Cysteinyl leukotriene receptors CysLT1 and CysLT2 are upregulated in acute neuronal injury after focal cerebral ischemia in mice1
Introduction
Cysteinyl leukotrienes (CysLT; including LTC4, LTD4 and LTE4), 5-lipoxygenase (5-LOX) metabolites of arachidonic acid, are the potent inflammatory mediators involved in various central nervous diseases, such as cerebral ischemia[1–3], brain trauma[4], brain tumors[5] and epilepsy[6]. After cerebral ischemia or ischemia-reperfusion, the production of CysLT in the ischemic brain is increased[1–3]. In addition, 5-LOX inhibitors ameliorate cerebral ischemic injuries via reducing the production of CysLT[7,8]. Since the actions of CysLT are mediated by activating their receptors (CysLT receptors), it is important to investigate the changes in CysLT receptors after cerebral ischemia.
Two types of CysLT receptors, CysLT1 and CysLT2, have been cloned and identified as G protein-coupled receptors[9–14]. The CysLT1 receptor is highly expressed in the human spleen and peripheral blood leukocytes, and weakly in the brain, lung, colon, heart, pancreas, prostate, skeletal muscle, kidney, liver, placenta and small intestine as determined by Northern blotting[9,10]. The mouse CysLT1 receptor has an expression pattern distinct from that of humans; it is highly expressed in the lung and skin, and weakly in other tissues including the brain[15]. Little is known about the expression and localization of the CysLT1 receptor in the brain. Recently, we reported that the CysLT1 receptor was primarily expressed in the microvascular endothelia in the human brain by immunohistochemical analyses, and its expression was increased in the neuron- and glial-appearing cells after traumatic injury[16]. Moreover, we have found that the CysLT1 receptor antagonists (pranlukast and montelukast) protect against cerebral ischemic injury in rats and mice, pharmacologically suggesting that the CysLT1 receptor may play a role in cerebral ischemia[17–22].
On the other hand, the CysLT2 receptor is highly expressed in the spleen, heart, adrenal glands and thymus, and weakly in the kidney, brain and peripheral blood leukocytes[23]. In the brain, the CysLT2 receptor expression is found in most regions and in the spinal cord[12,13]. The expression patterns of the CysLT2 receptor are similar in mouse and human tissues. We found that the CysLT2 receptor was primarily expressed in the smooth muscle cells of cerebral vessels in the human brain by immunohistochemical analyses, and its expression was also increased in the neuron- and glial-appearing cells after traumatic injury[24]. Because no selective antagonist is available for the CysLT2 receptor, its role in the brain is unknown.
However, no direct evidence shows the role of CysLT1 and CysLT2 receptors in the brain after cerebral ischemia. To clarify their role in acute ischemic neuronal injury, we investigated the temporal expressions of CysLT1 and CysLT2 receptor mRNA in the mouse brain from 1 h to 48 h (acute phase) after focal cerebral ischemia. We wanted to show the relationship between the temporal expressions and the development of ischemic neuronal injury. In addition, we observed the localization of the CysLT1 receptor 24 h after ischemia.
Materials and methods
Physiological parameter monitoring Male ICR (Institute of Cancer Researcch) mice weighing 25–30 g (Shanghai Experimental Animal Center, China, Shanghai, China; Certificate N
The mice were anaesthetized with chloral hydrate (400 mg/kg, ip). A polyethylene tube was inserted into the right femoral artery for continuous monitoring of blood pressure using a computer-assisted system (PC-Lab, Kelong Inc, Nanjing, China), and for measuring arterial blood Pao2, Paco2 and pH (Blood Gas Analyzer ABL 330, Leidu Inc, Copenhagen, Kobenhavn, Denmark). Blood glucose was monitored by a one touch basic blood glucose monitoring system (Lifescan Inc, New Brunswick, New Jersey, USA). Rectal (core) temperature was measured and maintained at 37.0±0.5 ºC with a heating pad and a heating lamp during the surgery.
Permanent focal cerebral ischemia After anesthesia, permanent focal cerebral ischemia was induced by middle cerebral artery occlusion (MCAO) as described previously[17]. Briefly, a 6–0 nylon monofilament suture, blunted at the tip and coated with 1% poly-L-lysine, was inserted into the right internal carotid artery, and advanced approximately 10 mm distal to the carotid bifurcation to occlude the origin of the middle cerebral artery. In the sham-operated mice, the same procedure was done, except for the insertion of the intraluminal filament. After surgery, the mice were kept for about 2 h in a warm box heated by lamps to maintain the body temperature.
Behavioral assessments Neurological deficit scores were evaluated 6, 24 and 48 h after MCAO[25]: 0, no deficit; 1, flexion of contralateral forelimb upon lifting of the whole animal by the tail; 2, circling to the contralateral side; 3, falling to contralateral side; and 4, no spontaneous motor activity. An inclined board test was performed to assess balance and coordination[20] based on modification of a reported method[26]. The mice were placed on a board (50×30 cm); once they were stable, the board was inclined from horizontal to vertical. The degree at which the animal fell from the board (holding angle) was recorded. The test was repeated 3 times and the average degree was used. Behavioral assessments were not observed 1 and 3 h after ischemia because the mice did not recover completely from the anesthesia.
