Interaction effects between estrogen receptor α and vitamin D receptor genes on age at menarche in Chinese women
Introduction
Menarche, the first occurrence of menstruation, is a marked characteristic of a woman’s sexual maturation and a biological signal for the onset of reproduction. Age at menarche is an important anthropological variable, which influences the total duration of women’s estrogen exposure and thus has major implications for a woman’s health later in life. Early menarche is associated with an increased risk of breast and endometrial cancer[1]. Delayed menarche increases the risk of Alzheimer’s disease[1] and osteoporosis[2], but decreases the incidence of coronary heart disease[1]. Therefore, from a clinical point of view, it is of interest to identify factors that influence the variation of menarcheal age.
Age at menarche is known to be a complex trait that is determined by multiple genetic and environmental factors, including nutrition, exercise, socioeconomic conditions, childhood experience, and general health[1,3–5]. The importance of genetic factors in determining menarcheal age has recently been recognized. Twin studies have shown that genetic factors can explain more than 50% of the variation of menarcheal age[4]. There are highly significant correlations between age at menarche in mothers and daughters[3], and family history is a strong predictor for early menarche[5]. Recently, some candidate genes, such as the androgen receptor (AR) gene[6], the cytochrome P450 c17α (CYP17) gene[7,8] and the CYP3A4 gene[8], have been tested for association with age at menarche. The results so far have been inconsi-stent, and the specific gene responsible for age at menarche is still not clear.
The onset of menstruation is determined by the hypothalamic-pituitary-gonadal axis and is initiated by an increased amplitude of estrogen exposure of tissues[9]. Estrogen signal receptors including estrogen receptor α (ER-α) play an important role in mediating the specific effects of the estrogen on development, proliferation and differentiation of reproductive tissues[10]. In addition, mice deficient in ER-α are infertile and exhibit atrophy of the oviduct and uterus[11]. Thus, the ER-α gene may be a candidate gene for the onset of menstruation. An association has been observed between the ER-α gene and age at menarche in Greek adolescent females[12] but not in other populations[7,13,14].
The vitamin D receptor (VDR) gene may be another potential candidate gene for age at menarche. The expression of VDR is detected in reproductive organs[15]. In vitamin D-deficient mice, uterine hypoplasia with impaired folliculogenesis was found in the female reproductive organs[16]. The VDR gene has been associated with several diseases that may be related to total tissue estrogen exposure, including breast cancer[17], cardiovascular disease[18], and osteoporosis[19]. Recently, the VDR gene has been associated with the age of menarche in Japanese girls[20].
In the present study, we intended to investigate whether the ER-α and VDR genes, as well as interactions between the two, had effects on the age at menarche in the Chinese female population.
Materials and methods
Subjects The study subjects were selected from 401 nuclear families used in our previous epidemiological study[21], which was approved by the Research Administration Departments of the Shanghai Sixth People’s Hospital and Hu-nan Normal University. All the subjects were recruited from the Shanghai urban area. They were all of the Han ethnic group, which accounts for more than 93% of the total Chinese population. Informed consent was obtained from each subject. For the study subjects, a detailed medical history, including menstrual history, was recorded by nurse-administered questionnaires. The sampling scheme and exclusion criteria have been detailed elsewhere[21].
For the present study, we randomly chose one pre-menopausal daughter from each nuclear family. Four hundred and one unrelated pre-menopausal women, who were all in good general health as defined by our exclusion criteria, were sampled. Among these women, 11 had no information of menarcheal age. Thus the 390 remaining pre-menopausal women were available for analysis. The women were unrelated and had regular menstrual cycles.
