Synergism of hydrochlorothiazide and nitrendipine on reduction of blood pressure and blood pressure variability in spontaneously hypertensive rats1
Introduction
Although many new drugs, or recently developed drugs were available, the classic antihypertensive drugs, such as hydrochlorothiazide and nitrendipine, still have their place in the treatment of hypertension. This is because of their effectiveness and their low cost. Clinically, only approximately 50% of patients achieve optimal blood pressure (BP) control with monotherapy. Therefore, treatment with two or more antihypertensive drugs of different classes is often necessary[1–3]. Hydrochlorothiazide, a thiazides diuretic and nitrendipine, a calcium antagonist, are two classic drugs widely used in the treatment of hypertension. They belong to two different classes of antihypertensives and the mechanisms of action are quite different. Generally speaking, a combination of two drugs with different action modes may be synergistic. However, there is no evidence to show whether a combination of hydrochlorothiazide and nitren-dipine is synergistic on BP reduction.
BP is not constant and there exists a spontaneous variation in BP. This spontaneous variation is defined as blood pressure variability (BPV). It is well known that BP level is an important determinant for the end-organ damage in hypertension. However, BP level is certainly not the unique determinant for hypertensive organ damage. Recently, it has been proposed that BPV may be an important factor determining organ damage in hypertension[4–7]. In other words, antihypertensive treatment should be aimed not only at reducing BP level but also at reducing BPV[7–9].
Therefore, the present work was designed to investigate the synergism of hydrochlorothiazide and nitrendipine on both BP and BPV reduction in spontaneously hypertensive rats (SHR). The possible pharmacological mechanisms of this synergism are also discussed.
Materials and methods
Animals and chemicals Seventy male SHR, at 16 weeks of age were provided by the animal center of our university. The animals were divided into seven groups with ten rats in each group. The rats were housed with controlled temperature (23−25 °C) and lighting (08:00–20:00 light, 20:00–08:00 dark) and with free access to food and tap water. All the animals used in this work received humane care in compliance with institutional animal care guidelines. Antihypertensive drugs used in this study are as follows: hydrochlorothiazide (Nanjing Pharmaceutical Co, Nanjing, China) and nitrendipine (Shanghai Sanwei Pharmaceutical Co, Ltd, Shanghai, China).
Drug administration The two drugs and the combination of these two drugs were dissolved in the 0.8% carboxymethylcellulose sodium (CMC). After a preliminary study, the dosages were as follow: hydrochlorothiazide 10, 20 mg/kg, nitrendipine 5 mg, 10 mg/kg and the combination of hydrochlorothiazide and nitrendipine 10+5, 20+10 mg/kg. Each group of rats received one dosage and 0.8% CMC was given to control rats (n=10 for each group). Drugs were administered by catheter of gastric fistula implanted 3 d before the experiment.
BP and BPV measurement Systolic BP (SBP), diastolic BP (DBP) and heart period (HP) were continuously recorded described in previous studies[10,11]. Rats were anesthetized with a combination of ketamine (40 mg/kg) and diazepam (6 mg/kg). A floating polyethylene catheter was inserted into the lower abdominal aorta via the left femoral artery for BP measurement, and another catheter was placed into the stomach via a mid-abdominal incision for drug administration (ig). The catheters were exteriorized through the interscapular skin. After operation, each animal was treated intramuscularly with a dose of sodium benzylpenicillin (6×104 IU) and housed individually with controlled temperature (23–25 °C) and with free access to food and tap water. After a 2-d recovery period, the animals were placed in individual cylindrical cages containing food and water for BP recording. The aortic catheter was connected to a BP transducer via a rotating swivel that allowed the animals to move freely in the cage. After approximately 4-h habituation, at 12:00 the BP signal was digitized by a computerized system (MPA 2000M, Alcott Biotech, Shanghai, China). Then, at 13:00 the drug was given via the catheter of gastric fistula. SBP, DBP, and HP values from every heartbeat were recorded for 4 h, up to 17:00, according to our preliminary studies. The mean values and standard deviation of these parameters for each rat were calculated. The standard deviation of all values obtained was denoted as the quantitative parameter of variability, ie, SBP variability (SBPV), DBP variability (DBPV).
