6-Hydroxydopamine-induced glutathione alteration occurs via glutathione enzyme system in primary cultured astrocytes1
Introduction
Parkinson’s disease is characterized by the selective demise of dopaminergic neurons. Recent findings in molecular genetics and neurochemistry have suggested that oxidative stress is possibly involved in the aging process and is one of the pathogenic mechanisms of Parkinson’s disease[1].
6-Hydroxydopamine (6-OHDA) is one of the most common neurotoxins used to model nigral degeneration experimentally in vitro as well as in vivo. 6-OHDA, like dopamine (DA), is a substrate for monoamine oxidase (MAO). This enzymatic reaction gives rise to hydrogen peroxide[2]. Astrocytes are essential for the cellular defense against reactive oxygen species (ROS)[3], within which there is a high concentration of the tripeptide glutathione (GSH; γ-L-glutamyl-L-cysteinylglycine)[4].
Glutathione in astrocytes is very important for cellular defense against ROS. Alterations of the GSH metabolism in the brain have been implicated in oxidative stress and neurodegenerative diseases such as Parkinson’s disease[5]. Sian et al found that GSH levels were reduced in substantia nigra in patients with Parkinson’s disease (reduced by 40% compared with control subjects)[6]. This depletion in GSH may increase the susceptibility of brain cells to other harmful events, such as the reduction of mitochondrial energy production.
In addition to the GSH levels, alterations in the specific activities of enzymes involved in GSH metabolism and defense against ROS have been reported. They are[7]: (1) γ-glutamylcysteine synthetase (γGCS), the rate-limiting enzyme and the first enzyme used in GSH synthesis, which converts glutamate and cysteine to γ-glutamylcysteine. (2) The ectoenzyme γGT (γ-glutamyltransferase), which converts GSH to cysteinylglycine (CysGly) and γ-glutamyl[8]. CysGly is a precursor for de novo GSH synthesis in both astrocytes and neurons[9]. Inhibition of γGT prevents the astroglia-induced effect on GSH levels in neurons[9]. (3) Glutathione peroxidase (GPx) and glutathione reductase (GR), which catalyze the SH/S-S exchange reactions and contribute to protein thiol protection. (4) Glutathione transferases (GST), which use GSH to detoxify peroxides and carbonyl-containing products of lipid peroxidation.
In the present study, we investigated the role of 6-OHDA in astroglial GSH metabolism, and the expression of glutathione-related enzymes, especially γGCS and γGT in primary cultured astrocytes induced by 6-OHDA.
Materials and methods
Materials Glutathione reductase, β-NADPH-Na4, and 6-OHDA were obtained from Sigma (St Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) was obtained from Gibco (Grand Island, NY, USA). γGT assay kits were purchased from Nanjing Jiancheng Biological Co (Nanjing, China). All PCR primers were purchased from Sangon (Shanghai, China). All reagents for RT-PCR were purchased from Promag (Madison, WI, USA). All other chemicals were obtained from standard commercial sources.
Cell culture Astrocyte-rich primary cultures obtained from the whole brains of neonatal Sprague-Dawley rats were prepared and maintained as described previously[10]. Briefly, the cortex was incubated in 0.125% trypsin at 37 °C for 8 min and mechanically disrupted by passing the tissue through nylon mesh. After centrifugation (1500×g, 5 min), the cell pellet was gently resuspended in a small volume of tissue growth medium (DMEM containing 10% fetal bovine serum, 100 kU/L penicillin and 100 mg/L streptomycin) and plated in the same medium at a density of 1×107 cells/L in 12-well plates precoated with poly-lysine for the GSH assay.
GSx assay GSH was assayed as total glutathione (GSx), which was the sum of the reduced and oxidized forms [GSH+2×glutathione disulfide (GSSG)] by a modified enzymatic recycling method of Tietze[11]. Cells were washed in PBS, dissolved in 200 µL PBS buffer (0.05 mmol/L edetic acid; 0.05% Triton-X 100, pH 8.0), and centrifuged to remove protein. According to the Bradford method[12], we used a volume of 50 µL of supernatant for assessing protein by using bovine serum albumin as a standard. A volume of 100 µL of supernatant was neutralized with 50 µL of a 5% (w/v) salicylsalicylic acid (SSA) solution. The solution was centrifuged, then the GSH content was determined after the addition of 4 µL 10 mmol/L β-NADPH, 3 µL 10 mmol/L 5, 5'-dithio-bis-2-nitrobenzoic acid (DTNB), 2 kU/L GSH reductase. The GSH content was determined by kinetic measurement of the absorbance changes at 412 nm for 5 min and calculated by comparison with standards.
