Effects of l-tetrahydropalmatine on locomotor sensitization to oxycodone in mice
Introduction
The term ‘behavioral sensitization’ is used to describe the augmented behavior activity produced by a given dose of an opioid-drug after repeated intermittent injections[1]. Recently, the importance of behavioral sensitization in drug abuse research has been realized. Studies have shown that behavioral sensitization has a close relationship with relapse, compulsive drug-seeking and drug-taking behaviors[2–5]. Investigation of sensitization may be helpful for better understanding of the relapse mechanisms and for providing new strategies for the treatment of drug addiction.
Oxycodone (4,5-epoxy-14-hydroxy-3-methoxy-17-methyl-morphinan-6-one) is a semi-synthetic derivative of the naturally occurring opium alkaloid, thebaine. Oxycodone is an opioid receptor agonist similar to morphine[6–8]. It is reported that the abuse potential of oxycodone is equivalent to that of morphine[9]. It has been found that withdrawal syndrome may occur in patients when high doses or the chronic treatment of oxycodone is broken or weakened, but the effects of oxycodone on locomotor behavior sensitivity in animals has not been documented. Therefore, the present study was designed to investigate whether acute injection of oxycodone would induce hyperlocomotor activity and chronic administration of oxycodone would induce locomotor sensitization in mice.
l-Tetrahydropalmatine (l-THP) is an active principle of Corydolis yanhusuo, a Chinese traditional herb used as an analgesic[10]. It is reported that l-THP possesses a blocking effect on dopamine D1 and D2 receptors and voltage-sensitive Ca2+ channels[11]. It has been suggested in recent studies that l-THP can inhibit physical dependence in morphine-dependent mice and significantly reduce the development of the conditional place preference induced by morphine in mice[12,13]. However, no research has been carried out on the effects of l-THP on locomotor sensitization. Therefore, it is interesting to determine whether pretreatment with l-THP prior to administration of oxycodone would inhibit the hyperactivity induced by oxycodone and prevent the development and expression of locomotor activity to oxycodone.
Materials and methods
Animals Kunming mice, initially weighing 18–22 g, were purchased from the Experimental Animal Center of Beijing Institute of Pharmacology and Toxicology. The animals were fed ad libitum and were housed in a room with a controlled ambient temperature (22±2 oC), humidity (50%±10%), and a 12-h light/dark cycle. Animals were acclimated to the housing conditions and handled for 3–4 d before experiments. All experiments were performed between 08.00 h and 16.00 h. All experiments were conducted according to the NIH Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996). The experimental procedures were approved by the local Committee on Animal Care and Use.
Drugs Oxycodone, obtained from Beijing Four-Ring Pharmaceutical Factory (Beijing), was dissolved in 0.9% saline injected subcutaneously. l-THP, kindly provided by Professor Guo-zhang JIN (Shanghai Insititute of Materia Medica, Chinese Academy of Sciences), was dissolved in distilled water and administered intragastrically.
Apparatus Locomotor activity was counted automatically with Small Animal Locomotion Recording Apparatus (Institute of Materia Medica, Chinese Academy of Medical Science), which consisted of four boxes (20 cm in diameter and 15 cm in height) with six photoelectric infrared sensors 2 cm above the floor of each box. The sensors detect the movements of the mice through infrared radiation.
Experimental procedures
Acute effects of oxycodone on locomotor activity in mice Mice were put into the test boxes immediately after treatment with saline or oxycodone (1.25, 2.5, and 5.0 mg/kg, sc). Locomotor counts were measured every 10 min for 90 min.
Development of locomotor sensitivity to oxycodone in mice Two groups of 10 mice each were given oxycodone or saline for 7 consecutive days, and their activity was measured for 60 min immediately after each administration. The experimental period for the 7 d remained at approximately the same time everyday during the daytime.
Effects of acute and chronic l-THP on locomotor activity in mice Four groups of mice were given l-THP (6.25, 12.5, and 18.75 mg/kg) or saline, respectively, once per day for 7 consecutive days, followed by a 5-d withdrawal period. On d 13, all animals were challenged with saline. On d 1, 7, and 13, after 40-min treatment with l-THP or saline, the mice were put into the test boxes and locomotor activity was monitored for 60 min.
