Phorbol-induced surface expression of NR2A subunit homologues in HEK293 cells1
Introduction
Three classes of subunits have been identified in the family of N-methyl-D-aspartate receptors (NMDAR): NR1, NR2 and NR3. The NR1 subunit is essential to the structure of NMDAR, whereas different NR2 subunits endow the receptor complexes with different characteristics[1]. The majority of functional NMDA receptors are generally thought to be tetrameric complexes composed of both NR1 and NR2 subunits[2]. Their structure is thought to be a dimer of dimers[3]. It was reported that expressing NR1-1a alone in Xenopus oocytes produces a glutamate and glycine-activated current, although the amplitude was only 1/10 compared with that in the NR1/NR2 coexpressed cells[4–6]. This evidence indicates that the NR1 subunit assembles to form functional homomeric receptors in oocytes. On the other hand, we have proven that NR2 subunits were assembled in HEK293 cells using fluorescent resonance energy transfer (FRET) technology. When we expressed NR2A or NR2B alone in HEK293 cells, FRET occurred in each transfection[7]. Previous studies also suggested that NR2 subunits were retained in the endoplasmic reticulum (ER) until they assembled with NR1 subunits[8]. However, there is no evidence indicating that NR2 subunits can express on the plasma membrane or form functional channels before assembling with NR1 subunits[1,9,10].
The surface expression of NMDAR has been shown to be regulated by the phosphorylation of the intracellular C-terminal regions of their composing subunits[11–14]. For example, the activation of protein kinase C (PKC) leads to the up-regulation of surface NMDAR through the effect of Src family kinases. Activation of the cAMP-dependent protein kinase (PKA) sites in the C1 cassette of NR1 subunits increases the synaptic targeting of NMDAR, while CaMKII can inactive synaptic Ras-GTPase activating protein and then downregulate synaptic NMDAR. 12-Myristate-13 acetate (PMA) is an activator of PKC, which are classified into 3 groups and 11 isozymes based on their structure[15]. PMA is thought to be a tumor promoter, playing roles in cell cycling, cell transformation, vesicle trafficking and gene expression. It has been shown that the application of PMA increases both the number and the open probability of the surface NMDA receptors composed of NR1 and NR2 subunits[16]. Biochemical studies have identified several PKC phosphorylation sites in the C-terminus of the NR2 subunits[17]. Many potential phosphorylation sites in NR2A C-terminus are thought to be important for PMA-based potentiation of NR2A in heteromeric NMDAR. Residues S1291, S1308 and S1312 are essential for PKC-mediated insulin potentiation of NR1/NR2A receptors[17]. Residues Y1105, Y1267 and Y1387 were utilized in Src-potentiation of whole cell NR1/NR2A currents in zinc sensitivity[18]. Also, the amino acids between 1400–1406 of NR2A are involved in PMA-potentiation of NR1/NR2A receptors[13]. However, since PMA-induced potentiation could hardly be blocked by site mutation[13], several indirect pathways may be involved in the processes. These regulatory mechanisms are largely uncertain.
In this study, we found that NR2A subunits were expressed on the cell surface after the cells were incubated with PMA. Interestingly, the surface expression does not require the coexpression of NR1 subunits. The NR2A C-terminal region required for the PMA-induced surface expression was further identified by a series of NR2A mutants with partial deletion of its C-terminus.
Materials and methods
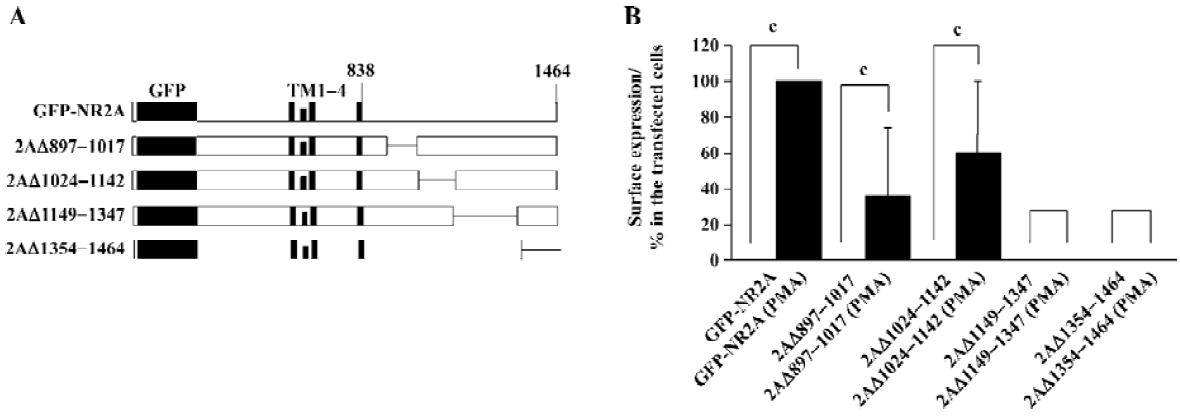
Molecular biology The generation of expression vectors for the green fluorescence protein (GFP) N-terminally tagged NR2A (GFP-NR2A) and a variety of GFP-NR2A mutants with C-terminal deletions used in this study have been described previously[19,20] (Figure 2A).
