Caveolin-1 is important for nitric oxide-mediated angiogenesis in fibrin gels with human umbilical vein endothelial cells1
Introduction
Caveolae are 50 to 100 nm non-clathrin-coated, flask-shaped invaginations of the plasma membrane playing key roles in vesicular transport and signal transduction. The structural protein of these plasmalemmal microdomains, caveolin, acts as a scaffold for many caveolar residents. A large body of evidence supports the idea that caveolins interacts with a number of signaling molecules, including receptor and non-receptor tyrosine kinases, heterotrimeric G-protein α-subunits, endothelial nitric-oxide synthase (eNOS) and protein kinase C[1]. Domain mapping studies revealed that the interaction of caveolins with these signaling molecules is mediated by a membrane-proximal region of the caveolins termed the “caveolin scaffolding domain” (residues 82–101 in caveolin-1 [Cav-1]). The Cav-1 isoform is particularly abundant in endothelial cells (EC) where it regulates various functions including angiogenesis.
Angiogenesis is the process of generating new blood vessels derived as extensions from the existing vasculature. The principal cells involved are EC, which line all blood vessels and constitute virtually the entirety of capillaries. Angiogenesis is essential for local tumour progression and the development of distant metastasis. Tumour angiogenesis involves the degradation of the basement membrane by activated tissue or circulating endothelial precursors, migration of endothelial cells and coalescence into vascular tubules[2].
The role of Cav-1 in angiogenesis has only been partially defined. Brouet et al[3] demonstrated that decreasing Cav-1 abundance was associated with augmented capillary formation. Endothelial-specific expression of Cav-1 impaired eNOS activation, endothelial barrier function and angiogenic responses to exogenous vascular endothelial growth factor (VEGF)[4]. Other research has shown that Cav-1 was essential for capillary formation but played different roles depending on the stage in the process of angiogenesis[5].
Nitric oxide (NO) is a highly diffusible intercellular signaling molecule that mediates a number of actions, such as vasodilatation, neurotransmission and immune response. It is generated by the enzyme nitric oxide synthase (NOS), which catalyzes the conversion of L-arginine to L-citrulline. NOS exists as 3 isoforms: the calcium-dependent endothelial (eNOS), neuronal (nNOS) and the calcium-independent inducible (iNOS).
Recently, eNOS, a caveolin-interacting protein, was demonstrated to play a predominant role in VEGF–induced angiogenesis and hyperpermeability[6,7]. Moreover, tumors implanted on eNOS-/- mice grew slower and exhibited reduced angiogenesis[8]. A number of angiogenic factors upregulate the endothelial expression of NOS and stimulate the release of endothelium-derived NO[9,10]. NO is a critical mediator of angiogenesis, although its role in tumor growth remains controversial[11]. The aim of the present study was to investigate the role of Cav-1 and the eNOS complex in NO-mediated angiogenesis modeled by human umbilical vein endothelial ells (HUVEC) in fibrin gels.
Materials and methods
Cell isolation and culture HUVEC were isolated from umbilical cords obtained from our hospital. The procedures were approved by the local authorities according to national regulations. Each extremity of the umbilical vein was cannulated and tightly maintained with string. Then the umbilical vein was flushed with 30 mL of warm Dulbecco’s phosphate buffered saline (PBS). 0.25% Trypsin (Hyclone, Logan, Utah, USA) was injected at 1 end until mildly distended. After 8–10 min incubation at 37 °C and 5% CO2, the cord was unclamped and the cells were collected by washing the vein with 20 mL of medium 199 (Gibco, Grand Island, NY, USA). The resultant endothelial cell suspension was centrifuged at 1000 rpm for 6 min and the cell pellet was resuspended in 5 mL endothelial cell basal medium (EBM) (Gibco, USA) supplemented with 20% fetal bovine serum (FBS; Gibco, USA), 100 U/mL penicillin (Invitrogen, San Diego, CA, USA), 100 µg/mL heparin and 50 µg/mL endothelial cell growth supplement (ECGS; BD Biosciences, Bedford, MA, USA). This suspension was seeded into a 25-cm2 gelatin-coated culture flask. The HUVEC were cultured at 37 °C and 95% air/5% CO2 and confirmed both by their characteristic cobblestone morphology under phase-contrast microscopy and by positive direct immunofluorescence staining for von Willebrand factor.