Effects of l-THP on the acute oxycodone-induced hyperactivity in mice Five groups of mice were administered with one of the following drug pairs: saline+saline, saline+oxyco-done, and l-THP (6.25, 12.5, and 18.75 mg/kg)+oxycodone with a 40-min interval between the two treatments. After the second treatment, the mice were put into the test boxes to record their locomotor activity for 60 min.
Effects of l-THP on the development of oxycodone sensitization To assess the effects of l-THP on the development of oxycodone sensitization, five groups of mice were administered for 7 consecutive days with one of the following drug pairs: saline+saline, saline+oxycodone, and l-THP (6.25, 12.5, and 18.75 mg/kg)+oxycodone. The interval between l-THP and oxycodone injections was 40 min, with l-THP given prior to the oxycodone. After 5 washout periods, all animals were injected with oxycodone (5 mg/kg) and then put into the test chambers to record their locomotor activity for 60 min.
Effects of l-THP on the expression of oxycodone sensitization Mice were injected with 5 mg/kg oxycodone for 7 consecutive days to induce locomotor sensitization. After 5 days of washout, the mice were challenged with 5 mg/kg oxyco-done, and with either saline or l-THP (6.25, 12.5, and 18.75 mg/kg), given 40 min prior to the oxycodone challenge. The locomotor activity of the mice was then measured for 60 min.
Statistics The results were expressed as the mean±SEM. In experiment acute effects of oxycodone on locomotor activity in mice and development of locomotor sensitivity to oxycodone in mice locomotor activity was analyzed using a two-way ANOVA. Post hoc comparisons were performed using Tukey’s test. For the other experiments, statistical analyses were performed using one-way ANOVA and a post hoc Tukey’s test. P<0.05 was considered statistically significant. Calculations were performed using the SPSS statistical package.
Results
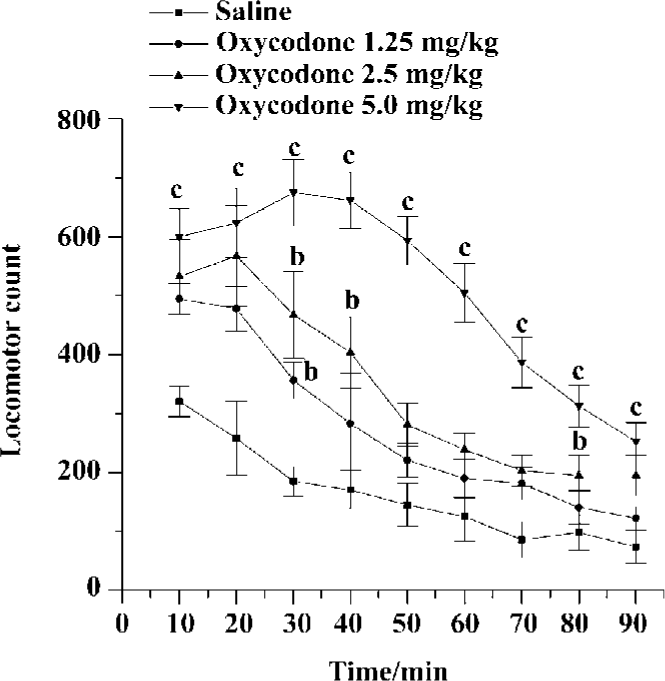
Acute effects of oxycodone on locomotor activity in mice Mice were given saline, oxycodone (1.25, 2.5, or 5 mg/kg), then locomotor activity was monitored for 90 min. Locomotor acounts showed a great difference between saline-treated mice and oxycodone-treated mice. Oxycodone dose-dependently induced locomotor response in mice during the 90-min test session [F (treatment) (3, 33)=16.598, P<0.01; F (treatment×time) (24, 424)=6.080, P<0.01]. During the first and the last 10 min, there was a significant difference between the saline-treated and oxycodone-treated group (5 mg/kg, sc). The climax of oxycodone-induced hyperactivity appeared approximately 30–40 min after the treatment of oxycodone. The psychomotor effect of 5 mg/kg oxycodone lasted about 90 min, and 1.25, 2.5 mg/kg oxycodone increased locomotor activity only at some time points (Figure 1).