RNA preparation and semiquantitative RT-PCR Semiquantitative RT-PCR with β-actin as an internal control was performed to examine the expression of messenger RNA for the γGCS, γGT, and other GSH enzymes in glial-rich primary cultures. Total RNA (2 µg) from primary cultured astrocytes was reverse transcribed into single-stranded cDNA in a 20-µL reaction mixture containing: 10 mmol/L dNTP, 1 µg oligo (dT) primer, 20 IU RNasin and 200 IU M-MLV reverse transcriptase. The mixture was incubated at 42 °C for 1 h and then the reverse transcriptase was inactivated by heating the reaction mixture to 95 °C for 10 min. PCR amplification was carried out with 1.5 µL cDNA product in a 30 µL reaction volume containing 3 pmol of each specific oligonucleotide primer, 10 mmol/L dNTP, and 1.5 IU Taq DNA polymerase. For all of the reactions, preliminary experiments were performed to determine the number of PCR cycles at which saturation occurred, and the experiments mentioned were carried out with a number of cycles that precedes saturation. The sequences of the primers, product size, and optimized number of PCR cycles for GSH-related enzymes and β-actin expression analyses were: (1) γGCS, forward primer: 5'-AGACA-CGGCATCCTCCAGTT-3'; reverse primer: 5'-CTGACACGTA-GCCTCGGTAA-3' (GenBank accession no: NM_012815; product size: 801 bp). The thermal cycler unit was programmed for 30 cycles at 95 °C for 1 min, 60 °C for 1 min, then 72 °C for 1 min. (2) γGT: we used nested RT-PCR for γGT, in which the reverse transcriptase reaction and two sequential PCR procedures were carried out. The first-round primer pairs were designed from the target mRNA, and the second-round primers were designed from the first-round amplified PCR products. Forward and reverse primers were selected from the coding domain to identify any γGT mRNA nonselectively; the first-round primers were 5'-GCTTTGTGC-GAGGTGTTCTG-3' and 5'-CCATCGTCTGGAAGGTAGA-3'; the second-round primers were 5'-CTCAGCGGGCCCGTG-CTG-3' and 5'-GGCGGTTGGGTGAGTGGT-3', in the primary and the secondary PCR reactions, respectively (GenBank accession no: BC078768; product size: 261 bp). The thermal cycler unit was programmed for 30 cycles at 95 °C for 1 min, 55 °C for 1 min, and 72 °C for 1.5 min. (3) GPx, forward primer: 5'-GTATGTCTGCTGCTCGGCTCTC-3'; reverse primer: 5'-AAATGATGTACTTGGGGTCGGTC-3' (GenBank accession no: NM_030826; product size: 450 bp). The thermal cycler unit was programmed for 26 cycles at 94 °C for 45 s, 61 °C for 45 s, and 72 °C for 45 s. (4) GR, forward primer: 5'-ACGA-GGAAGACGAAATGCGTGATG-3'; reverse primer: 5'-AGGA-TGAATGGCGACCCTATTGTC-3' (GenBank accession no: U73174; product size: 171 bp). The thermal cycler unit was programmed for 24 cycles at 94 °C for 1 min, 56 °C for 1 min, and 72 °C for 1 min. (5) GST, forward primer: 5'- CCAAA-TTGAGAATTCCACAGCGC-3'; reverse primer: 5'- TGCCTG-CAGGATCCAATGTGGA-3' (GenBank accession no:NM_017014; product size: 205 bp). The thermal cycler unit was programmed for 22 cycles at 95 °C for 30 s, 63 °C for 45 s, and 72 °C for 30 s. (6) β-actin, forward primer: 5'-CACGATG-GAGGGGCCGGACTCATC-3'; reverse primer: 5'-TAAAGA-CCTCTATGCCAACACAGT-3' (GenBank accession no: NM_031144; product size: 240 bp). The thermal cycler unit was programmed for 24 cycles at 94 °C for 30 s, 61 °C for 30 s, and 72 °C for 30 s. PCR products were separated by electrophoresis on a 2% agarose gel and visualized after ethidium bromide staining over UV light.