Probability sum test To determine whether the combination was synergistic, we used the probability sum test (q test)[10,12]. This test came from classic probability analysis and it was proposed for evaluating the synergism of the combination of 2 drugs. In the present work, we used the following criteria. Compared with the mean values of 1-h baseline (before drug administration), rats with a decrease of the mean value of 4 h ≥20 mmHg (in SBP) and ≥15 mmHg (in DBP) were defined as responders. For SBPV and DBPV, the rats with a decrease or increase ≥1.5 mmHg were defined as responders. The formula is as follows: q=PA+B/(PA+PB–PA×PB). Here, A and B indicate drug A and drug B; P (probability) is the percentage of responders in each group. PA+B is real percentage of responder and (PA+PB–PA×PB) is expected response rate. (PA+PB) indicates the sum of the probabilities when drug A and drug B is used alone. (PA×PB) is the probability of rats responding to both drugs when they were used alone, that is assuming the two drugs act independently. When q<0.85 the combination is antagonistic; when q>1.15 it is synergistic. And when q is between 0.85 and 1.15, it is additive.
Statistical analysis Data were expressed as mean±SEM. Comparisons among groups were made by using ANOVA and comparisons between values obtained in the same group before and after drug administration were made by using the paired t-test. P<0.05 was considered statistically significant.
Results
Effects of nitrendipine and hydrochlorothiazide alone and in combination on BP in SHR The baseline SBP in control SHR was 175±12 mmHg and DBP was 110±14 mmHg. BP remained unchanged after administration of vehicle (Table 1). The baseline SBP and DBP in all 6 treated groups of SHR were similar to those in the control group. SBP and DBP (means of a period of 4 h) were significantly decreased in rats treated with the combination and with the high dose of hydrochlorothiazide or nitrendipine alone. The minimal decrease in SBP (-6 mmHg) was observed in rats treated with a small dose (10 mg/kg) of hydrochlorothiazide and the maximal decrease in SBP (-41 mmHg) in rats treated with the combination of hydrochlorothiazide and nitrendipine at high doses (20+10 mg/kg). A significant dose-effect relationship was found in the combination group. The decrease in 4-h DBP was not as profound as the decrease in SBP. Only the combination of hydrochlorothiazide and nitrendipine at high doses (20+10 mg/kg) decreased the DBP of rats more than 20 mmHg. In terms of HP, only a slight tachycardia (shortened HP) was seen in rats treated with large-dose nitrendipine and the combination at a large dose (Table 1).

Full table
Effects of nitrendipine and hydrochlorothiazide alone, and in combination on SBPV and DBPV after administration in SHR The mean values of BPV over 1 h just before drug administration served as the baseline. The mean values of BPV over 4 h after drug administration were compared with baseline values. The baseline SBPV and DBPV in control SHR were 9.67±0.16 mmHg and 6.51±0.19 mmHg, respectively. BPV remained unchanged after administration of the vehicle. The baseline BPV in all 6 treated groups of SHR was similar to those in the control group. It was found that SBPV significantly decreased in rats treated with the combination and with the high dose of nitrendipine alone and DBPV significantly decreased only in rats treated with the combination (Figure 1).