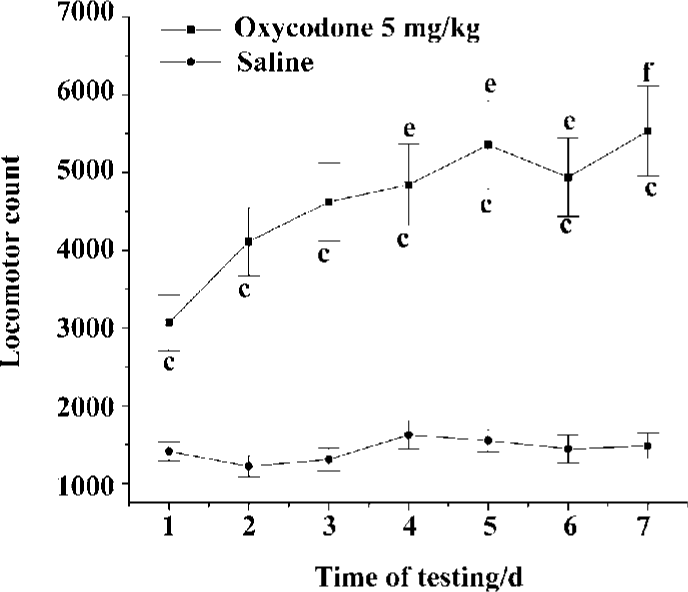
Development of locomotor sensitivity to oxycodone in mice Figure 2 showed the total 60-min activity counts after 7 repeated administrations of oxycodone or saline to the mice in the test boxes. The activity counts were dependent on the drug [F (1,126) =20.764, P<0.01] and number of administrations [F (6,126)=73.246, P<0.01]. There was a significant interaction between the drug given and the number of administrations [F (6,126)=45.00, P<0.01]. The locomotor activity showed significant enhancement in the fourth injection compared to the initial injection. There was no significant difference among saline groups.
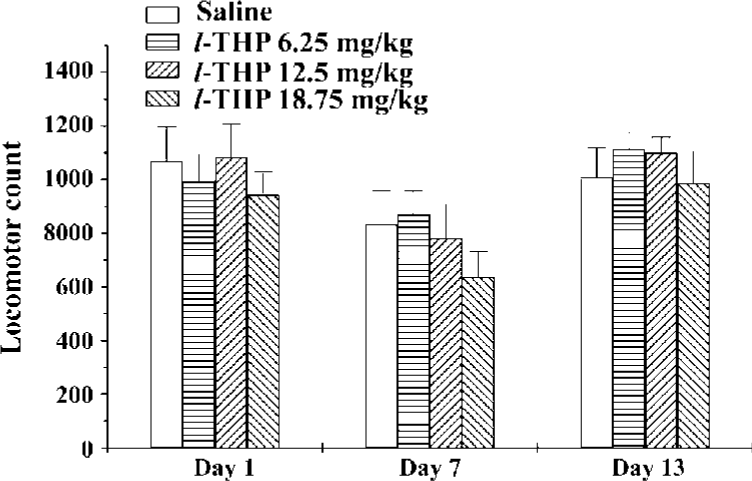
Effects of acute and chronic l-THP on locomotor activity in mice Mice were given l-THP (6.25, 12.5, and 18.75 mg/kg, ig) for 7 consecutive days, then subjected to withdrawal from l-THP for 5 d. On d 13, all animals were challenged with saline. On d 1, 7, and 13, 40 min after injection of l-THP or saline, the mice were put into the test boxes and locomotor counts were measured for 60 min. On d 1 and 7, there was no difference between the l-THP groups (6.25, 12.5, and 18.75 mg/kg) and saline group [F (3, 37)=1.360, P>0.05, F (3, 37)=0.348, P>0.05, respectively]. On d 13, there was also no significant difference between l-THP groups and saline groups after administration of saline [F (3, 37)=1.532, P>0.05]. These results indicated that acute or chronic pretreatment with l-THP at the dose of 6.25, 12.5, and 18.75 mg/kg might not affect locomotor activity in mice (Figure 3).
