Genistein inhibits carotid sinus baroreceptor activity in anesthetized male rats1
Introduction
Phytoestrogens are plant-derived diphenolic compounds that are structurally and functionally similar to estradiol. Accumulating evidence indicates that phytoestrogens may confer cardiovascular protection[1-3]. Genistein (GST), one of the most well-known phytoestrogens, is an isoflavone that is also a specific inhibitor of protein tyrosine kinase (PTK)[4]. It has been demonstrated that GST has a hypocho-lesterolemic effect in animals and humans, and is able to inhibit low density lipoprotein (LDL) oxidation, endothelial cell proliferation and angiogenesis[5], and to enhance the dilator response to acetylcholine of atherosclerotic arteries[6]. All of these effects may predict a favorable impact on the cardiovascular system. Li et al reported that GST decreased the contractile response of the aortic artery in vitro[7]. Furthermore, our previous studies showed that GST decreased the vascular tone in the femoral, renal and mesenteric vascular beds via protein tyrosine kinase (PTK) inhibition[8], reduced infarct size and apoptosis of myocytes in ischemia/reperfusion rabbit heart[9], and inhibited the voltage-dependent Ca2+ channel in isolated guinea pig ventricular myocytes[10]. Whether GST affects carotid baroreceptor activity (CBA) remains to be clarified. The aim of the present study was to observe the effects of GST on CBA in anesthetized male rats with perfusing isolated carotid sinus, and to elucidate the mechanism involved.
Materials and methods
Animals Sprague-Dawley rats (♂, 350 ± 20 g, Grade II, Certificate N
Perfusion of left carotid sinus The method of isolating the carotid sinus was described in our previous study[11,12]. The left carotid sinus areas were fully exposed by turning the trachea and the esophagus rostrally. Sternohyoideus muscles and superior laryngeal nerves were sectioned. The bilateral aortic nerves, right carotid sinus nerves, cervical sympathetic nerves and recurrent laryngeal nerves were all sectioned. The common, external and internal carotid arteries and smaller arteries originating from these vessels were exposed and ligated, while carefully leaving the left carotid sinus nerve undisturbed. Ligation of the occipital artery at its origin from the external carotid artery excluded chemoreceptors from the isolated carotid sinus, thereby preventing chemoreceptor activation secondary to decreased carotid sinus pressure. A plastic catheter introduced into the left common carotid artery in the anterograde way (serving as an inlet tube) was attached to a peristaltic pump that controlled the intrasinus pressure (ISP). ISP was monitored by a polygragh (RM-6240, Chengdu Instrument Factory, Chengdu, China) connected to the inlet tube. A plastic catheter inserted into the external carotid artery served as an outlet tube. The carotid sinus was then perfused with warm (37 °C) modified Krebs-Henseleit (K-H) solution (NaCl 118.0 mmol/L, NaHCO3 25.0 mmol/L, KCl 4.7 mmol/L, KH2PO41.2 mmol/L, MgSO4 1.2 mmol/L, CaCl2 2.5 mmol/L, glucose 5.6 mmol/L, pH 7.35?7.45) bubbled with 95% O2 and 5% CO2.
Recording of sinus nerve afferent discharge The left carotid sinus nerve was cut near the glossopharyngeal nerve and desheathed carefully. The isolated sinus nerve and surrounding structures were immersed in warm (37 °C) liquid paraffin to avoid drying of the tissues. The sinus nerve was placed on a bipolar platinum electrode and the bioelectrical signal was recorded on a polygragh (RM-6240, Chengdu Instrument Factory), with an integral time of 5 s. ISP and discharge of sinus nerve were recorded synchronously and at the end of the experiment, the integral of sinus nerve activity (ISNA) was obtained and measured.