3-D angiogenesis assay in fibrin gels Human fibrinogen (20 mg/mL in PBS) was dissolved in serum-free EBM to a concentration of 2.0 mg/mL, supplemented with heparin (100 µg/mL) and ECGS (50 µg/mL); 6-well plates were used for the assay. The bottom of each well was first covered with 1.0 mL of fibrinogen solution, and the clotting was induced by the addition of thrombin (0.5 U/mL, Sigma, St Louis, MO, USA). After 10 min of polymerization, another 1.0 mL of fibrinogen solution containing about 3×105 HUVEC was added. After the HUVEC were evenly distributed on the surface of the gels, polymerization was induced as described earlier. The gels were soaked in EBM medium containing 20% FBS, heparin (100 µg/mL) and ECGS (50 µg/mL) and cultured for periods of up to 5 d in the presence or absence of 5 mmol/L NG-nitro-L-arginine methyl ester (L-NAME; Sigma, USA) and 20 ng/mL VEGF. Cultures were preincubated for 30 min with vehicle or L-NAME before being exposed to VEGF.
Quantification of endothelial capillary tubule formation HUVEC in fibrin gels formed 3-D structures, which were called tubule formation. The tubule formation index was determined by measuring the length of tubules (≥30 µm) in random fields from each well. Images were captured using an Olympus Inverted Research Microscope (Olympus, Tokyo, Japan) coupled to an Olympus C-5050 Zoom digital camera. Images were prepared in Adobe Photoshop 7.0 (Adobe, San José, CA, USA) and exported to an image analysis software package (Image J program, NIH, Bethesda, MD, USA) for identification of endothelial cell tubule-like networks. The total tubule length was derived for each of the 4 randomly chosen fields, and the total area of the culture surface covered by HUVEC was determined in the same fields. The tubule formation index was calculated as the ratio of the total tubule length over the cell area for each field, and a mean tubule formation index value was obtained for each culture well[12].
Cell treatment with antisense oligonucleotides Translation of Cav-1 mRNA was inhibited using Cav-1 antisense oligonucleotide (ASON), ATGTCCCTCCGAGTCTA, directed against nucleotides 20–36 of the open reading frame. The nonsense oligonucleotide (NSON), ATGATGAGGAA-CGACCGAACTGTGC, was used as controls to assess nonspecific effects of oligonucleotide treatments[13]. Before transfection, HUVEC were seeded at a density of 1×105–2×105 cells/mL in 6-well plates. When cells were 90%–95% confluent, the 500 µL of diluted DNA and Lipofectamine 2000 (Invitrogen, San Diego, CA, USA) mixture was added to each well containing cells and medium. HUVEC were incubated at 37 °C in a CO2 incubator for 18–48 h prior to testing for transgene expression by RT-PCR. Densitometric analysis of the bands was performed by TANON GIS software (Shang-hai Tanon Co, Shanghai, China) and indicated as the percentage inhibition with respect to Cav-1 levels in untreated HUVEC and Lipofectamine-treated HUVEC.
Semiquantitative RT-PCR analysis Fibrin gels were dissolved with 10×trypsin EDTA for 5–10 min to release HUVEC[14], and total RNA was isolated from HUVEC using TRIzol (Invitrogen, USA) according to protocol. The concentration of RNA extracted was determined at wavelength of 260 nm using Biophotometer (Eppendorf, Netheler-Hinz, Hamburg, Germany). First-strand complementary DNA (cDNA) were synthesized by the Reverse Transcription System (Promega, Madison, WI, USA). Total RNA (1 µg) was reverse transcribed to first-strand cDNA in a 20 µL mixture containing 25 mmol/L MgCl2 (4 µL), reverse transcription 10×Buffer (2 µL), 10 mmol/L deoxyribonucleotide triphosphate (dNTP) mixture (2 µL), recombinant RNase inhibitor (0.5 µL), avian myeloblastosis virus (AMV) reverse transcriptase (15 U), and Oligo(dT)15 primers (0.5 µg). The reactions were incubated at 42 °C for 60 min, and then the samples were heated at 95 °C for 5 min. Cav-1 and eNOS mRNA was performed by the RT-PCR technique using a housekeeping gene, GAPDH, as an internal standard. The total reaction volume was 25 µL for PCR reaction, which was performed in a DNA thermal cycler (MJ Research PTC200, Waltham, MA, USA). Primer sequences and PCR reaction conditions are shown in Table 1. The PCR samples, together with a 2000 bp DNA ladder, were analyzed by 1.5% agarose gel and visualized by ethidium bromide staining. The intensity of the bands was measured by densitometry, and the relative value of the Cav-1 or eNOS band to the GAPDH band was calculated in each sample.