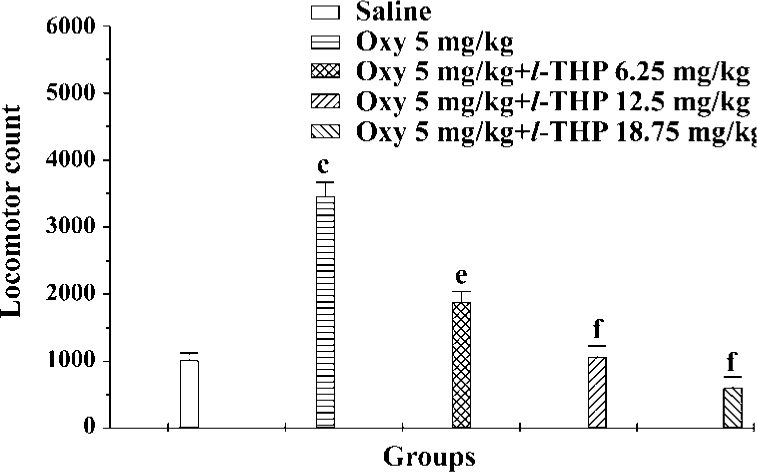
Effects of l-THP on acute oxycodone-induced hyperactivity in mice Locomotor counts were greatly increased in the oxycodone group compared with the saline group. l-THP at doses of 6.25, 12.5, and 18.75 mg/kg antagonized hyperactivity induced by oxycodone [F (4, 60)=15.76, P<0.01] (Figure 4).
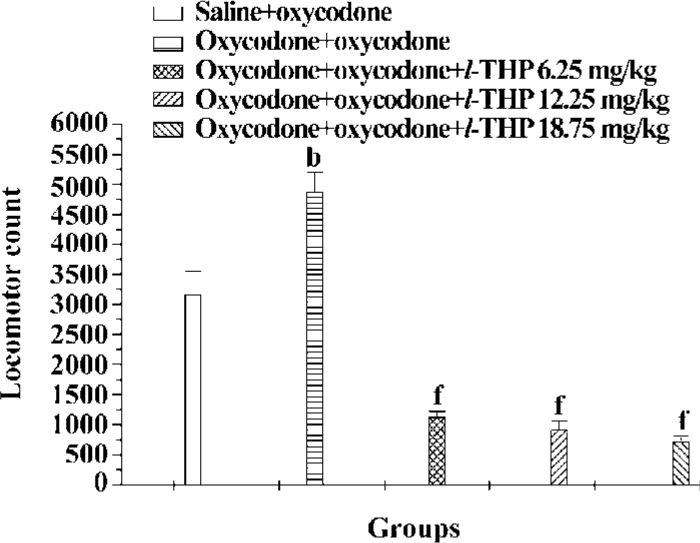
Effects of l-THP on the development of oxycodone sensitization Figure 5 showed that the psychomotor effect of oxycodone was significantly enhanced in mice pretreated with oxycodone (5 mg/kg×7, sc), 5 d cessation of treatment. l-THP (6.25, 12.5 mg/kg) did not affect the magnitude of sensitization, but there was a marked difference between oxycodone+oxycodone group and l-THP (18.75 mg/kg)+oxycodone+oxycodone group, indicating that l-THP (18.75 mg/kg) greatly inhibited the development of oxycodone sensitization [F (4, 62) =8.766, P<0.01].