Protocols With a computer-controlled program[13], ISP was altered in a stepwise manner by perfusing the left carotid sinus with K-H solution. After ISP was lowered from 100 mmHg to 0 mmHg, it began to increase slowly to 240 mmHg in a staircase manner, and then decreased to 0 mmHg in the same manner, and again stabilized at 100 mmHg. Each step of the staircase changed the ISP by 30 mmHg and lasted for 15 s. The functional curve for the ISP-ISNA relationship was constructed, and the functional parameters of carotid baroreceptor activity, such as peak slope (PS), peak integral value (PIV), threshold pressure (TP), saturation pressure (SP) and operation range (OR) were determined. TP was the ISP at which ISNA began to increase by 15% in response to an increase in ISP. SP was the ISP at which ISNA just showed no further increase with an increase in ISP. OR was calculated as the difference between SP and TP.
On perfusing the carotid sinus with K-H solution, the functional curve of carotid baroreceptor (FCCB) was drawn, obtaining the control parameters of TP, SP, OR, PS, and PIV. ISP was then fixed at 100 mmHg for 20 min, and K-H solution containing GST at 50, 100, and 200 µmol/L was then perfused to examine the changes in ISNA, followed by measurement of the parameters again. Finally, the carotid sinus was perfused with K-H solution as a postcontrol.
The effect of NG-nitro-L-arginine methyl ester (L-NAME) on the response to GST was examined. After the control parameters of CBA were obtained, the isolated carotid sinus was perfused with K-H solution containing 100 µmol/L L-NAME for 20 min, and the above parameters were measured. Then GST 100 µmol/L was added to perfuse the sinus area. The parameters were measured within 15 min, and the drugs were then washed out with K-H solution. To determine whether Ca2+ was involved in the effect of GST, one experimental group was treated with 500 nmol/L Bay K8644 for 20 min before GST was added. To further determine the involvement of PTK, pretreatment with a potent inhibitor of tyrosine phosphatase, sodium orthovanadate (1 mmol/L), was carried out.
Drugs Genistein (purity 99%, Sigma, St Louis, MO, USA) was prepared with dimethyl sulphoxide. The final concentration of dimethyl sulphoxide in the perfusing solution was lower than 0.05%. L-NAME (Sigma) and sodium orthovanadate (Sigma) were dissolved in saline. Bay K8644 (Sigma) was dissolved in 99% ethyl alcohol. No changes in ISNA were observed during perfusion with ethyl alcohol (1:2000).
Statistical analysis All data are presented as mean±SD. The significance of group differences was determined by ANOVA and t-test. Differences were considered significant when P<0.05.
Results
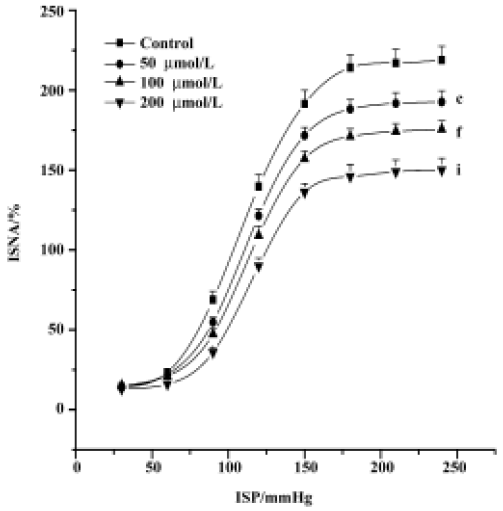
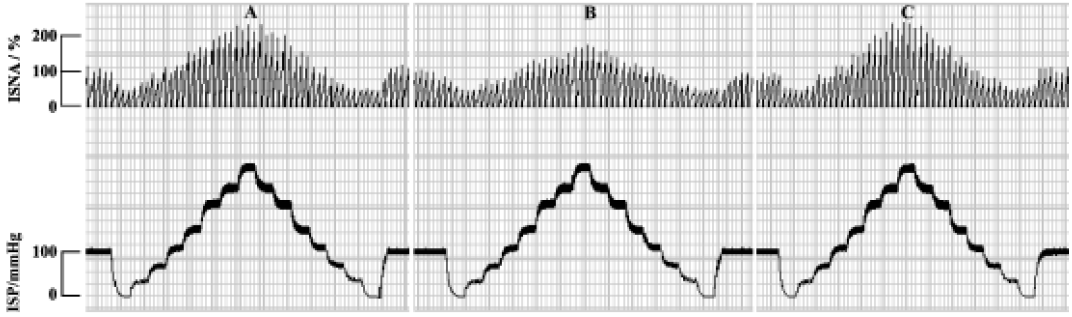
Effect of GST on carotid baroreceptor activity By perfusing carotid sinus with K-H solution and elevating ISP from 0 mmHg to 240 mmHg in a stepwise manner, ISNA was increased. There was no difference in CBA parameters among the controls. As compared with control groups, treatment with GST decreased PIV and PS, and increased TP and SP, shifting FCCB to the right and downward (Table 1, Figure 1). The above effects occurred within 5 min of perfusing the carotid sinus with GST, and reached a peak during 8?12 min. Figure 2 is an original tracing showing the effects of GST on ISNA.