Full table
NO assay Nitrite, a stable end product of NO, was measured in the culture medium using the NO Colorimetric Assay (Jiancheng Bio, Nanjing, China) according to the manufacturer’s protocol.
Data analysis and statistics Data are presented as mean±SD. Statistical comparisons between the groups were performed using the Student’s t test or one-way ANOVA. Differences among means were considered significant when P<0.05.
Results
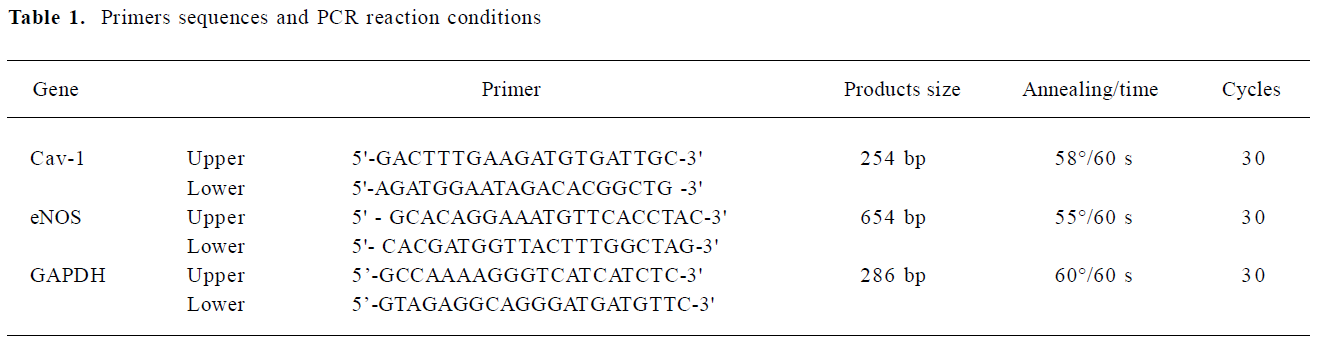
Cav-1 and eNOS mRNA expression during the formation process of capillary-like tubules The time-dependent formation of capillary-like tubules of HUVEC in fibrin gels in the presence of VEGF (20 ng/mL) is shown in Figure 1. These tubular structures first appeared after 1 d of incubation and developed into a well-organized capillary-like network after 5 d of incubation (Figure 1A). RT-PCR analysis revealed that Cav-1 levels steadily increased in a time-dependent manner and reached their maximum after 5 d of incubation, but there were no obvious changes in eNOS mRNA expression in response to VEGF in the fibrin gel model (Figure 1B,C; n=4).
