Effects of AMP579 and adenosine on L-type Ca2+ current in isolated rat ventricular myocytes
Recent studies showed that AMP579 was a novel adenosine agonist with high affinity for adenosine A1 and A2 receptors[1,2]. Experiments in animal models have demonstrated that AMP579 reduced infarct size by 50% to 98% when administered before a final ischemic event (mediation of ischemic preconditioning) or just before reperfusion (attenuation of reperfusion injury)[3,4]. Further experiments on pigs, dogs, and rabbits suggested that AMP579 was more powerful than adenosine in attenuating polymorphonuclear neutrophil-mediated inflammatory responses, dilating the coronary artery, reducing myocardial contracture and limiting infarct size[5,6] . Although the protective effect of AMP579 required adenosine receptor activation, adenosine could not duplicate the effects.
The difference between pharmacologic effect of AMP579 and adenosine might reflect the differences in ionic mechanisms. It has been established that adenosine could cause an attenuation of basal ICa-L only in unstimulated atrial myocytes, but under conditions of isoproterenol stimulation, adenosine could markedly attenuate isoproterenol induced-ICa-L in both atrial and ventricular myocytes. However, little is known about the electrophysiological effects of AMP579 so far. This study will examine the effects of AMP579 and adenosine on L-type calcium channel and elucidate the mechanisms underlying the cardioprotective effect of AMP579 and its utility in treatment of myocardial ischemia-reperfusion injury.
Materials and methods
Rat myocardial cell isolation Ventricular myocytes were obtained from Wistar male rats (250–300 g) by enzymatic isolation procedure. In brief, rats were killed by cervical dislocation and the heart was then immediately removed, cannulated through the aorta and perfused through the coronary artery with Ca2+-free Tyrode’s solution for 10 min. The composition of Ca2+-free Tyrode’s solution was: NaCl 140.0 mmol/L, KCl 5.4 mmol/L, MgCl2 1.0 mmol/L, NaH2PO4 0.3 mmol/L, glucose 10.0 mmol/L, HEPES 5.0 mmol/L; pH adjusted to 7.4 with NaOH at room temperature. The heart was then perfused with enzymatic solution, which was low Ca2+ (CaCl2 150 µmol/L) Tyrode’s solution with collagenase P (0.3g/L) for about 8−10min. The left ventricle was then removed. The cells were isolated by gentle agitation and kept in Krebs buffer (KB) solution, which contained: KOH 85.0 mmol/L, L-glutamic acid 50.0 mmol/L, KCl 30.0 mmol/L, taurine 20.0 mmol/L, KH2PO4 30.0 mmol/L, MgCl2 1.0 mmol/L, HEPES 10.0 mmol/L, glucose 10.0 mmol/L and egtazic acid 0.5 mmol/L; pH adjusted to 7.4 by KOH.
Electrophysiological measurement Whole-cell patch-clamp was used to record ICa-L (L-type Ca2+ currents) and membrane capacitance was measured with a P-clamp 5.51 software package (Axon Instruments, USA). Patch electrodes were made from thin-walled glass capillaries (1.5 mm outside diameter) using a two-stage vertical microelectrode puller (model PP-83, Narishige Scientific Instruments, Japan). The electrode resistance ranges 3 MΩ¸ when filled with pipette solution.
For the measurement of ICa-L, the extracellular solution contained: NaCl 140.0 mmol/L, CaCl2 1.8 mmol/L, MgCl21.0 mmol/L, KCl 5.4 mmol/L, glucose 10.0 mmol/L, NaH2PO4 0.3 mmol/L, and HEPES 10.0 mmol/L; pH adjusted to 7.4 with NaOH. The pipette solution contained: egtazic acid 10.0 mmol/L, KCl 140.0 mmol/L, Na2ATP 2.0 mmol/L, HEPES 5.0 mmol/L, 4-AP 5.0 mmol/L, MgCl2 1.0 mmol/L; pH adjusted to 7.4 with KOH. The calcium current was expressed as membrane current density (pA/pF). The cell capacitance was measured by the method previously described by Coetzee et al[9]. ICa-L was measured according to the method described by Hartzell et al[10]. The AMP579 was a gift from Department of Cardiothoracic Surgery Research Laboratory, Emory University School of Medicine, USA. AMP579 was dissolved in small volumes of Me2SO, then diluted to the desired final concentration before each experiment.