Genotyping Genomic DNA was isolated using the phenol-chloroform extraction method from whole blood. All subjects were genotyped by polymerase chain reaction followed by restriction fragment length polymorphism procedures (PCR-RFLP). For the ER-α gene, the forward primer (5'-CTG CCA CCC TAT CTG TAT CTT TTC CTA TTC TCC-3') and reverse primer (5'-TCT TTC TCT GCC ACC CTG GCG TCG ATT ATC TGA-3') were used to amplify a 1.3 kb DNA fragment in intron 1. For the ApaI inside the VDR gene, a 745 bp DNA fragment was produced using the forward primer in intron 8 (5'-CAG AGC ATG GAC AGG GAG CAA G-3') and the reverse primer in exon 9 (5'-GCA ACT CCT CAT GGC TGA GGT CTC A-3'). The PCR mix contained 50 ng genomic DNA, each of the four deoxyribonucleotides (dNTPs; 0.2 mmol/L), 0.6 U Taq polymerase (Sangon, Shanghai, China), MgCl2 (1.5 mmol/L), each primer (0.4 µmol/L), and 1×PCR buffer in a total volume of 25 µL. PCR was performed in 38 cycles as follows: denaturation at 95 °C for 30 s, annealing at 50 °C for 30 s, and extension at 72 °C for 60 s on a PE9700 Thermal Cycler (Perkin-Elmer Cetus, Norwalk, CT, USA). After amplification, 8 µL of the PCR products was digested with the respectively restriction endonucleases PvuII, XbaI, and ApaI (Promega, Madison, WI, USA) at 65 °C for 4 h and electrophoresed on 2% agarose gel, stained with ethidium bromide, and visualized under UV light. The genotypes were designated as PP, Pp, and pp for PvuII, XX, Xx, and xx for XbaI, and AA, Aa, and aa for ApaI. Uppercase and lowercase letters represent the absence and presence of restriction sites, respectively.
Statistical analysis The χ2 test was performed to test for the Hardy-Weinberg equilibrium (HWE) at the studied marker loci. The phenotypic values were verified for normal distribution by using the Shapiro-Wilks test. The Program SimWalk2 (available at http://www.genetics.ucla.edu/home/software.htm
Results
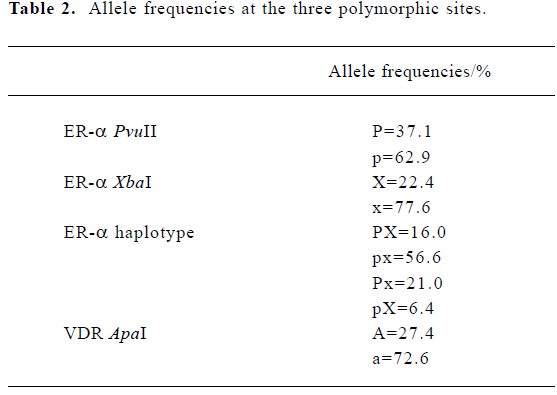
Descriptive characteristics of the study subjects The basic characteristics of the 390 unrelated pre-menopausal women are summarized in Table 1. Allele frequencies at the three polymorphic sites in our subjects are summarized in Table 2. All three loci were in HWE (P>0.10). When the genotypes of the ER-α gene were defined according to the haplotypes, ten genotypes were found, with pxpx as the most common (frequency=0.331) and pXpX as the least common (frequency=0.002).