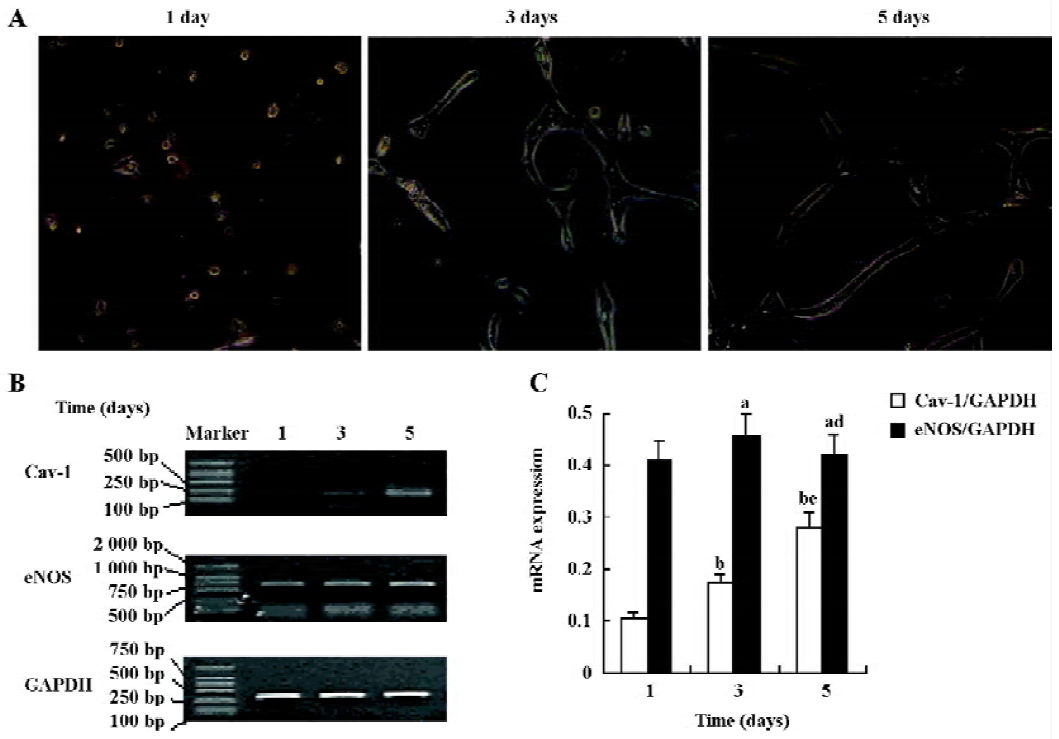
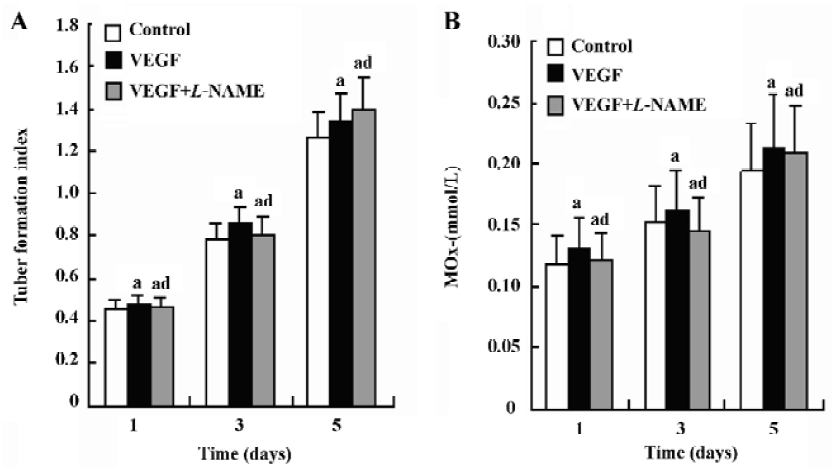
Effects of NO on the formation of capillary-like tubules by HUVEC In the absence or presence of VEGF (20 ng/mL), HUVEC cultured in fibrin gels for 5 d exhibited classical capillary-like tubules. But the addition of VEGF resulted in the more extensive formation of capillary-like tubules. This promoting effect of VEGF on angiogenesis was almost completely blocked by the co-incubation with the NO synthesis inhibitor L-NAME (Figure 2A). The tubule formation index of HUVEC in fibrin gels is shown in Figure 2B (n=4).
NO production by HUVEC in fibrin gels is shown in Figure 2C (n=4). NO production was significantly increased by VEGF in HUVEC. The addition of L-NAME (5.0 mmol/L) to the culture medium significantly attenuated nitrite accumulation in response to VEGF.