Statistic analysis Data were expressed as mean±SD. Statistical significance was determined by Student’s t-test and P<0.05 was considered significant.
Results
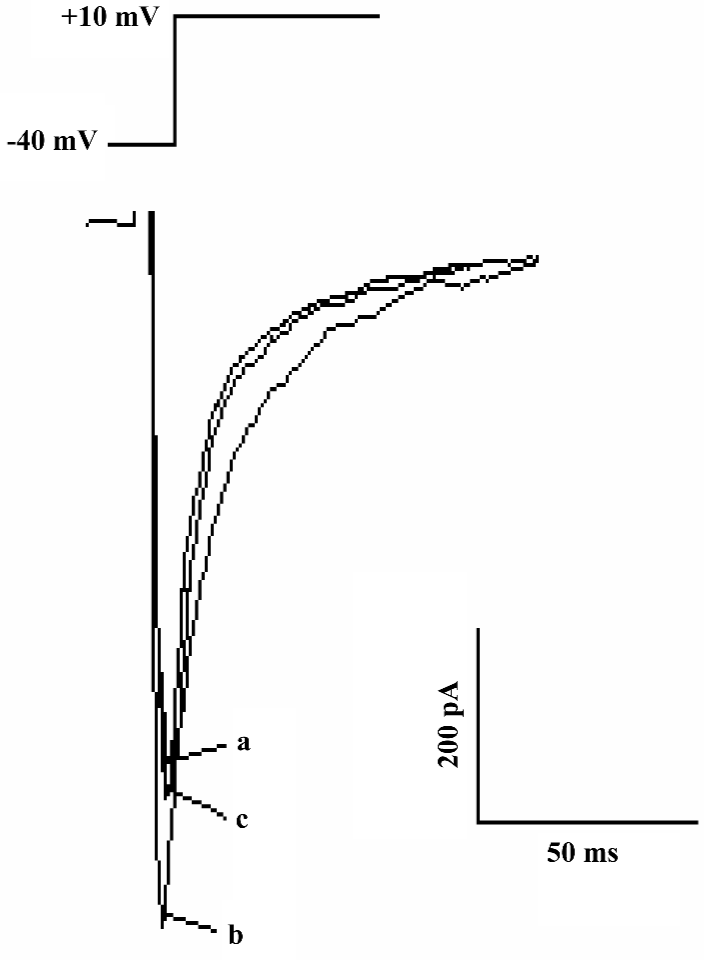
Detection of L-type calcium channel current The calcium current was activated by depolarizing pulse from a holding potential of -40 mV to +10 mV at 50 mV step-voltage. This inward current could be completely inhibited by 1 µmol/L verapmil, the basic characteristics indicated that the current present in rat ventricular myocytes was L-type Ca2+ current .
Effect of AMP579 and adenosine on L-type calcium current In the presence of adenosine at 10 nmol/L, 1, 10, and 50 µmol/L, ICa-L varied from 4.9±0.9 to 4.8±0.9, 4.9±0.9, 4.9±0.9, 4.7±0.9 pA/pF, respectively (n=5, P>0.05). Adenosine had no effect on basal ICa-L. However, when ICa-L was augmented to 2.7±0.6 pA/pF by 10 nmol/L isoproterenol, adenosine at 10 nmol/L, 1, 10, and 50 µmol/L significantly reduced it to 2.4±0.6, 2.1±0.6, 2.0±0.5, and 1.9±0.5 pA/pF, respectively (n=4, P<0.05). Adenosine showed an inhibitory effect on isoproterenol-induced ICa-L in a concentration-dependent manner with the IC50 of 13.06 µmol/L (Figure 1, 2).