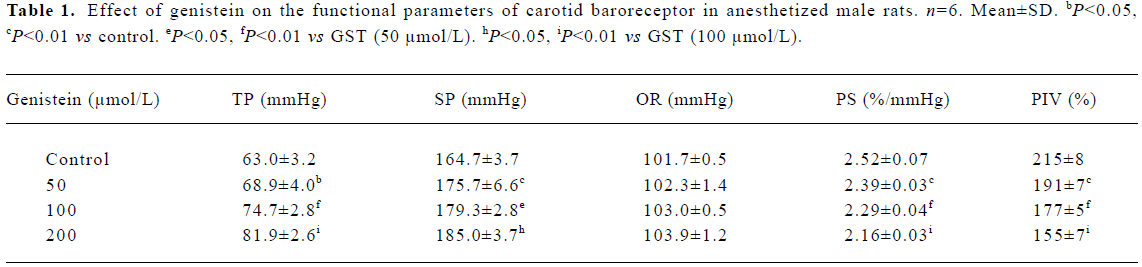
Full table
Effect of L-NAME on GST responses L-NAME (100 µmol/L) did not induce any change in the functional parameters of the carotid baroreceptor, and also did not influence the effect of 100 µmol/L GST (Table 2).
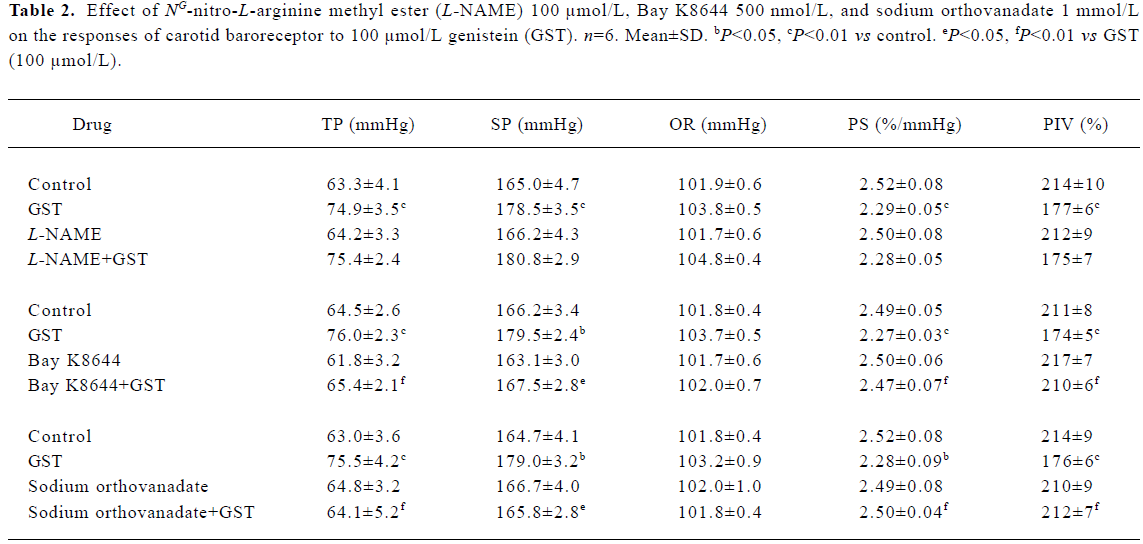
Full table
Effect of Bay K8644 on GST responses Bay K8644 (500 nmol/L) did not produce any change in the functional parameters of the carotid baroreceptor, but completely blocked the action of GST (Table 2).