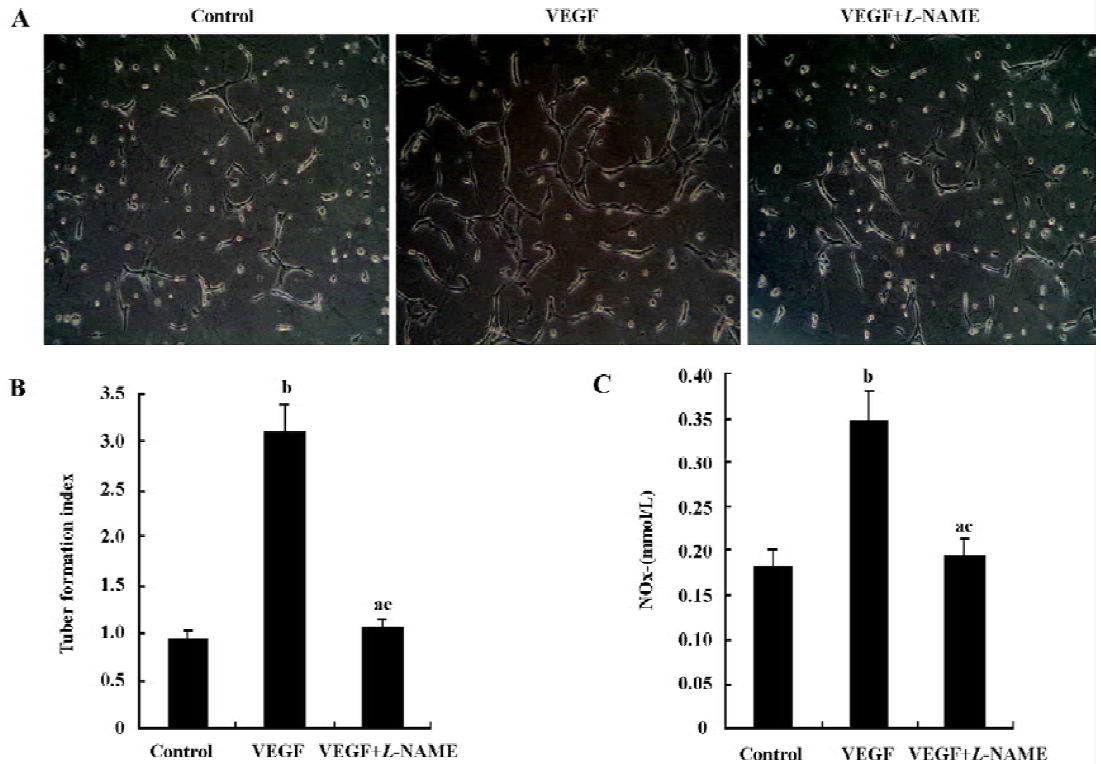
The role of downregulated Cav-1 on the formation of capillary-like tubules To further examine the effect of Cav-1 on the formation of capillary-like tubules of HUVEC in fibrin gels, we next used an antisense approach. The level of Cav-1 mRNA was reduced by 73% when HUVEC were infected with the antisense Cav-1 oligonucleotides (Figure 3A).The transduced HUVEC with ASON were then plated in the fibrin gels and incubated for 5 d; the capillary-like tubules were significantly fewer than those of non-transduced control cells and transduced HUVEC with NSON (Figure 3B,C; n=4).