Transfection of heterologous cells HEK293 cells were cultured and plated on polylysine-coated coverslips (8 mm×8 mm) in 35 mm dishes 1 d before transfection. The plasmids (1.5 μg in amount) for the NR2 subunits were transfected alone or with NR1-1a at a molar ratio of 1:1 using Lipofectamine 2000 (Invitrogen, Los Angeles, CA, USA) according to the manufacturer’s instruction. After transfection, cells were grown in the presence of 0.5 mmol/L ketamine and 1 mmol/L kynurenic acid (Sigma-Aldrich, St Louis, MO, USA). The transfected cells were used for electrophysiological recording and immunocytochemical staining 24–48 h after transfection.
Immunofluorescent staining For live cell surface staining, GFP-tagged NMDAR on the cell surface were labeled using immunofluorescent staining in live cells as described previously[19]. The transfected HEK293 cells grown on coverslips were incubated with rabbit anti-GFP antibody for 7 min, rinsed 3 times, and then incubated with Cy3-conjugated goat anti-rabbit secondary antibody (Chemicon, Los Angeles, CA, USA) for another 7 min. After brief washing and fixation, the cells were observed and imaged under a confocal microscope. The imaging analysis software used was FluoView (FV500, Olympus, Tokyo, JAPAN). All procedures were performed at room temperature. The surface expression of GFP-tagged NMDA receptors in HEK293 cells was measured by counting the number of surface-labeled cells in the population of GFP-positive cells.
For permealized labeling, HEK293 cells were washed with PBS, fixed on ice with 4% paraformaldehyde for 20 min, washed 3 times with PBS, and permeabilized with 0.2% Triton X-100 for 10 min at room temperature. After 3 washes with PBS, the cells were blocked with 10% bovine serum albumin (BSA) in PBS for 1 h and then incubated in primary antibody for 15 h at 4 °C. After another 3 washes with PBS, the cells were incubated with the appropriate secondary antibodies for 1 h at room temperature. The cells were then observed and imaged using a confocal microscopy.
Results
PMA-induced surface expression of NR2A in HEK293 cells We cotransfected HEK293 cells with NR1-1a and GFP-NR2A by Lipofectamine 2000 and then measured surface expressed receptors 24–48 h after transfection using a live surface staining method. GFP-tagged NMDAR were expressed on the cell membrane, with N-terminal GFP tags exposed extracellularly. The exposed GFP tags were stained using polyclonal antibodies. Positive labeling was indicated by numerous red puncta on the cell surface, indicating the surface expression of the NMDAR (Figure 1A,1B). After quantification, we found that 49%±4% of transfected HEK293 cells were surface labeled (Figure 1G).
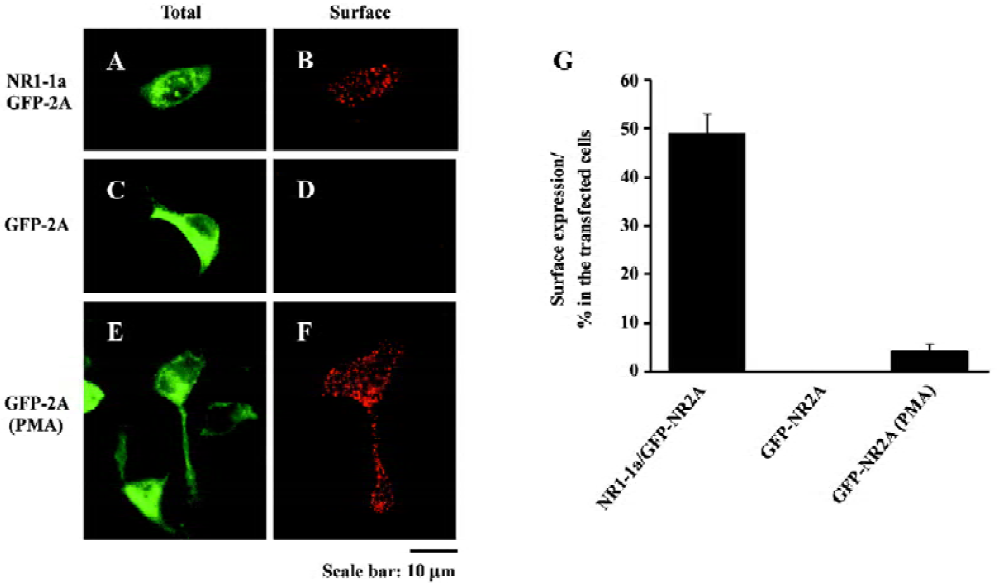
We then transfected HEK293 cells with GFP-NR2A alone and measured surface expressed receptors 24 h after transfection. No surface staining was detected in these HEK293 cells expressing GFP-NR2A alone (Figure 1C, 1D,1G).