Full table
Full table
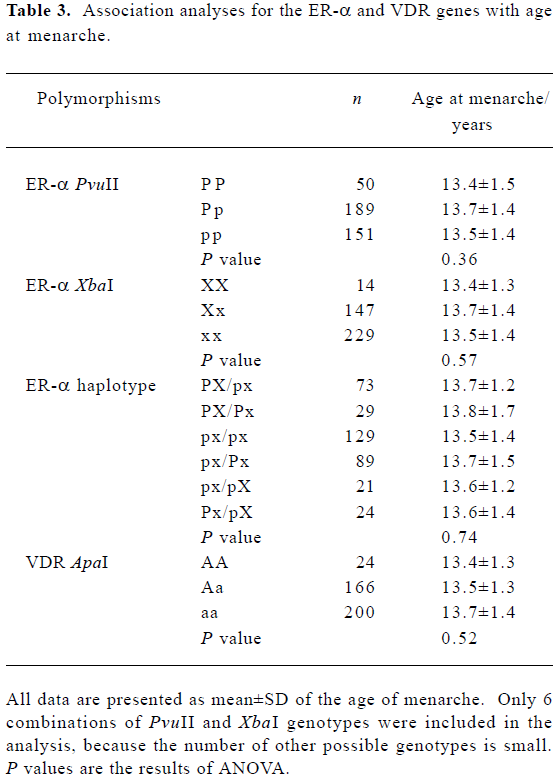
Association of the ER-α and VDR genes with the age at menarche We did not find a significant association between age at menarche and either the ER-α individual polymorphisms or the ER-α haplotypes, or the VDR ApaI locus (P>0.10) (Table 3). Evidence of an interaction between the ER-α and VDR genes was observed. With the aa genotype at the VDR gene, subjects with haplotype PX at the ER-α gene had, on average, 6 months later onset of menarche than the non-carriers (P=0.01) (Table 4).
Full table

Full table
Discussion
Age at menarche is an important complex trait, which is controlled by both genetic and environmental factors. Some genetic studies have been performed in white and black women, and significant differences between different ethnic populations have been observed, that is, the average onset of menstruation in African-American girls is 9 months earlier than that in Caucasians[23]. However, few genetic studies have been conducted in Chinese. In this study, the potential interaction effects between the ER-α and VDR genes were observed, although neither of them was significantly associated with the age at menarche individually.
A preliminary study in Greek adolescent females has shown an association between ER-α XbaI and PvuII and age at menarche[12]. Subjects with the genotype XX had later onset of menarche than those with genotypes Xx or xx. The study also found that haplotype PX homozygotes were correlated with later onset of age at menarche. However, such an association was not observed in Japanese women[7] or Dutch women[13,14]. Similar to these findings, we did not find any significant association between the ER-α XbaI or PvuII polymorphisms and age at menarche in Chinese women.
For the VDR ApaI locus, no association was obtained in the present study. However, in a Japanese population, a significant association was found between the VDR gene and age at menarche[20].
Discrepancies between our study and the other studies may be due to differences in ethnic background, sample size, ascertainment schemes, and statistical methods. For example, if the sample sizes are limited, the power to detect the association will be very low. However, for a single candidate gene that can explain approximately 10% of the variation of menarcheal age, our study sample has about 70% power to detect its association with age at menarche. In addition, gene-by-gene or gene-by-environment interactions may also influence the results of association. For example, interaction between the ER-α and VDR genes was observed in our study. To our best knowledge, this is the first study to find an interaction effect between these two genes on age at menarche. This finding suggests that the magnitude and the direction of the effects of genotypes at one locus may be affected by the specific genotype at the other loci. It also implies that the combination of genotypes at several loci may be more important than a single one. The gene-by-gene interactions may be different in different populations, so the interaction effects observed here have yet to be confirmed by separate analyses in various populations or ethnic groups.
The mechanism by which these two genes interact with each other to affect the onset of menstruation is not clear; however, from a physiological point of view, interaction is possible. An estrogen-responsive promoter region has been characterized in the VDR gene. Transcription of the VDR promoter is dependent on the estrogen receptor[24]. On the other hand, vitamin D may influence the balance between androgens and estrogens, which in turn modulates the availability of steroid hormones for their receptors[25]. In addition, vitamin D may act at several points along the estrogen response pathway, affecting the levels of estrogen receptor as well as their ability to function as enhancers of transactiva-tion[26].
Noticeably, there was a multiple-testing problem, because we tested multiple alleles in our analyses. If we use the Bonferroni correction to adjust for the multiple testing (P ≤0.006 as the significant level), the interaction between the ER-α and VDR genes (P=0.01) will be close to, but not reach, statistical significance. In this situation, the Bonferroni correction is likely to be too conservative because it assumes that all variants are independent[27]. However, the statistical tests in the present study are expected to be highly correlated. For example, the XbaI and PvuII polymorphisms are only 45 bp apart and in strong LD. Thus multiple testing corrections may lead to power loss and an increased rate of false-negative associations. To report some potentially important associations that are likely to be worthwhile pursuing further, we report the original statistical results.