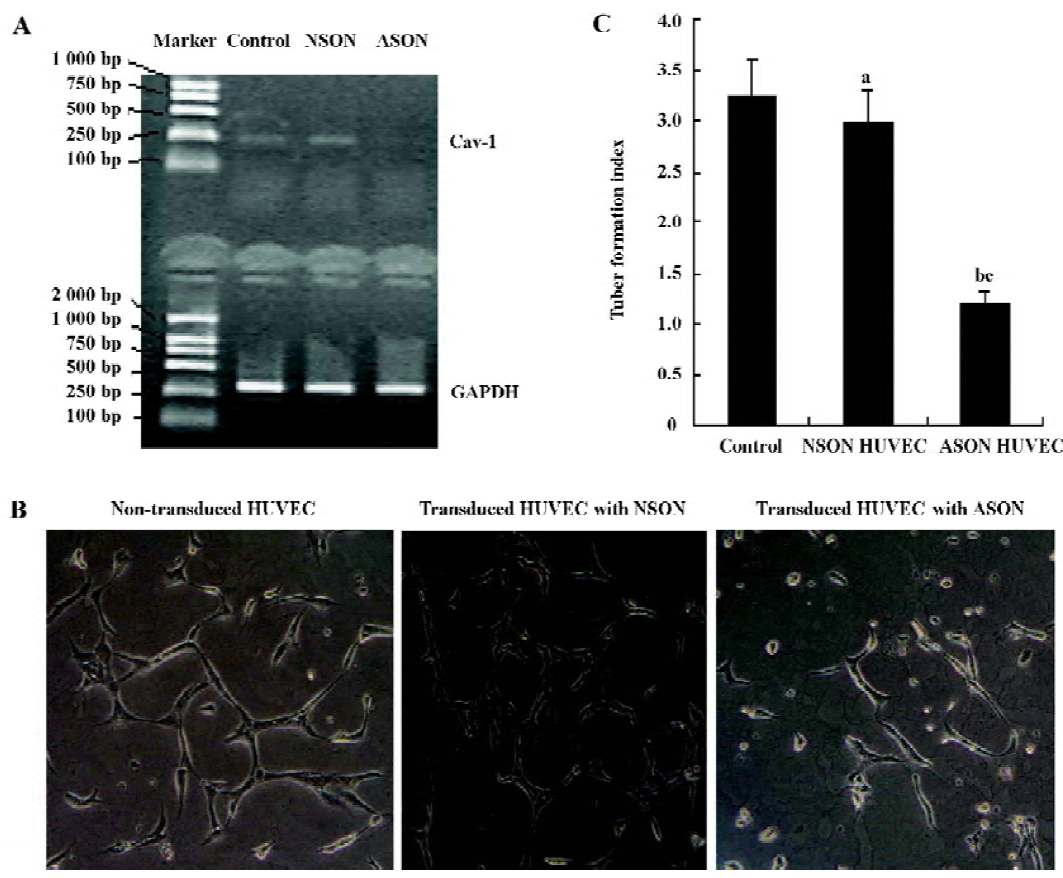
Effects of VEGF and L-NAME on capillary-like tubule formation and NO production of transduced HUVEC with ASON in fibrin gels In order to examine the role of Cav-1 in the VEGF/NO-mediated angiogenesis, we cultured the transduced HUVEC with ASON in fibrin gels in the presence of VEGF (20 ng/mL) and L-NAME (5.0 mmol/L) for 5 d. The tubule formation index and NO production were measured at different times. The transduced HUVEC with ASON cultured in fibrin gels in the presence of VEGF (20 ng/mL) and L-NAME (5.0 mmol/L) did not alter the formation of the capillary-like tubules and NO production significantly (Figure 4A,B; n=4).
Discussion
Previous studies of the role of NO in the angiogenic response have provided conflicting results. It has been reported that the NO-donor compound sodium nitroprusside (SNP) inhibited angiogenesis in the chick chorioallantoic membrane and reduced vascular tubule formation of HUVEC grown on Matrigel[15]. On the other hand, NO is an endothelial survival factor, inhibiting apoptosis[16], and increasing endothelial cell proliferation[17] and migration[18]. Further-more, the vasodilator effects of NO may play a role in its angiogenic effects. These studies indicate that NO is a critical pro-angiogenic factor. In the present study, HUVEC in a 3-D gel produced more NO and formed more capillary-like tubules when stimulated by VEGF. The capillary tubule formation was abolished by the NOS antagonist L-NAME. Our study showed that VEGF stimulated the endothelial elaborate of NO, and NO was a critical angiogenic mediator.
NO has been identified as a downstream mediator of various growth factors initiating the angiogenic signaling cascade in endothelial cells[19]. Cav-1 is a key player in signal transduction which regulates various functions including transcytosis, permeability, vascular tone and angiogenesis[20]. The role of Cav-1 in NO-mediated angiogenesis has only been partially defined. The caveolin scaffolding domain (CSD) could block NO-mediated vascular permeability[8], which is known as an early event in the process of angio-genesis. Brouet and colleagues reported that the same CSD peptides could block the NO-dependent angiogenesis in a model of EC cultured on Matrigel[3]. Our study shows that the levels of Cav-1 mRNA in HUVEC expression increased steadily from 1 to 5 d culture, and the maximum level of Cav-1 expression occurred just prior to the formation of extensive capillary-like tubules. Furthermore, the downregulation of Cav-1 expression, via an antisense approach, reduced the number of capillary-like tubules significantly. These results support that Cav-1 plays an important positive role in capillary-like tubules formation.