Twenty-four hours after transfection, HEK293 cells transfected with GFP-NR2A alone were incubated with fetal bovine serum (FBS)-free culture medium plus 200 nmol/L PMA (Chemicon, Los Angeles, CA, USA) for 30 min. After briefly washing with PBS, the cells were incubated with PMA-free culture medium for 2 h. Finally, surface expressed GFP-NR2A subunits were measured by live surface staining (Figure 1E,1F). We surprisingly found that 4.1%±1.5% of GFP expressing cells exhibited positive labeling (Figure 1G). These results indicate that some of the transfected cells could deliver NR2A subunits to the cell surface after incubating with PMA.
Segment between 1149D and 1464V was necessary for PMA mediated surface expression of homomeric NR2A subunits It has been reported that the region between amino acids 1105–1400 or 1267–1458 of NR2A is sufficient for PKC-mediated potentiation of NR1/NR2A heteromers[13,21]. We used a similar method to study whether this region also plays a role in the PMA-induced potentiation of NR2A subunits.
We generated a series of GFP-NR2A truncations: 2AΔ897–1017, 2AΔ1024–1142, 2AΔ1149–1347, and 2AΔ1354–1464[20]; the numbers following 2AΔ indicating the first and last residues of the deleted segments, respectively. Each mutation contained a GFP tag in the extracellular N-termini (Figure 2A). HEK293 cells were transfected with these mutants. PMA effects were assessed by incubation of parallel transfection with or without PMA (200 nmol/L, 30 min). We did not find any surface stained cells in any transfection without PMA incubation (Figure 2B). However, surface expression was found in cells expressing 2AΔ897–1017 or 2AΔ1024–1142 after incubating with PMA. In contrast, HEK293 cells expressing 2AΔ1149–1347 or 2AΔ1354–1464 did not show surface labeling after incubating with PMA (Figure 2B). After incubating with PMA, the percentage of surface-labeled cells from transfected cells was normalized to 100%. The relative percentage of surface-labeled cells from transfected cells expressing 2AΔ897–1017, 2AΔ1024–1142, 2AΔ1149–1347, or 2AΔ1354–1464 was 36.3%±37.4%, 60.1%±39.7%, 0%±0%, or 0%±0%, respectively (Figure 2B).
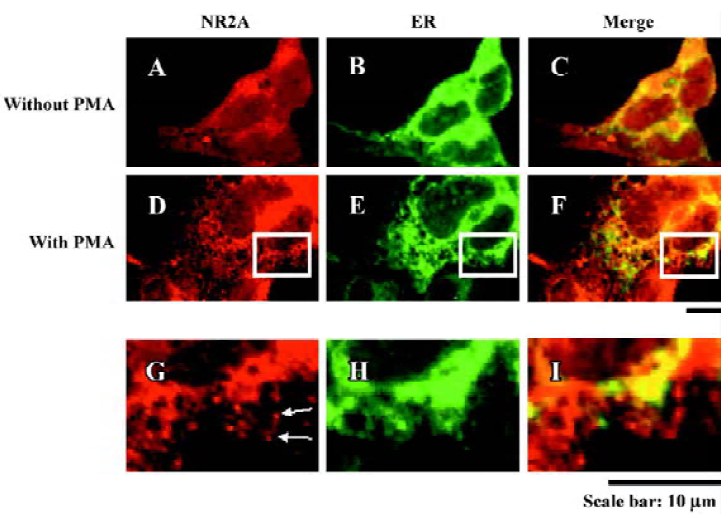
PMA-induced NR2A subunits exporting from the ER Previous studies have provided evidence that NR1 dimers and NR2 dimers are retained in the ER until they coassemble[7,22]. However, whether NR2 subunits export from the ER or not after incubating with PMA remains unknown. Our immunocytochemical staining of HEK293 cells transfected with NR2A alone showed extensive colocalization of NR2A subunits with an ER marker (Figure 3). We also fixed the cells 2 h after PMA treatment (200 nmol/L, 30 min) and then performed immunocytochemical staining. Our results showed that many NR2A puncta did not colocalize with an ER marker (Figure 3). These results suggest that PMA induces NR2A subunit export from the ER position.