Effect of AMP579 on ICa-L Isoproterenol 10 nmol/L augmented ICa-L to 3.8±0.7 pA/pF. AMP579 10 µmol/L reduced ICa-L to 2.4±0.1 pA/pF (P<0.05, n=3, Figure 3), AMP579 also showed an inhibitory effect on isoproterenol-induced ICa-L. AMP579 and adenosine (both 10 µmol/L) suppressed isoproterenol-induced ICa-L by 11.1% and 5.2%, respectively. AMP579 had a stronger inhibitory effect. In contrast to adenosine, AMP579 possessed a direct inhibitory effect on basal ICa-L in a concentration-dependent manner with the IC50 of 1.17 µmol/L (Table 1, Figure 4).


Full table

AMP579 10 μmol/L markedly reduced basal ICa-L from 2.5±1.2 to 2.0±1.0 pA/pF (n=5, P<0.05). Infusion of PD116948 30 µmol/L, an adenosine A1 receptor blocker, did not abolish the inhibitory effects of AMP579 on ICa-L (1.9±0.6 vs 2.0±1.0 pA/pF, P>0.05). But under the same conditions AMP579 10 µmol/L markedly reduced the ICa-L from 2.4±0.4 to 1.8±0.4 pA/pF (n=4, P<0.01). Infusion of 0.4 µmol/L GF109203X, a PKC blocker, significantly reversed it to 2.2±0.4 pA/pF (P<0.05, Figure 5). So GF109203X could abolish the inhibitory effect of AMP579, indicating that the inhibitory effect on basal ICa-L by AMP579 was induced through activating PKC but not linked to the adenosine A1 receptor.

Discussion
In cardiac tissue, a direct inhibition of basal ICa-L by adenosine has only been demonstrated in guinea-pig atrial and ferret ventricular myocytes[11,12]. But in the presence of isoproterenol stimulation, adenosine has prominent inhibitory effects on ICa-L in ventricular myocytes[13]. These may reflect differences in receptor-effector coupling mechanisms, the level of basal adenylate cyclase activity, the basal phosphorylated state of Ca2+ channels and/or the effect of phosphorylation on the gating of L-type Ca2+ channel. Consistent with previous reports, our experiment shows that adenosine has no direct inhibitory effect on basal ICa-L in the rat ventricle, but in the condition that isoproterenol was previously administered, adenosine shows an inhibitory effect on the ICa-L induced by isoproterenol with an IC50 of 13.06 µmol/L, suggesting that adenosine exerts an indirect inhibitory effect on ICa-L in the rat ventricle by inhibition of isoproterenol stimulation.
In contrast to adenosine, AMP579 shows a direct inhibitory effects on basal ICa-L in the rat ventricle with IC50 of 1.17 µmol/L. The blocking of Ca2+ influx by L-type Ca2+ channel could serve as an efficient method for protecting the ischemic myocyte by minimizing ischemia-induced Ca2+ overload and irreversible cell contracture and autodigestion by Ca2+-dependent proteases[14]. Therefore, by reducing both basal ICa-L and isoproterenol-induced ICa-L, AMP579 will play a more important role in negative chronotropic and negative dromotropic effects. These action mechanism differences between AMP579 and adenosine may account for the contribution of AMP579 in reducing neutrophil-mediated inflammatory reaction, inhibiting cardiac contraction, dilating coronary vessels, attenuating ischemia and reperfusion injury.
Our study does not show that adenosine A1 receptor is linked to inhibition of AMP579 on basal ICa-L. At present, available data indicate that three pathways are involved in receptor-linked downstream mechanisms for inhibition of ICa-L by adenosine. The first is cAMP-PKA, as PKA increase ICa-L by phosphorylation on the gating of the L-type calcium channel, inhibitions of adenylate cyclase and reductions of cAMP and PKA levels by adenosine result in attenuation on ICa-L[12]. Second is that activation of guanylate cyclase results in increments of intracellular cGMP and PKG concentration, which in turn inhibits phosphorylation on the gating of the L-type calcium channel[15]. The third is modulated by PKC, because there are different PKC subunits which result in different effects[16]. Our experiment finds that special PKC antagonist GF109203X can totally eliminate inhibitory effects of AMP579 on ICa-L, suggesting that AMP579 exerts a direct inhibitory effects on the L-type calcium channel through the PKC pathway.