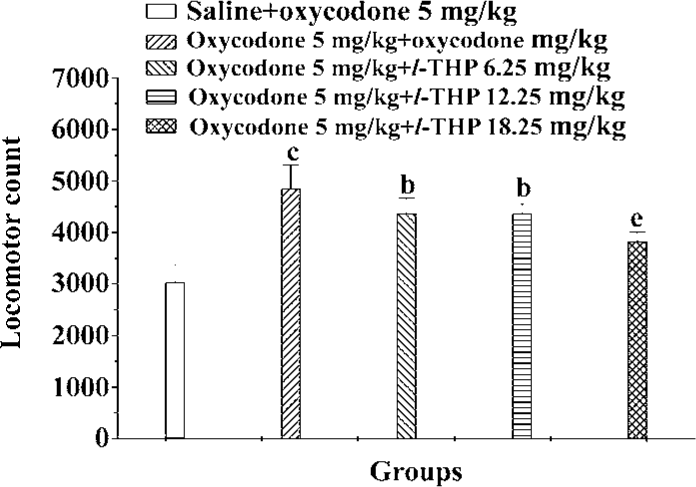
Effects of l-THP on expression of oxycodone sensitization Our protocol induced great locomotor sensitization to oxycodone in oxycodone+oxycodone group compared to the saline+oxycodone group. There were great differences between oxycodone+oxycodone group and l-THP (6.25, 12.5, 18.75 mg/kg)+oxycodone+oxycodone groups [F (4, 65)=24.128, P<0.01]. In all, l-THP (6.25, 12.5, and 18.75 mg/kg), administered 40 min before the challenge doses of oxycodone, inhibited the expression of oxycodone sensitization (Figure 6).
Discussion
In our research, acute administration of oxycodone increased locomotor activities in mice and those effects were progressively enhanced by the repeated injection of oxycodone, indicated by the development of behavioral locomotor activity (Figure 1). In addition, locomotor activities were increased when the mice were treated with oxycodone after a 7-d period of washout, which attributed to the expression phase (Figure 2). Oxycodone has been used clinically for over 80 years, but its pharmacological properties are still poorly characterized. The present results showed that the repeated administration of oxycodone in mice induced behavioral locomotor sensitization similar to morphine.
l-THP, an active principle of Corydolis yanhusuo, at doses of 6.25, 12.5, and 18.75 mg/kg per se did not affect locomotor activity in mice treated with acute or chronic administration, but inhibited hyperactivity, and the development and expression of locomotor sensitivity induced by oxycodone (5 mg/kg, sc).
Behavioral sensitization consists of two phases: development/induction and expression. There is evidence suggesting that the induction and the expression of sensitization to opioids involve different anatomical and physiological mechanisms. The development of sensitization consists of the immediate molecular and/or cellular effects that induce behavioral sensitization and are altered by drug actions in the somatodendritic regions of the A10/A9 dopamine neurons[14]. Changes in dopamine transmission within the nucleus accumbens seems to be responsible for the expression of sensitization, which refers to the long-term consequences of molecular and/or cellular effects that induce behavioral sensitization[14]. The present results demonstrated that pretreatment with l-THP not only inhibited the development, but also inhibited the expression of oxycodone.