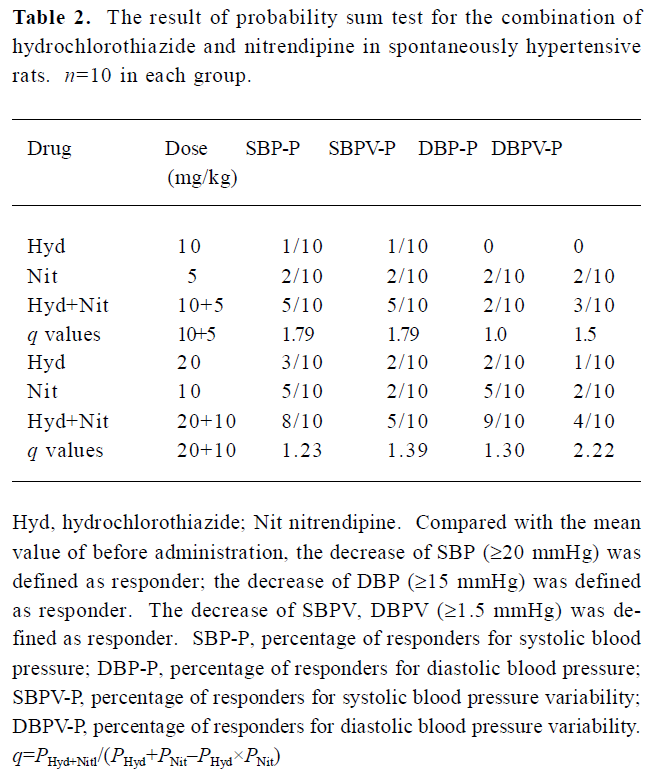
Synergism of hydrochlorothiazide and nitrendipine on SBP and SBPV in SHR Based on the results presented in Table 2, the effectiveness of the decrease in SBP was calculated individually. Rats with a decrease in SBP (≥20 mmHg) were defined as responder and with a decrease in SBP (<20 mmHg) as non-responder. According to this criterion, the probability was defined as the ratio of responder by the total number rats studied in a group. The result of the probability is presented in Table 2. Using the formula described in the method section, we have q-values of 1.79 for the low-dose group (10+5 mg/kg) and 1.23 for the high-dose group (20+10 mg/kg) with the combination of hydrochlorothiazide and nitrendipine. These q values were >1.15, and the combination was synergistic. However, q values for DBP were not the same as for SBP. The synergism was only seen in the large-dose group (q=1.30). BPV was calculated using the data obtained during a period of 4 h after the drug administration. Compared with the baseline, a decrease of SBPV (≥1.5 mmHg) was defined as responder. According to this criterion and using the formula described in the Method section, we have the q-values 1.79 for the low dose group and 1.39 for the high-dose group with the combination of hydrochlorothiazide and nitrendipine. In terms of the effect on DBPV, we have q values of 1.50 for the low-dose group and 2.22 for the large-dose group. As all q values were >1.15, the combination was judged as synergistic.
Full table
Hourly analysis of the effects of nitrendipine and hydro-chlorothiazide alone and in combination on BP Figure 2 shows hourly analysis of the effects of hydrochlorothiazide and nitrendipine alone and in combination on BP. In the control group, SBP remained unchanged in the 1st to 4th hour after vehicle administration (data not shown). It was found that SBP was significantly decreased in the 2nd hour after the treatment with a small dose of hydro-chlorothiazide, in the 2nd and 3rd hour after the treatment with a small dose of nitrendipine, and in the 2nd to 4th hour after the treatment with a small dose of the combination (upper panel of Figure 2). In large dose groups, SBP was significantly decreased in the 1st to 3rd hour after the treatment with hydrochloro-thiazide, in the 2nd and 3rd hours after the treatment with nitrendipine, and in all 4 hour studied after the treatment with combination (bottom panel of Figure 2). From the hourly analysis, it was found that the combination of hydrochlorothiazide and nitrendipine not only potentiated but also prolonged BP reduction.

Discussion
The main findings of the present work may be summarized as follows: (i) there existed an important synergism of hydrochlorothiazide and nitrendipine on SBP reduction. This synergism was greater when treated with small doses; (ii) there existed a synergism of hydrochlorothiazide and nitrendipine on BPV reduction; (iii) the combination of these two drugs prolonged the antihypertensive effect in SHR.
Theoretically, using two antihypertensive agents with different modes of action can increase blood pressure control. Hydrochlorothiazide and nitrendipine are two drugs widely used and often used in combination[12,13]. It was reported that the combination was found to be effective in lowering BP to desirable levels with minimal side effects[13]. Practically, however, it is not easy to judge whether a combination of two drugs is synergic or not. The probability sum test was first proposed by Jin in 1980[10] and adapted for evaluating the synergism of two antihypertensive drugs recently. With this method, we have studied the synergic effects on BP lowering and BPV reduction of two combinations: atenolol and nitrendipine, atenolol and amlodipine[12,14,15]. This method was also used in the present work to evaluate the possible synergism of hydrochlorothiazide and nitrendipine.
Usually, antihypertensive drugs are used orally in hypertensive patients. To mimic this clinical administration, drugs are given by gavage through the mouth in animal studies. However, stress is unavoidable when the gavage is used. In the present study, drugs were given ig by using a gastric catheter implanted 3 d before the experiments[10,15]. This method allows us to record the observing variables during or immediately after drug administration.
