione: importance and transport. Semin Liver Dis 1998;18:389-401.
- Maher JJ. Leukocytes as modulators of stellate cell activation. Alcohol Clin Exp Res 1999;23:917-21.
- Svegliati Baroni G, D’Ambrosio L, Ferretti G, Casini A, Di Sario A, Salzano R, et al. Fibrogenic effect of oxidative stress on rat hepatic stellate cells. Hepatology 1998;27:720-6.
- Wright MC, Issa R, Smart DE, Trim N, Murray GI, Primrose JN, et al. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology 2001;121:685-98.
- Czaja MJ. Induction and regulation of hepatocyte apoptosis by oxidative stress. Antioxid Redox Signal 2002;4:759-67.
- Canbay A, Guicciardi ME, Higuchi H, Feldstein A, Bronk SF, Rydzewski R, et al. Cathepsin B inactivation attenuates hepatic injury and fibrosis during cholestasis. J Clin Invest 2003;112:152-9.
- Canbay A, Higuchi H, Bronk SF, Taniai M, Sebo TJ, et al. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology 2002;123:1323-30.
- Faouzi S, Burckhardt BE, Hanson JC, Campe CB, Schrum LW, Rippe RA, et al. Anti-Fas induces hepatic chemokines and promotes inflammation by an NF-kappa B-independent, caspase-3-dependent pathway. J Biol Chem 2001;276:49077-82.
- Zhou YJ, Chen HS, Sha BX. Antiapoptotic mechanisms of idoxifene on cultured rat hepatocytes undergoing oxidative stress. Chin Pharm J 2005;40:278-81.
- Xu JW, Gong J, Chang XM, Luo JY, Dong L, Hao ZM, et al. Estrogen reduces CCl4-induced liver fibrosis in rats. World J Gastroenterol 2002;8:883-7.
- Burger HG. Selective oestrogen receptor modulators. Horm Res 2000;53 Suppl 3:25-9.
- Smith CL, O’Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev 2004;25:45-71.