It has been hypothesized that dopamine (DA) is one of the important neurotransmitters involved in locomotion. Measurement of spontaneous locomotor activity has been used to obtain preliminary information on the behavioral properties of drugs acting on dopaminergic system[15,16]. Morphine is known to activate ventral tegmental area dopamine neurons indirectly as a consequence of inhibiting non-dopamine, presumably γ-GABA, neurons of the ventral tegmental area, leading to increased dopamine release in the nucleus accumbens[17]. Direct infusions of morphine or μ-receptor-selective peptides into the ventral tegmental area elicit locomotion, which can be blocked by DA receptor antagonist administration into the nucleus accumbens. Following repeated administration of morphine, there is a marked increase in the induction of locomotor-stimulating effects of morphine, there is general agreement that the mesoac-cumbens DA system is the anatornical locos for sensitized locomotion[18]. Kalivas and Stewart (1991) presented preliminary results showing that either systemic or intra-ventral tegmental area administration of sch23390 prevented sensitization to systemic morphine, when the combinations were given every other day for 8 d. They also suggested DA D1 receptor involvement in the development of morphine sensitization[14]. In other studies, the blockade of the dopamine D2 receptor by haloperidol significantly antagonized the effects of opioid on locomotor activity[19]. Thus, dopamine D1 and D2 receptors play important roles in the acceleration of opioid sensitization[20]. Although the mechanisms of action through which l-THP attenuates the psychomotor effect of oxycodone are not clear, one reason maybe relevant to dopamine D1 and D2 receptors, which are involved in oxycodone-induced hyperactivity and locomotor sensitivity. l-THP, which has affinity for D1 as well as D2 receptors, is a dopamine receptor antagonist[11]. l-THP may inhibit mesolimbic dopamine D1 and D2 receptors and attenuate psychomotor effects of oxycodone. Our recent studies also showed that oxycodone (2.5 mg/kg, sc) increased dopamine-concentrations of dialysates with microdialysis in the striatum of rat. l-THP (25 mg/kg, ig) per se, did not affect dopamine release, but pretreatment of rats with l-THP (25 mg/kg, ig) significantly inhibited oxycodone-induced increases in extraceullar dopamine concentrations [F(3,18)=5.068, P<0.05] (Liu et al, unpublished data, 2004). These results indicate that the inhibiting locomotor sensitivity effect of l-THP might be connected with the DA system. l-THP could inhibit dopamine release in the mesolimbic system, induced by oxycodone, and then inhibit the development and expression of locomotor sensitization.
Another reason might be connected with L-type Ca2+ channels. Recent reports show that L-type Ca2+ channels may play an important role in the development of morphine behavioral sensitization. Co-administration with L-type Ca2+ channel blockers attenuates the development of morphine tolerance, dependence, and sensitization, suggesting that the L-type Ca2+ channel might play a role in morphine-induced neural and behavioral plasticity[21,22]. In contrast, L-type Ca2+ channel blockers have antidopaminergic properties[23]. L-type Ca2+ channel blockers such as nimodipine, nifedipine, and verapamil, dose-dependently antagonize apomorphine-induced yawning and penile erections in rats[24,25]. Nimodipine and verapamil inhibits locomotor activity induced by morphine[26]. Therefore, L-type Ca2+ channel blockers could attenuate morphine-induced hyperactivity through an antidopaminergic action. The inhibitory effect of l-THP on the development and expression of sensitivity of oxycodone might also be a result of the inhibitory effect of l-THP on L-type Ca2+ channel. Previous studies show that l-THP is also an L-type calcium antagonist. Using the patch-clamp technique, l-THP causes both tonic and use-dependent reduction of Ca2+ current in single ventricular myocytes of guinea pigs, has a moderate inhibitory effect on L-type Ca2+ current, and has inhibitory effects on [Ca2+]i in myocytes by blocking voltage-dependent calcium channels similar to verapamil[27–29]. Therefore, the inhibitory effect of l-THP on L-type Ca2+ channel might be included in mechanisms of action through which l-THP attenuated locomotor sensitization to oxycodone.
In conclusion, the present data indicates that l-THP attenuated psychomotor effects of oxycodone, and the development and expression of locomotor sensitivity of oxycodone. The exact mechanisms of the inhibitory effect of l-THP on oxycodone sensitivity need further investigation.
Acknowledgement
We thank Prof Guo-zhang JIN for kindly providing l-THP.
References
- Spyraki C, Fibinger HC, Phillips AG. Attenuation of heroin reward in rats by disruption of the mesolimbic dopamine system. Psychopharmacology 1983;79:278-83.
- De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci 1998;10:3565-71.
- Kalivas PW, Pierce RC, Cornish J, Sorg BA. A role for sensitization in craving and relapse in cocaine addiction. J Psychopharma-col 1998;12:49-53.
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of drug addiction. Brain Res Brain Res Rev 1993;18:247-91.
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 2000;95:S91-117.
- Kalso E, Vainio A. Morphine and oxycodone hydrochloride in the management of cancer pain. Clin Pharmacol Ther 1990;47:639-46.
- Glare PA, Walsh TD. Dose-ranging study of oxycodone for chronic pain in advanced cancer. J Clin Oncol 1993;11:973-8.