The present work clearly demonstrated the synergism of hydrochlorothiazide and nitrendipine on SBP reduction in SHR. This synergism is greater when the treatment is with small doses (q=1.79) than with large doses (q=1.23). Inter-estingly, the combination of these drugs could prolong their antihypertensive effect. Even in large doses, there was no effect in the 4th hour after the administration of hydro-chlorotiazide or nitrendipine. However, the antihypertensive effect of the combination of these drugs lasted more than 4 h even in the small-dose group. In terms of DBP, the synergism was not obvious as SBP, and it was only seen in the large-dose group. It is well known that hydrochlorothiazide reduces the blood volume in acute treatment and nitrendipine dilate the resistant arteries. The great difference in their action modes might be the basis of synergism when they were used in combination.
BPV is associated with hypertensive organ damage in SHR[7,16]. Furthermore it was found that high BPV alone, without hypertension, could also induce organ damage in sinoaortic denervated normotensive rats[15,16]. Recently, it was reported that BPV reduction contributed importantly to the organ protective effects of some antihypertensive drugs[7–9]. Therefore, we propose BPV reduction as a new strategy for the treatment of hypertension[7,8]. In the present work, it was found that the monotherapies with hydrochlorotiazide or nitrendipine had no or minor effects on BPV reduction. However, the combination of hydrochlorotiazide and nitrendi-pine possessed a significant effect on BPV reduction. This effect will be beneficial in the treatment of hypertension.
In conclusion, the combination of hydrochlorothiazide and nitrendipine possessed an obvious synergism on both BP lowering and BPV reduction in conscious free-moving SHR.
References
- Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, et al. Single drug therapy for hypertension in men. A comparison of six anti-hypertensive agents with placebo: the Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med 1993;328:914-21.
- Prisant LM. Fixed low-dose combination in first-line treatment of hypertension. J Hypertens 2002;20:S11-9.
- Moser M, Black HR. The role of combination therapy in the treatment of hypertension. Am J Hypertens 1998;11:73S-78S.
- Parati G, Lantelme P. Blood pressure variability, target organ damage and cardiovascular events. J Hypertens 2002;20:1725-9.
- Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, et al. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension 2000;36:901-6.
- Parati G, Mancia G. Blood pressure variability as a risk factor. Blood Press Monit 2001;6:341-7.
- Su DF, Miao CY. Reduction of blood pressure variability: a new strategy for the treatment of hypertension. Trends Pharmacol Sci 2005;26:388-90.
- Julien C. Pharmacological attenuation of blood pressure variability. Acta Pharmacol Sin 2005;26:1288-9.
- Liu JG, Xu LP, Chu ZX, Miao CY, Su DF. Contribution of blood pressure variability to the effect of nitrendipine on end-organ damage in spontaneously hypertensive rats. J Hypertens 2003;21:1961-7.
- Su DF, Xu LP, Miao CY, Xie HH, Shen FM, Jiang YY. Two useful methods for evaluating antihypertensive drugs in conscious freely moving rats. Acta Pharmacol Sin 2004;25:148-51.
- Su DF, Cerutti C, Barres C, Vincent M, Sassard J. Blood pressure and baroreflex sensitivity in conscious hypertensive rats of Lyon strain. Am J Physiol 1986;251:H1111-7.
- Jin ZJ. About the evaluation of drug combination. Acta Pharmacol Sin 2004;25:146-7.
- Staessen J, Bert P, Bulpitt C, De Cort P, Fagard R, Fletcher A, et al. Nitrendipine in older patients with isolated systolic hypertension: second progress report on the SYST-EUR trial. J Hum Hypertens 1993;7:265-71.
- Schoenberger JA. Calcium antagonists: use in hypertension evaluation of calcium antagonists in combination with diuretics. Angiology 1988;39:87-93.
- Xie HH, Miao CY, Jiang YY, Su DF. Synergism of atenolol and nitrendipine on hemodynamic amelioration and organ protection in hypertensive rats. J Hypertens 2005;23:193-201.
- Shen FM, Xie HH, Ling G, Xu LP, Su DF. Synergistic effects of atenolol and amlodipine for lowering and stabilizing blood pressure in 2K1C renovascular hypertensive rats. Acta Pharmacol Sin 2005;26:1303-8.