Determination of γGT activity After removal of the medium, cells were washed twice with 4 mL ice-cold PBS [10 mmol/L potassium phosphate buffer containing 150 mmol/L NaCl (pH 7.4)] and were lysed by incubation with 500 µL 20 mmol/L potassium phosphate buffer (pH 7.0) containing 0.2 mmol/L edetic acid for 10 min on ice. The cell lysates were scraped off the flask and transferred to Eppendorf tubes. After centrifugation (15 000×g, 5 min, 4 °C), the supernatant was discarded and the pellets were resuspended in 100 µL of 20 mmol/L potassium phosphate buffer (pH 7.0) containing 200 µmol/L edetic acid and 1% (w/v) Triton X-100. During 20-min incubation on ice the lysates were resuspended several times. After centrifugation (15 000×g, 5 min, 4 °C) the activity of γGT was measured in 50 µL aliquots of the supernatants according to the method described by Meister et al[8]. The samples were determined by γGT assay kits from the Nanjing Jiancheng Biological Co. The protein content of the cultured cells was determined using the Bradford method[12].
Data analysis All values were presented as mean±SD. The t-test was used for comparisons and differences were considered significant if P<0.05.
Results
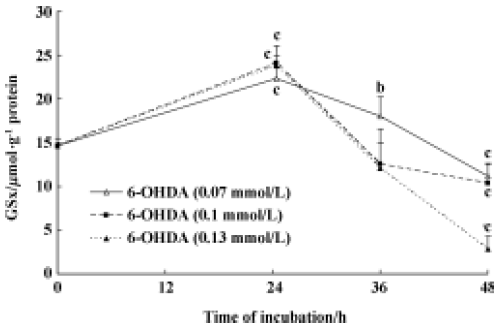
Effect of 6-OHDA on endogenous GSx contents in astrocytes To elucidate the relationship between endogenous GSx and 6-OHDA-induced cell damage, we measured the GSx content of astrocytes. The GSx concentration increased from 14.62 µmol·g-1·protein to 24.02 µmol·g-1 protein after incubation of the astrocytes with 6-OHDA for 24 h (Figure 1) and decreased to 10.35 µmol·g-1 protein after exposure to 6-OHDA for 48 h (Figure 1). The GSx content further reduced to 20% of control levels when cells were incubated with 0.13 mmol/L 6-OHDA for 48 h (Figure 1).
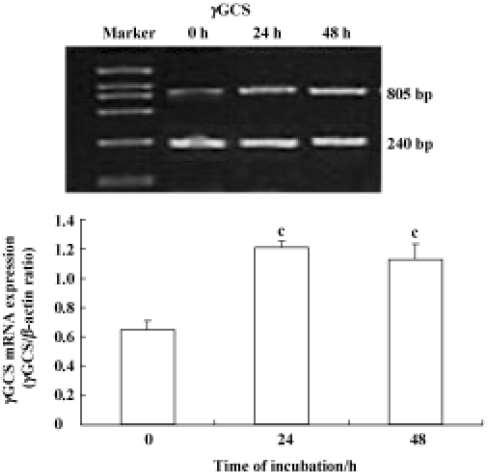
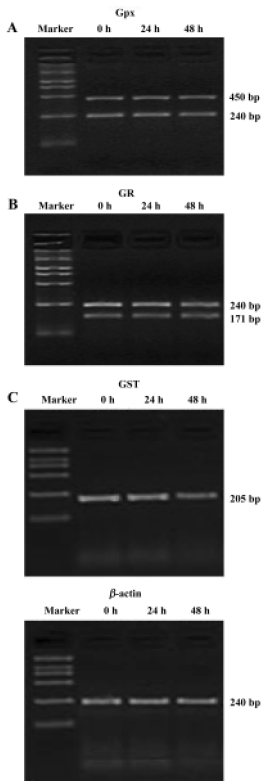
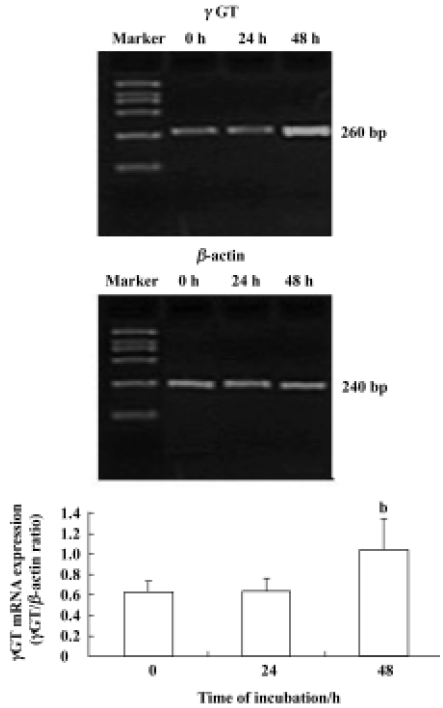
Effect of 6-OHDA on glutathione enzyme expression in astrocytes To further address whether the 6-OHDA had an effect on glutathione enzymes, we detected the mRNA expression of glutathione enzymes. In this study, all RT-PCR data were normalized relative to levels of β-actin mRNA. Astrocytes were exposed to 6-OHDA for 24 h or 48 h. We found that the levels of cellular GSx and γGCS mRNA were increased after the cells were incubated with 6-OHDA for 24 h; however, the levels of cellular GSx decreased markedly, whereas γGCS mRNA expression increased again after exposure for 48 h (Figure 2). However, 6-OHDA failed to alter the levels of GPx, GR, and GST mRNA, after exposure for both 24 h and 48 h (Figure 3). The levels of γGT mRNA expression in astrocytes were up-regulated after exposure to 6-OHDA for 48 h, but not for 24 h (Figure 4).