Discussion
Although the NR1 subunit homologues have been studied in Xenopus oocytes and mammalian cells[23], it still remains unclear whether there are functional homomeric NMDAR existing in physiological or pathological conditions. Previous studies have shown that transiently expressing NR1-1a in COS cells did not produce functional NR1-1a homomeric receptors[24,25]. In agreement with these reports, we did not detect surface expressed NR2A after transfecting HEK293 cells with GFP-NR2A alone, even after transfecting as much as 5 μg of GFP-NR2A plasmid into HEK293 cells. However, using the live surface staining method, some of the cells were found to be surface labeled with GFP-NR2A subunits after incubating with PMA (200 nmol/L, 30 min).
The molecular mechanisms underlying the PMA mediated potentiation of NR2 subunits remains unclear. Phorbol PMA is an activator of PKC. Previous studies have provided evidence that PMA induces the phosphorylation of several serine and tyrosine sites[17,18]. It is possible that some phosphorylation sites act in concert to mediate the potentiation of surface NR2 subunits. This may explain why the PMA-potentiation of NR2A subunits could not be induced by deleting the amino acids between 1149–1347 or between 1354–1464. Our results suggest that the surface expression of NR2A subunits was mediated by the simultaneous phosphorylation of several residues between 1149–1464.
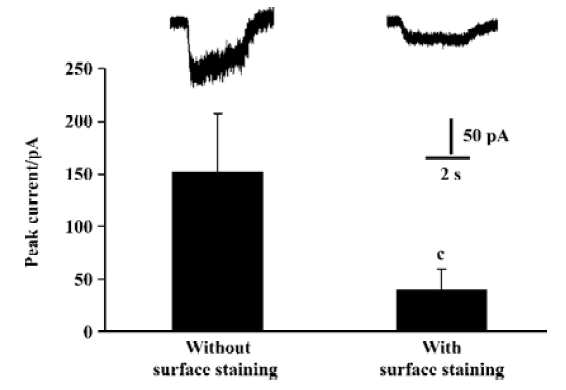
We detected surface NR2 subunits in HEK293 cells by immunochemical methods. However, it seems difficult to identify whether or not the surface NR2 subunits form ion channels in HEK293 cells. Voltage clamp is one of the most direct ways to identify ion channels. In NR1/NR2B coexpressing HEK293 cells, our data showed that the current amplitudes were reduced significantly after surface staining (Figure 4), suggesting that surface labeling reduced the sensitivity of the whole cell recording. After PMA incubation, we could not produce significant NMDA currents in surface-labeled HEK293 cells expressing NR2 alone. There are 2 possible explanations for this: First, although some of the NR2A subunits export from the ER by PMA-based induction, they fail to assemble an NMDA channel. There may be monomeric, dimeric, trimeric or some other formation of homomeric NR2A complexes that express on the cell surface. However, they do not have normal NMDAR function and can not be activated by glutamate. Second, the NR2A subunits may assemble into homomeric receptors, but the current is far more less than normal NMDA currents. So after surface staining, they could hardly be recorded.
In summary, our experiments demonstrate that in a small fraction of transfected cells, NR2A subunits can traffic to the cell membrane after PMA incubation. This process is mediated by the NR2A C-terminal region between amino acids 1149 and 1464. Thus, our work has provided further evidence that the NR2A C-terminus plays an important role in PMA-induced potentiation of NR2A-containing NMDAR. In addition, it would be interesting to explore whether NR2A subunits express on the surface of neurons under proper conditions in vivo in the future.
Acknowledgment
Gratitude is expressed to Prof Shu-min DUAN, Shanghai Institute for Biological Sciences, Chinese Academy of Sciences, for support and valuable advice.
References
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, et al. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science 1992;256:1217-21.