Caveolin is known to repress the catalytic activity of various enzymes, and caveolae are thought to facilitate and amplify signaling cascades through the compartmentation of receptors with their effectors/mediators[21], a process named “the caveolar paradox”[22]. eNOS is anchored in part to caveolae by cotranslational N-myristoylation and posttranslational palmitoylation[23] and catalyzes the NADPH-dependent production of NO in the process of converting L-arginine to L-citrulline. So the role of Cav-1 in the process of angiogenesis is associated with eNOS and NO production. The relationship between Cav-1 and eNOS and NO in the process of angiogenesis is still unknown. In the present study, we show that NO production increased steadily during the process of capillary-like tubules formation. With the increase of NO production, the levels of Cav-1 mRNA in HUVEC expression increased steadily from 1 to 5 d of culture. Although VEGF augments the endothelial expression of NOS and stimulates the biosynthesis of NO from cultured HUVEC[9,10], we did not see obvious changes in eNOS mRNA expression in response to VEGF in the fibrin gel model. The equilibrium between eNOS bound to caveolin and caveolin-free eNOS determines the basal component of eNOS-dependent NO release in EC. In addition, tonic repression of basal activity and facilitation of agonist-evoked stimulation of eNOS are 2 apparently paradoxical tasks in the context of the caveolar/eNOS interaction[22]. Furthermore, NO interferes with the integrity of Cav-1 scaffolding function and dissociates Cav-1 and the eNOS complex[24]. These studies account for the increase of NO production during the process of angio-genesis. Our study shows that Cav-1 plays a synergetic role with NO in VEGF-induced angiogenesis.
In the present study, we show that the capillary-like tubules formation and NO production of transduced HUVEC cultured in fibrin gels had no responses to the addition of VEGF (20 ng/mL) and L-NAME (5.0 mmol/L). Our results were quite different from some studies[4,25]. Under different conditions, the interaction of Cav-1 with eNOS plays different roles in eNOS activity[22]. Sonveaux et al[26] demonstrated that the lack of caveolin prevented the time-dependent VEGF-induced phosphorylation of extracellular signal-regulated kinase (ERK) and that VEGF-induced phosphorylation of eNOS on Ser1177 and dephosphorylation on Thr495, both considered as hallmarks of eNOS activation, were abrogated in Cav-/- EC, but not in Cav+/+ EC. Cav-1 is critical for VEGF and eNOS activation, and both VEGF and eNOS play an important role in NO-mediated angiogenesis. Our results further demonstrate that Cav-1 is indispensable in VEGF/NO-mediated angiogenesis.
In conclusion, our angiogenesis model suggests that NO is a critical angiogenic mediator. In this NO-mediated angio-genesis, Cav-1 played an important positive role in EC differentiation and capillary-like tubules formation. Cav-1 is critical for the role of VEGF and eNOS in NO-mediated angiogenesis and may be an important target of anti-angiogenesis therapy.
References
- Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem 1998;273:5419-22.
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumourigenesis. Cell 1996;86:353-64.
- Brouet A, Sonveaux P, Dessy C, Moniotte S, Balligand JL, Feron O. Hsp 90 and caveolin are key targets for the proangiogenic nitric oxide-mediated effects of statins. Circ Res 2001;89:866-73.
- Bauer PM, Yu J, Chen Y, Hickey R, Bernatchez PN, Looft-Wilson R, et al. Endothelial-specific expression of caveolin-1 impairs microvascular permeability and angiogenesis. Proc Natl Acad Sci USA 2005;102:204-9.
- Liu J, Razani B, Tang S, Terman BI, Ware A, Lisanti MP. Angiogenesis activators and inhibitors differentially regulate caveolin-1 expression and caveolae formation in vascular endothelial cells. J Biol Chem 1999; 274: 15 781–5.