Effect of sodium orthovanadate on GST res-ponses Sodium orthovanadate (1 mmol/L) did not change CBA, but completely blocked the effect of GST (Table 2).
Discussion
The present study demonstrated that GST inhibited CBA in a concentration-dependent manner. By perfusing the left carotid sinus baroreceptor with GST, the FCCB were shifted to the right and downward, with a reduction in PS and PIV, indicating the inhibitory action of GST on CBA. It is well established that the arterial baroreceptors play an important role in the short-term control of cardiovascular activity. Inhibition of CBA is inclined to cause increased arterial blood pressure that can antagonize hypotensive effects caused by other ingredients.
As nitric oxide synthase (NOS) is present in afferent baroreceptor fibers innervating the carotid sinus[14], and increasing evidence has shown that nitric oxide (NO) may suppress the action potential of baroreceptors[15,16]. Further-more, our previous study demonstrated that 17β-estradiol inhibited CBA via endothelial NO release. NO suppressed Na+ current in baroreceptor neurons and activated the calcium-dependent K+ channels localized in vascular smooth muscle, then hyperpolarized baroreceptor neurons[17]. Both of these mechanisms may account for the inhibitory effect of 17β-estradiol on CBA. In the present study, pretreatment with L-NAME, a non-selective inhibitor of NOS, did not affect the action of GST, thus suggesting that locally released NO was not involved in the effect of GST on CBA. This result indicated that GST and 17β-estradiol inhibited CBA via different pathways.
It has been reported that a mechanosensitive ion channel is localized on the baroreceptor neurons, and that vascular distention is effectively translated to the deformation of the afferent nerve endings as arterial pressure rises. Deformation then depolarizes the nerve endings by opening non-selective cation channels to create a generator potential that triggers action potential discharge[18,19]. Moreover, it has been demonstrated that stretching of the walls of the carotid sinus may induce an increase in Ca2+ influx on baroreceptor neurons, which is mediated by stretch-activated channels[16]. Suppressing Ca2+ influx through the stretch-activated channels may be an important pathway in regulating CBA. Studies have shown that agmatine[20], cholecystokinin octapeptide[21] and aminoglycoside antibiotics (such as streptomycin)[22] can inhibit CBA in this way. We have observed that pretreatment with L-type calcium channel agonist Bay K8644 completely blocks the inhibitory effect of GST on CBA. Based on the above observations, it may be concluded that GST inhibits CBA through a decrease in Ca2+ influx by blocking the stretch-activated channels.
Genistein has also proved to be a specific inhibitor of PTK. Evidence has been presented to suggest that enhanced tyrosine phosphorylation participates in the mechanisms that regulate the contraction of smooth muscle[5]. Vanadate, an inhibitor of tyrosine phosphatase, can enhance protein tyrosine phosphorylation[6]. PTK has been described as an important modulator regulating the tone of vascular smooth muscle[23]. Our present study showed that the effect of GST on CBA was inhibited by pretreatment with sodium orthova-nadate, suggesting that the PTK pathway is involved. From this data together with our previous findings, it may be inferred that GST inhibits PTK, relaxes the vascular smooth muscle, and then attenuates the stretch-activated Ca2+ channels on the baroreceptor neurons.
In summary, the present study has revealed that GST inhibits CBA, and the effect may be mediated by PTK inhibition and a decrease in Ca2+ influx through the stretch-activated channels.
References
- Anthony MS, Clarkson TB, Williams JK. Effects of soy isoflavones on atherosclerosis: potential mechanisms. Am J Clin Nutr 1998;68 Suppl:S1390-3. [PubMed]
- Anthony MS, Clarkson TB. Association between plasma isoflavone and plasma lipoprotein concentrations. J Med Food 1999;2:263-6. [PubMed]
- Figtree GA, Griffiths H, Lu YQ, Webb CM, Macleod K, Collins P. Plant-derived estrogens relax coronary arteries by a calcium antagonistic mechanism. J Am Coll Cardiol 2000; 35: 1977-85.