- Luo J, Wang Y, Yasuda RP, Dunah AW, Wolfe BB. The majority of N-methyl-D-aspartate receptor complexes in adult rat cerebral cortex contain at least three different subunits (NR1/NR2A/NR2B). Mol Pharmacol 1997;51:79-86.
- Huggins DJ, Grant GH. The function of the amino terminal domain in NMDA receptor modulation. J Mol Graph Model 2005;23:381-8.
- Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature 1991;354:31-7.
- Karp SJ, Masu M, Eki T, Ozawa K, Nakanishi S. Molecular cloning and chromosomal localization of the key subunit of the human N-methyl-D-aspartate receptor. J Biol Chem 1993;268:3728-33.
- Yamazaki M, Mori H, Araki K, Mori KJ, Mishina M. Cloning, expression and modulation of a mouse NMDA receptor subunit. FEBS Lett 1992;300:39-45.
- Qiu S, Hua YL, Yang F, Chen YZ, Luo JH. Subunit assembly of -methyl--aspartate receptors analyzed by fluorescence resonance energy transfer. J Biol Chem 2005; 280: 24 923–30.
- Hawkins LM, Prybylowski K, Chang K, Moussan C, Stephenson FA, Wenthold RJ. Export from the endoplasmic reticulum of assembled -methyl--aspartic acid receptors is controlled by a motif in the C terminus of the NR2 subunit. J Biol Chem 2004; 279: 28 903–10.
- Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, et al. Molecular diversity of the NMDA receptor channel. Nature 1992;358:36-41.
- Meguro H, Mori H, Araki K, Kushiya E, Kutsuwada T, Yamazaki M, et al. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature 1992;357:70-4.
- Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci 2001;21:3063-72.
- Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, et al. Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci 2001;4:382-90.
- Grant ER, Guttmann RP, Seifert KM, Lynch DR. A region of the rat N-methyl-D-aspartate receptor 2A subunit that is sufficient for potentiation by phorbol esters. Neurosci Lett 2001;310:9-12.
- Ali DW, Salter MW. NMDA receptor regulation by Src kinase signalling in excitatory synaptic transmission and plasticity. Curr Opin Neurobiol 2001;11:336-42.
- Liu WS, Heckman CA. The sevenfold way of PKC regulation. Cell Signal 1998;10:529-42.
- Zheng X, Zhang L, Wang AP, Bennett MV, Zukin RS. Protein kinase C potentiation of -methyl--aspartate receptor activity is not mediated by phosphorylation of -methyl--aspartate receptor subunits. Proc Natl Acad Sci USA 1999; 96: 15 262–7.
- Jones ML, Leonard JP. PKC site mutations reveal differential modulation by insulin of NMDA receptors containing NR2A or NR2B subunits. J Neurochem 2005;92:1431-8.
- Zheng F, Gingrich MB, Traynelis SF, Conn PJ. Tyrosine kinase potentiates NMDA receptor currents by reducing tonic zinc inhibition. Nat Neurosci 1998;1:185-91.
- Luo JH, Fu ZY, Losi G, Kim BG, Prybylowski K, Vissel B, et al. Functional expression of distinct NMDA channel subunits tagged with green fluorescent protein in hippocampal neurons in culture. Neuropharmacology 2002;42:306-18.
- Zheng CY, Luo JH, Fu T, Yang W, Shen HQ. Surface expression of NMDA receptors composed of NR1 subunit and NR2A subunit mutants with partially deleted C-terminus in HEK293 cells. J Zhejiang Univ Med Sci 2003;32:475-9. Chinese..
- Grant ER, Bacskai BJ, Anegawa NJ, Pleasure DE, Lynch DR. Opposing contributions of NR1 and NR2 to protein kinase C modulation of NMDA receptors. J Neurochem 1998;71:1471-81.
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol 2003;43:335-58.
- Zannat MT, Locatelli F, Rybak J, Menzel R, Leboulle G. Identification and localisation of the NR1 subunit homologue of the NMDA glutamate receptor in the honeybee brain. Neurosci Lett 2006;398:274-9.
- Ishmael JE, Franklin PH, Murray TF, Leid M. High level expression of the NMDAR1 glutamate receptor subunit in electroporated COS cells. J Neurochem 1996;67:1500-10.
- Liao GY, Wagner DA, Hsu MH, Leonard JP. Evidence for direct protein kinase-C mediated modulation of N-methyl-D-aspartate receptor current. Mol Pharmacol 2001;59:960-4.