References
- Nakamura M, Zhao ZQ, Clark KL, Velez DV, Guyton RA, Vinter-Johansen J. A novel adenosine analog, AMP579, inhibits neutrophil activation, adherence and neutrophil-mediated injury to coronary vascular endothelium. Eur J Pharmacol 2000;397:197-205.
- Sledeski AW, Kubiak GG, O’Brien MK, Powers MR, Powener TH, Truesssdale LK. Efficient synthesis of AMP579, a novel adenosine A1/A2 receptor agonist. J Org Chem 2000;65:8114-9.
- Budde JM, Velez DA, Zhao ZQ, Clark KL, Morris CD, Muraki S, et al. Comparative study of AMP579 and adenosine inhibition of neutrophil-mediated vascular and myocardial injury during 24 h of reperfusion. Cardiovasc Res 2000;47:294-305.
- Xu Z, Downey JM, Cohen MV. AMP579 reduces contracture and limits infarction in rabbit heart by activating adenosine A2 receptors. J Cardiovasc Pharmacol 2001;38:474-81.
- Mcvey MJ, Smiths GJ, Cox BF, Kitzen JM, Clark KL, Perrone MH. Cardiovascularpharmacology of the adenosine A1/A2- receptor agonist AMP579: coronary hemodynamic and cardiopro-tective effects in the canine myocardium. J Cardiovasc Pharmacol 1999;33:701-10.
- Smits GJ, Mcvey M, Cox BF, Perrone MH, Clark KL. Cardio-protective effects of the novel A1/A2 receptor agonist AMP579 in a porcine model of myocardial infarction. J Pharmacol Exp Ther 1998;286:611-8.
- Pelleg A, Belardinelli C. Cardiac electrophysiology and pharmacology of adenosine: basic and clinical aspects. Cardiovasc Res 1993;27:54-61.
- Belardinelli L, Linden J, Berne RM. The cardiac effects of adenosine. Prog Cardiovasc Dis 1989;32:73-97.
- Coetzee WA, Ichikawa H, Hearse DJ. Oxidant stress inhibits Na+/Ca2+ exchange in cardiac myocytes: mediation by sulfhydryl groups? Am J Physiol 1994;266:H909-19.
- Hartzell HC, Simmons MA. Comparison of effects of acetylcholine on calcium and potassium currents in frog atrium and ventricle. J Physiol 1987;89:411-22.
- Cerbai E, Klockner U, Isenberg G. Ca2+-antagonistic effects of adenosine in guinea-pig atrial cell. Am J Physiol 1988;255:H872-8.
- Qu Y, Campbell DL, Whorton AR, Strauss HC. Modulation of basal L-type Ca2+ current by adenosine in ferret isolated right ventricular myocytes. J Physiol 1993;471:269-93.
- Isenberg G, Belardinelli L. Ionic basis for the antagonism between adenosine and isoproterenol on isolated mammalian ventricular myocytes. Circ Res 1984;55:309-25.
- Eckert R, Utz J, Trautwin W, Mentzer RM, Wis M, Saar H. Involvement of intracellular Ca2+ release mechanism in adenosine-induced cardiac Ca2+ current inhibition. Surgery 1993;114:334-42.
- Shen JB, Pappano AJ. On the role of phosphatase in regulation of cardiac L-type calcium current by cyclic GMP. J Pharmacol Exp Ther 2002;301:501-6.
- Kameyama M, Hofmann F, Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca2+ channel in the guinea-pig heart. Pflugers Arch 1985;405:285-93.