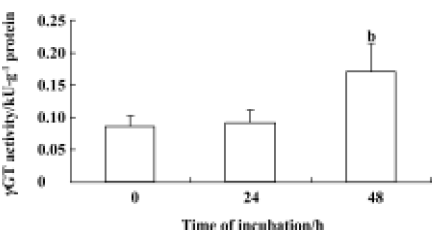
Measurement of γGT activity in astrocytes Specific γGT activity was measured in astrocytes in the presence and absence of 6-OHDA. We treated astrocytes with 100 µmol/L 6-OHDA for 24 h or 48 h. γGT activity did not alter after treatment with 6-OHDA for 24 h, but it increased significantly from an initial value of 0.08 kU·g-1 protein to a plateau value of 0.17 kU·g-1 protein after exposure to 6-OHDA for 48 h. The increase in γGT activity in astrocytes correlated well with the levels of γGT mRNA expression in cells (Figure 5).
Discussion
A high intracellular concentration of glutathione protects against a variety of different ROS. GSH reacts directly with radicals in nonenzymatic reactions. It should be noted that GSx, including GSH and GSSG, is very important for cellular defense against ROS. Glutathione levels in the substantia nigra pars compacta of patients with Parkinson’s disease is significantly reduced, but the levels do not change in patients with multiple system atrophy or progressive supranuclear palsy[13]. It has been found that oxidative stress might originate in astrocytes rather than in neurons. Astrocytes surrounding dopaminergic neurons in the brain may be involved in the selective vulnerability of these neurons by scavenging ROS and releasing CysGly by using γGT, and CysGly is the precursor for GSH synthesis in neurons. Hence, astrocyte function is an important contributor to the pathogenesis of Parkinson’s disease.
6-OHDA is known to cause oxidative stress to DA neurons, and it is usually thought to cross cell membrane through dopamine transporters, to inhibit mitochondrial respiration and to generate intracellular ROS[14]. A previous study has shown that in 6-OHDA-treated rats, the decreased levels of GSH could be due to an increased level of free radical-generated lipid peroxidation[15].
To investigate the relationship between glutathione and 6-OHDA-induced cell damage, we measured the GSx content of cells by using the enzymatic recycling method with some modifications[11]. We found that in primary cultured astrocytes, the cytosolic GSx content increased in response to treatment with different concentrations of 6-OHDA (0.07, 0.1, and 0.13 mmol/L) after incubation for 24 h, but decreased significantly after incubation for 48 h (Figure 1). The data presented here are consistent with the previous findings that treatment with 6-OHDA for 24 h induced up-regulation of GSx levels in astrocytes[15]. Shimizu et al also found a delayed increase in GSH levels after the addition of 6-OHDA in human neuroblastoma SK-N-SH cells[16]. However, the mechanism by which GSx fluctuates in primary culture astrocytes in response to 6-OHDA is not clear. Some investigators have suggested that in the primary astrocytes, 6-OHDA toxicity may not result from the inhibition of mitochondrial respiration, but may be secondary to auto-oxidation and ROS generation[17].