For genetic investigations of complex traits, phenotype definition is a critical issue. In the present study, age at menarche was determined retrospectively based on self-report, and recall error may be inevitable. However, menarche is one of the most important milestones in a woman’s life and retrospective recall is known to be reasonably accurate: recent studies have shown a high correlation between the recalled and actual ages at menarche[28]. Furthermore, it seems unlikely that this recall bias differs in different genotypes. Thus, our results based on the recalled age at menarche may be valid. Menarcheal age is affected by nutritional level and other living environmental factors. Such information was not recorded and was not used as covariates to adjust the raw data in the present study. In this study, we tried to limit the unrelated subjects to a similar age, so they would have a similar living environment. Such a method of subject ascertainment will have improved the accuracy of our association results.
In summary, the ER-α and VDR genes individually were not associated with the age of menarche in Chinese women. However, potential interaction effects between them were observed. Further studies in other populations with larger sample sizes and denser markers are required to confirm the findings reported here.
Acknowledgements
The study was partially supported by a key project grant (30230210), an Outstanding Young Scientist Award (30025025), a general grant (30470534) from the National Science Foundation of China, three projects from the Scientific Research Fund of Hunan Provincial Education Department (02A027, 03C226, 04B039), and a grant from the Natural Science Foundation of Hunan Province (04JJ1004). Ji-rong LONG and Hong-wen DENG were partially supported by grants from the Health Future Foundation of the USA, grants from the National Institutes of Health (K01 AR02170-01, R01 GM60402-01 A1), and grants from the State of Nebraska Cancer and Smoking Related Disease Research Program (LB595), the State of Nebraska Tobacco Settlement Fund (LB692), and the US Department of Energy (DE-FG03-00ER63000/A00). We thank all of the study subjects for volunteering to participate in the study.
References
- Rees M. The age of menarche. ORGYN 1995;4:2-4.
- Ito M, Yamada M, Hayashi K, Ohki M, Uetani M, Nakamura T. Relation of early menarche to high bone mineral density. Calcif Tissue Int 1995;57:11-4.
- Treloar SA, Martin NG. Age at menarche as a fitness trait: nonadditive genetic variance detected in a large twin sample. Am J Hum Genet 1990;47:137-48.
- Kaprio J, Rimpela A, Winter T, Viken RJ, Rimpela M, Rose RJ. Common genetic influences on BMI and age at menarche. Hum Biol 1995;67:739-53.
- Chie WC, Liu YH, Chi J, Wu V, Chen A. Predictive factors for early menarche in Taiwan. J Formos Med Assoc 1997;96:446-50.
- Jorm AF, Christensen H, Rodgers B, Jacomb PA, Easteal S. Association of adverse childhood experiences, age of menarche, and adult reproductive behavior: does the androgen receptor gene play a role? Am J Med Genet 2004;125B:105-11.
- Gorai I, Tanaka K, Inada M, Morinaga H, Uchiyama Y, Kikuchi R, et al. Estrogen-metabolizing gene polymorphisms, but not estrogen receptor-alpha gene polymorphisms, are associated with the onset of menarche in healthy postmenopausal Japanese women. J Clin Endocrinol Metab 2003;88:799-803.
- Kadlubar FF, Berkowitz GS, Delongchamp RR, Wang C, Green BL, Tang G, et al. The CYP3A4*1B variant is related to the onset of puberty, a known risk factor for the development of breast cancer. Cancer Epidemiol Biomarkers Prev 2003;12:327-31.
- Stoll BA. Western diet, early puberty, and breast cancer risk. Breast Cancer Res Treat 1998;49:187-93.