- Akiyame T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, et al. Genistein: a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 1987;262:5592-5. [PubMed]
- Fotsis T, Pepper M, Adlercreuta H, Fleischmann G, Hase T, Montesano R, et al. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci USA 1993;90:2690-4. [PubMed]
- Honore EK, Williams JK, Anthony MS, Clarkson TB. Soy isoflavones enhance coronary vascular reactivity in atherosclerotic female macaques. Fertil Steril 1997;67:148-54. [PubMed]
- Li HF, Wang LD, Qu SY. Phytoestrogen genistein decreases contractile response of aortic artery in vitro and arterial blood pressure in vivo. Acta Pharmacol Sin 2004;25:313-8. [PubMed]
- Ji ES, Zhang LH, Wang YH, Yue H, He RR. Responses of regional vascular beds to local injection of genistein in rats. Acta Physiol Sin 2003;55:255-9. [PubMed]
- Ji ES, Yue H, Wu YM, He RR. Effects of phytoestrogen genistein on myocardial ischemia/reperfusion injury and apoptosis in rabbits. Acta Pharmacol Sin 2004;25:306-12. [PubMed]
- Ji ES, Wang C, He RR. Effects of genistein on intracellular free calcium concentration in guinea pig ventricular myocytes. Acta Physiol Sin 2004;56:204-9. [PubMed]
- Zhao G, Ho SY. The facilitating effect of atrial natriuretic peptide in the carotid sinus baroreflex function. Acta Physiol Sin 1991;43:360-7. [PubMed]
- Zhang H, Liu YX, Wu YM, Wang ZM, He RR. Capsaicin facilitates carotid sinus baroreceptor activity in anesthetized rats. Acta Pharmacol Sin 2004;25:1439-43. [PubMed]
- Yi XL, Fan ZZ, He RR. An automatic system controlled by computer for carotid sinus perfusion. Chin J Appl Physiol 1993;9:156-9.
- Hohler B, Mayer B, Kummer W. Nitric oxide synthase in the rat carotid body and carotid sinus. Cell Tissue Res 1994;276:559-64. [PubMed]
- Li Z, Chapleau MW, Bates JN, Bielefeldt K, Lee HC, Abboud FM. Nitric oxide as an autocrine regulator of sodium currents in baroreceptor neurons. Neuron 1998;20:1039-49. [PubMed]
- Chapleau MW, Hajduczok G, Sharma RV, Wachtel RE, Cunningham JT, Sullivan MJ, et al. Mechanisms of baroreceptor activation. Clin Exp Hypertens 1995;17:1-13. [PubMed]
- Wang S, Fan ZZ, He RR. 17β-Estradiol inhibits carotid sinus baroreceptor activity in anesthetized male rats. Acta Pharmacol Sin 2001;22:440-4. [PubMed]
- Grigg P. Biophysical studies of mechanoreceptors. J Appl Physiol 1986;60:1107-15. [PubMed]
- Drummond HA, Welsh MJ, Abboud FM. EnaC subunits are molecular components of the arterial baroreceptor complex. Ann NY Acad Sci 2001;940:42-7. [PubMed]
- Qin XM, Fan ZZ, He RR. Agmatine inhibits the afferent activity of carotid baroreceptor in rats. Acta Physiol Sin 2001;53:137-41. [PubMed]
- Liu YX, Zhang H, Dong JH, Li Q, He RR. Cholecystopinin octapeptide inhibits carotid sinus baroreceptor activity in rats. Chin J Pharmacol Toxicol 2005;19:18-23.
- Qin XM, He RR. Role of calcium in the mechanism underlying the inhibitory effect of streptomycin on carotid sinus baroreflex in rats. Acta Physiol Sin 2000;52:463-7. [PubMed]
- Hughes A, Wijetunge S. Role of tyrosine phosphorylation in excitation-contraction coupling in vascular smooth muscle. Acta Physiol Scand 1998;164:457-69. [PubMed]