- Mather LE. Analgesic drug. In: Anaesthesia. Nimmo WS, Smith G, editors. Oxford: Blackwell Scientific Publications; 1989. p 73–104.
- Fishbain DA, Goldberg M, Rosomoff RS, Rosomoff H. Atypical withdrawal syndrome (organic delusional syndrome) secondary to oxycodone detoxification. J Clin Psychopharmacol 1988;8:441-2.
- Chen MX, Jin GZ. Role of d-tetrahydropalmatine in the inhibition of dopamine and serotonin uptake of synaptosomes in the rat brain. Chin J Physiol Sci 1996;12:25-30.
- Jin GZ. (-)-Tetrahydropalmatine and its analogues as new dopamine receptor antagonists. TIPS 1987;8:81-2.
- Ge XQ, Zhang HQ, Zhou HZ, Xu ZG, Bian CF. Experimental study of tetrahydroprotoberberines inhibiting morphine withdrawal syndromes. Chin J Drug Depend 1999;8:178-84.
- Jin GZ. Discoveries in the voyage of reseach. Shanghai: Shanghai Scientific & Technical Publishers; 2001. p351-2.
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev 1991;16:223-44.
- Kalivas PW, Duffy P. Sensitization to repeated morphine injection in the rat: possible involvement of A 10 dopamine neurons. J Pharmacol Exp Ther 1987;241:204-12.
- Vezina P, Stewart J. The effect of dopamine receptor blockade on the development of sensitization to the locomotor activating effects of amphetamine and morphine. Brain Res 1989;499:108-20.
- Scheel-Kruger J. Dopamine-GABA interactions: evidence that GABA transmits, modulates and mediates dopaminergic functions in the basal ganglia and the limbic system. Acta Neuron Scand 1986.Suppl 107:1-54.
- Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J Neurosci 1993;13:266-75.
- Magnus-Ellenbroek B, Havemann-Reinecke U. Morphine-induced hyperactivity in rats-a rebound effect? Naunyn Schmie-debergs Arch Pharmacol 1993;347:635-42.
- Kuribara H. Modification of morphine sensitization by opiod and dopamine receptor antagonists: evaluation by studying ambulation in mice. Eur J Pharmacol 1995;275:251-8.
- Martin MI, Lizasoain L, Leza JC. Calcium channel blockers: effects on morphine-induced hyperactivity. Psychopharmacol 1990;101:267-70.
- Michaluk J, Karokewicz B, Antkiewicz-Michaluk L, Vetulani J. Effects of various Ca2+ channel antagonists on morphine analgesia, tolerance and dependence and on blood pressure in the rat. Eur J Pharmacol 1998;352:189-97.
- Pucilowski O. Psychopharmacological properties of calcium channel inhibitors. Psychopharmacology 1992;109:12-29.
- Argiolas A, Melis MR, Gessa GL. Calcium channel inhibitors prevent apomorphine- and oxytocin-induced penile erection and yawning in male rats. Eur J Pharmacol 1989;166:515-8.
- Czyrak A, Mogilnicka E, Maj J. Some central pharmacological effects of the calcium channel antagonist flunarizine. J Neural Transm Gen Sect 1990;83:179-88.
- Zhang Q, Li JX, Zheng JW, Lliu RK, Liang JH. L-type Ca2+ channel blockers inhibit the development but not the expression of sensitization to morphine in mice. Eur J Pharmacol 2003;467:145-50.
- Huang K, Dai GZ, Li XH, Fan Q, Cheng L, Feng YB, et al. Blocking L-calcium current by l-tetrahydropalmatine in single ventricular myocyte of guinea pigs. Acta Pharmacol Sin 1999;20:907-11.
- Li XT, Wang YL, Wang JX, Yang SJ. Effects of tetrahydroproto-berberines on cytosolic free calcium in cultured rat single myocardial cells. Acta Pharm Sin 1995;30:567-72.
- Sun F, Li DX. Effects of l-tetrahydropalmatine on isolated rabbit arterial strips. Acta Pharmacol Sin 1989;10:30-3.