- Luo JD, Chen AF. Nitric oxide: a newly discovered function on wound healing. Acta Pharmacol Sin 2005;26:259-64.
- Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA 2001;98:2604-9.
- Gratton JP, Lin MI, Yu J, Weiss ED, Jiang ZL, Fairchild TA, et al. Selective inhibition of tumor microvascular permeability by cavtratin blocks tumor progression in mice. Cancer Cell 2003;4:31-9.
- Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates eNOS message, protein, and NO production in human endothelial cells. Am J Physiol 1998;274:H1054-8.
- van der Zee R, Murohara T, Luo Z, Zollmann F, Passeri J, Lekutat C, et al. Vascular endothelial growth factor/vascular permeability factor augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation 1997;95:1030-7.
- Alexandrova R, Mileva M, Zvetkova E. Nitric oxide and cancer (mini review). Exp Pathol Parasitol 2001;4:13-8.
- Babaei S, Teichert-Kuliszewska K, Monge JC, Mohamed F, Bendeck MP, Stewart DJ. Role of nitric oxide in the angiogenic response in vitro to basic fibroblast growth factor. Circ Res 1998;82:1007-15.
- Phillips PG, Birnby LM. Nitric oxide modulates caveolin-1 and matrix metalloproteinase-9 expression and distribution at the endothelial cell/tumor cell interface. Am J Physiol Lung Cell Mol Physiol 2004;286:L1055-65.
- Nakatsu MN, Sainson RC, Aoto JN, Taylor KL, Aitkenhead M, Perez-del-Pulgar S, et al. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of bibroblasts and angiopoietn-1. Microvas Res 2003;66:102-12.
- Pipili-Synetos E, Sakkoula E, Haralabopoulos G, Andriopoulou P, Peristeris P, Maragoudakis ME. Evidence that nitric oxide is an endogenous antiangiogenic mediator. Br J Pharmacol 1994;111:894-902.
- Dimmeler S, Hermann C, Galle J, Zeiher AM. Upregulation of superoxide dismutase and nitric oxide synthase mediates the apoptosis-suppressive effects of shear stress on endothelial cells. Arterioscler Thromb Vasc Biol 1999;19:656-64.
- Ziche M, Parenti A, Ledda F, Dell’Era P, Granger HJ, Maggi CA, et al. Nitric oxide promotes proliferation and plasminogen activator production by coronary venular endothelium through endogenous bFGF. Circ Res 1997;80:845-52.
- Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest 1994;94:2036-44.
- Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. NO production contributes to the angiogenic properties of VEGF in human endothelial cells. J Clin Invest 1997;100:3131-9.
- Frank PG, Woodman SE, Park DS, Lisanti MP. Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol 2003;23:1161-8.
- Deurs B, Roepstorff K, Hommelgaard AM, Sandvig K. Caveolae: anchored, multifunctional platforms in the lipid ocean. Trends Cell Biol 2003;13:92-100.
- Feron O, Kelly RA. The caveolar paradox: suppressing, inducing, and terminating eNOS signaling. Circ Res 2001;88:129-31.
- Feron O, Michel JB, Sase K, Michel T. Dynamic regulation of endothelial nitric oxide synthase: complementary roles of dual acylation and caveolin interactions. Biochemistry 1998;37:193-200.
- Li H, Brodsky S, Basco M, Romanov V, De Angelis DA, Goligorsky MS. Nitric oxide attenuates signal transduction: possible role in dissociating caveolin-1 scaffold. Circ Res 2001;88:229-36.
- Miyawaki-Shimizu K, Predescu D, Shimizu J, Broman M, Predescu S, Malik AB. siRNA-induced caveolin-1 knockdown in mice increases lung vascular permeability via the junctional pathway. 2006; 290: L405–13.
- Sonveau X, Martinive P, DeWever J, Batova Z, Daneau G, Pelat M, et al. Caveolin-1 expression is critical for VEGF-induced ischemic hindlimb collateralization and nitric oxide-mediated angiogenesis. Circ Res 2004;95:154-61.