More importantly, our results showed that the mRNA expression of γGCS was up-regulated significantly after incubation of the astrocytes with 6-OHDA for 24 h, which correlated well with the alteration of GSx in the presence of 6-OHDA in astrocytes (Figure 2). A previous study has shown that 6-OHDA induced an elevation of GSx contents in astrocyte cells because of an increase of the expression of γGCS mRNA[16]. It is well known that γGCS is the first and the rate-limiting enzyme in the synthesis of GSH. Induction of γGCS by oxidative stress could play a key role in determining cellular glutathione content[16]. Recent studies have demonstrated that the antioxidant response element (ARE), a cis-acting enhancer sequence, mediates the transcriptional activation of genes in cells exposed to oxidative stress. The 5'-flanking region of the γGCS gene also contains several transcription binding sites, including the ARE[18,19]. These findings support the idea that transcriptional regulation of the γGCS gene is involved in the antioxidant system of astrocyte cells. Hence, the increased γGCS levels in 6-OHDA-treated astrocytes may occur by activating ARE, and regulating the γGCS gene, thereby activating the antioxidative system including GSx. Therefore, the elevation of GSx in astrocytes appears to reflect the resistance and defense mechanisms of astrocytes in the brain against oxidative stress in the early stages. If the toxicity of 6-OHDA continues, massive production of ROS may contribute to the decline in GSx levels in cells.
However, our results showed that γGCS mRNA expression did not decrease after 48-h incubation with 6-OHDA (Figure 2). This finding was not consistent with the change in GSx in 6-OHDA-treated astrocytes. GSH redox-system genes play a key role in the control of the oxidation and reduction of the SH groups of proteins. For this reason, we detected the mRNA expression levels of other glutathione enzymes, including GPx, GR, GST, and γGT in astrocytes treated with 6-OHDA for 24 h and 48 h. Our finding that 6-OHDA failed to alter the mRNA levels of GPx, GR, and GST (Figure 3) confirmed the previous finding that the expression levels of GPx mRNA were not changed in astrocytes after paraquat exposure[20]. However, other studies have shown that GPx and GR expression increased after the exposure of cultured astrocytes to H2O2. With regard to this experimental result, we believe that the different results may be related to differences in preparation techniques, to differences in experimental methods, or to differences in culture conditions.
For further investigation, we measured the activity of γGT in astrocytes treated with 6-OHDA for 24 h and 48 h. The increase in γGT activity after 6-OHDA treatment for 48 h (Figure 5) was consistent with the change in γGT mRNA levels. A previous study reported that the activities of enzymes involved in the glutathione cycle were normal with the exception of γGT, the activity of which was increased in Parkinson’s disease[21]; this was a similar finding to our results. So we conclude that the increase in mRNA levels and the activity of γGT induced GSx loss after exposure to 6-OHDA for 48 h. The dipeptide CysGly, which is generated from extracellular GSH by a γGT reaction, may be the most important among the exogenous precursors of GSH, because it is efficiently utilized by neurons in micromolar concentra-tions. However, dipeptides could be hydrolyzed by neuronal ectopeptidase-generating amino acids such as cystein, glutamate, and glycine[22]. Many such amino acids possibly also have toxic effects on neurons[28].
It has been found that γGT activity increases in astroglial cultures treated with an nitric oxide donor for 24 h[23]. Ruedig et al suggested that TNFα synthesis in the brain also induced γGT expression in astrocytes[24]. Such a scenario can be considered for Parksinson’s disease, where an elevated level of TNFα in the substantia nigra and increased immunoreactivity for TNFα in astrocytes[25] is accompanied by an elevated specific γGT activity[6]. Previous findings have shown that 6-OHDA can increase the generation of NO and TNFα in mesencephalic cells[26,27]. Thus, 6-OHDA may up-regulate γGT via the activation of the NO or TNFα signaling pathway followed by GSH loss. Increased intracellular GSH content and γGT activity in astrocytes exposed to 6-OHDA may help to protect neurons in vitro by supplying more GSH precursors, and thus elevating neuronal GSH levels.
In the present study, we found that glutathione played an important role in mediating 6-OHDA-induced cell damage in a Parkinson’s disease model. We also found that an increase in γGCS, the rate-limiting enzyme in GSH synthesis, correlated with an increase in GSx after exposure of the cells to 6-OHDA for 24 h. Interestingly, GSx levels decreased because of increased γGT mRNA levels and γGT activity, despite up-regulated γGCS mRNA levels after 48-h incubation with 6-OHDA. The findings in this study may provide useful information for identifying new therapeutic targets for neurodegenerative diseases such as Parkinson’s disease.