- Pelletier G, El-Alfy M. Immunocytochemical localization of estrogen receptors alpha and beta in the human reproductive organs. J Clin Endocrinol Metab 2000;85:4835-40.
- Rosenfeld CS, Roberts RM, Lubahn DB. Estrogen receptor- and aromatase-deficient mice provide insight into the roles of estrogen within the ovary and uterus. Mol Reprod Dev 2001;59:336-46.
- Stavrou I, Zois C, Ioannidis JP, Tsatsoulis A. Association of polymorphisms of the oestrogen receptor alpha gene with the age of menarche. Hum Reprod 2002;17:1101-5.
- Weel AE, Uitterlinden AG, Westendorp IC, Burger H, Schuit SC, Hofman A, et al. Estrogen receptor polymorphism predicts the onset of natural and surgical menopause. J Clin Endocrinol Metab 1999;84:3146-50.
- Boot AM, van der Sluis IM, de Muinck Keizer-Schrama SM, van Meurs JB, Krenning EP, Pols HA, et al. Estrogen receptor alpha gene polymorphisms and bone mineral density in healthy children and young adults. Calcif Tissue Int 2004;74:495-500.
- Walter MR. Newly identified actions of the vitamin D endocrine system. Endocr Rev 1992;13:719-64.
- Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 1997;16:391-6.
- Bretherton-Watt D, Given-Wilson R, Mansi JL, Thomas V, Carter N, Colston KW, et al. Vitamin D receptor gene polymorphisms are associated with breast cancer risk in a UK Caucasian popula-tion. Br J Cancer 2001;85:171-5.
- Uitterlinden AG, Fang Y, Van Meurs JB, Van Leeuwen H, Pols HA. Vitamin D receptor gene polymorphisms in relation to vitamin D related disease states. J Steroid Biochem Mol Biol 2004;89–90:187-93.
- Qin YJ, Zhang ZL, Huang QR, He JM, Hu YQ, Zhao Q, et al. Association of vitamin D receptor and estrogen receptor-alpha gene polymorphism with peak bone mass and bone size in Chinese women. Acta Pharmacol Sin 2004;25:462-8.
- Kitagawa I, Kitagawa Y, Kawase Y, Nagaya T, Tokudome S. Advanced onset of menarche and higher bone mineral density depending on vitamin D receptor gene polymorphism. Eur J Endocrinol 1998;139:522-7.
- Qin YJ, Shen H, Huang QR, Zhao LJ, Zhou Q, Li MX, et al. Estrogen receptor alpha gene polymorphisms and peak bone density in Chinese nuclear families. J Bone Miner Res 2003;18:1028-35.
- Zhang YY, Long JR, Liu PY, Liu YJ, Shen H, Zhao LJ, et al. Estrogen receptor alpha and vitamin D receptor gene polymorphisms and bone mineral density: association study of healthy pre- and postmenopausal Chinese women. Biochem Biophys Res Commun 2003;308:777-83.
- Anderson SE, Dallal GE, Must A. Relative weight and race influence average age at menarche: results from two nationally representative surveys of US girls studied 25 years apart. Pediatrics 2003;111:844-50.
- Wietzke JA, Welsh J. Phytoestrogen regulation of a vitamin D3 receptor promoter and 1, 25-dihydroxyvitamin D3 actions in human breast cancer cells. J Steroid Biochem Mol Biol 2003;84:149-57.
- Willing M, Sowers M, Aron D, Clark MK, Burns T, Bunten C, et al. Bone mineral density and its change in white women: estrogen and vitamin D receptor genotypes and their interaction. J Bone Miner Res 1998;13:695-705.
- Swami S, Krishnan AV, Feldman D. 1alpha, 25-Dihydroxyvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF-7 human breast cancer cells. Clin Cancer Res 2000;6:3371-9.
- Nyholt DR. Genetic case-control association studies: correcting for multiple testing. Hum Genet 2001;109:564-7.
- Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B, et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol 2002;155:672-9.