References
- Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol 2003;53:S26-S38.
- Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol 2001;65:135-72.
- Cooper AJL. Role of astrocytes in maintaining cerebral glutathione homeostasis and in protecting the brain against xenobiotics and oxidative stress. In: Shaw CA, editor. The role of glutathione in the nervous system. Washington: Taylor and Francis; 1998. p 91−115.
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. New York: Oxford University Press; 1999.
- Bains JS, Shaw CA. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Rev 1997;25:335-58.
- Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, et al. Alteration in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol 1994;36:348-55.
- Aksenov MY, Markesbery WR. Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 2001;302:141-5.
- Anderson ME, Meister A. Transport and direct utilization of γ-glutamylcyst(e)ine for glutathione synthesis. Proc Natl Acad Sci USA 1983;80:707-11.
- Dringen R, Kranich O, Hamprecht B. The 7-glutamyl trans-peptidase inhibitor acivicin preserves glutathione released by astroglial cells in culture neurochemical research. Neurochem Res 1997;22:727-33.
- Hamprecht B, Loffler F. Primary glial cultures as a model for studying hormone action. Methods Enzymol 1985;109:341-5.
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 1969;27:502-22.
- Kruger NJ. The Bradford method for protein quantitation. Methods Mol Biol 1994;32:9-15.
- Uitti RJ, Calne DB. Pathogenesis of idiopathic Parkinsonism. Eur Neurol 1993;33 Suppl l:6-23.
- Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol 2000;62:649-71.
- Iwata-Ichikawa E, Kondo Y, Miyazaki I, Asanuma M, Ogawa N. Glial cells protect neurons against oxidative stress via transcriptional up-regulation of the glutathione synthesis. J Neurochem 1999;72:2334-44.
- Shimizu E, Hashimoto K, Komatsu N, Iyo M. Roles of endogenous glutathione levels on 6-hydroxydopamine induced apoptotic neuronal cell death in human neuroblastoma SK-N-SH cells. Neuropharmacology 2002;43:434-43.
- Sagara J, Miura K, Bannai S. Cysteine uptake and glutathione level in fetal brain cells in primary culture and in suspension. J Neurochem 1993;61:1667-71.
- Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Phamacol Toxicol 2003;43:233-60.
- Mulcahy RT, Gipp JJ. Identification of a putative antioxidant response element in the 5'-flanking region of the human gamma-glutamylcysteine synthetase heavy subunit gene. Biochem Biophys Res Commun 1995;209:227-33.
- Schmuck G, Rohrdanz E, Tran-Thi QH, Kahl R, Schluter G. Oxidative stress in rat cortical neurons and astrocytes induced by paraquat in vitro. Neurotox Res 2002;4:1-13.
- Owen AD, Schapira AH, Jenner P, Marsden CD. Oxidative stress and Parkinson’s disease. Ann NY Acad Sci 1996;786:217-23.
- Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci 1999;19:562-69.
- Gegg ME, Beltran B, Salas-Pino S, Bolanos JP, Clark JB, Moncada S, et al. Differential effect of nitric oxide on glutathione metabolism and mitochondrial function in astrocytes and neurones: implications for neuroprotection/neurodegeneration. J Neurochem 2003;86:228-37.
- Ruedig C, Dringen R. TNFα increases the activity of γ-glutamyl transpeptidase in cultured rat astroglial cells. J Neurosci Res 2004;75:536-43.
- Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci Lett 1994;172:151-54.
- Nobre HV Jr, Cunha GM, Maia FD, Oliveira RA, Moraes MO, Rao VS. Catechin attenuates 6-hydroxydopamine (6-OHDA)-induced cell death in primary cultures of mesencephalic cells. Comp Biochem Physiol C Toxicol Pharmacol 2003;136:175-80.
- Mogi M, Togari A, Tanaka K, Ogawa N, Ichinose H, Nagatsu T. Increase in level of tumor necrosis factor (TNF)-alpha in 6-hydroxydopamine-lesioned striatum in rats without influence of systemic L-DOPA on the TNF-alpha induction. Neurosci Lett 1999;268:101-4.
- Puka-Sundvall M, Eriksson P, Nilsson M, Sandberg M, Lehmann A. Neurotoxicity of cysteine: interaction with glutamate. Brain Res 1995;705:65-70.