Histopathology analyses The brains were removed at 1, 3, 6, 24, and 48 h after MCAO, fixed in 4% paraformaldehyde overnight, and cut by cryostat into serial 8 μm-thick coronal sections, then stained with 1% toluidine blue. The apparently surviving neuron-appearing cells in the temporoparietal cortex III, IV layers, the hippocampal CA1 region and the striatum (1.8–2.0 mm caudal from the bregma) were counted using the ImageTool 2.0 analysis program (University of Texas Health Science Center in San Antonio, San Antonio, Texas, USA).
RT-PCR The ischemic and the contralateral non-ischemic hemispheres were dissected on ice 1, 3, 6, 24 and 48 h after MCAO, and stored at -80 °C for RNA extraction. Total RNA was extracted using Trizol reagents (Invitrogen, Carlsbad, California,USA) according to the manufacture’s protocol. For cDNA synthesis, aliquots of total RNA (1 µg) were mixed with 1 mmol/L dNTP, 0.2 µg random primer, 20 U RNase, 200 U M-MuLV reverse transcriptase in 20 µL of the reverse reaction buffer. The mixture was incubated at 42 °C for 60 min, and then 70 °C for 10 min to inactivate the reverse trans-criptase. As a negative control, the reaction was performed at the absence of reverse transcriptase.
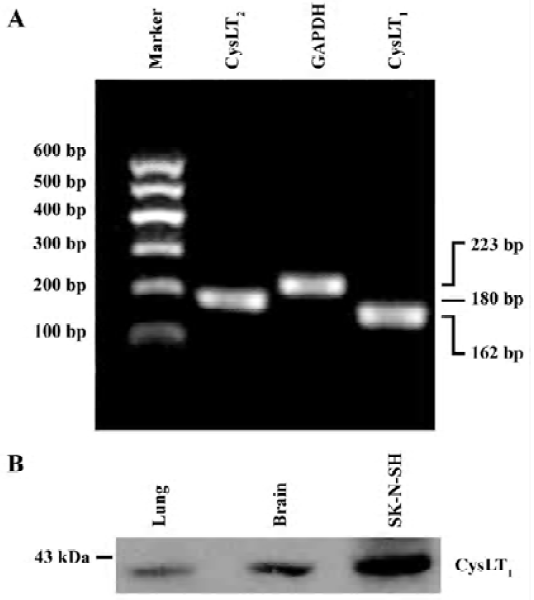
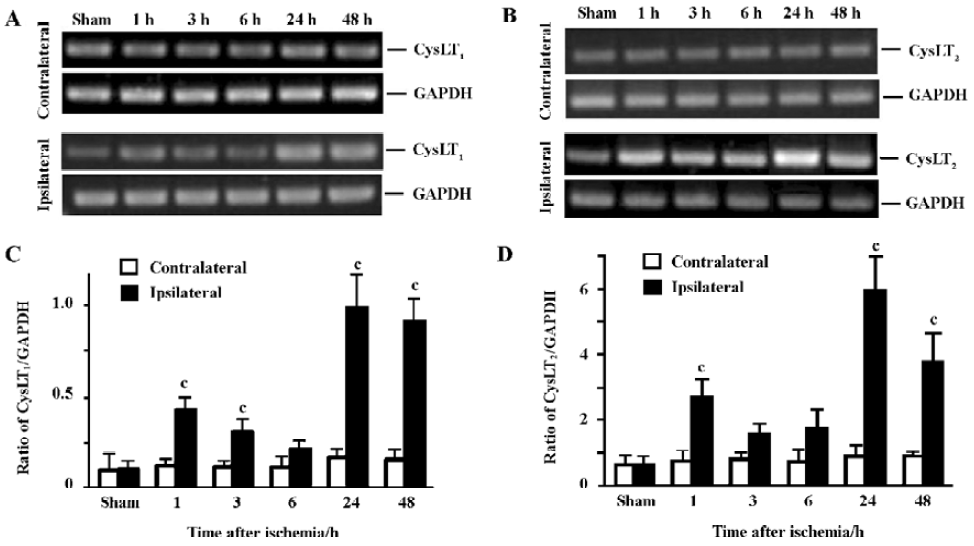
PCR was performed in a 40 µL reaction mixture containing PCR buffer, 4 µL cDNA, 200 µmol/L dNTP, 1.5 mmol/L MgCl2, 10 pmol of each primer and 1 U of Taq DNA polymerase (Takara, Kyoto, Japan). PCR reactions were performed as follows: 94 ºC for 2 min followed by 35 cycles of 93 ºC for 10 s, 66 ºC for 30 s and 72 ºC for 30 s, and finally extended at 72 ºC for 10 min. The primer sequences for mouse CysLT1 and CysLT2 receptors[13] were synthesized by Sangon Biotech Co (Shanghai, China) as follows: mCysLT1 (+): 5'-CAA CGA ACT ATC CAC CTT CAC C-3', mCysLT1 (–): 5'-AGC CTT CTC CTA AAG TTT CCA C-3'; mCysLT2 (+): 5'-GTC CAC GTG CTG CTC ATA GG-3', mCysLT2 (–): 5'-ATT GGC TGC AGC CAT GGT C-3'. GAPDH was used as the control; its primer sequences were GAPDH (+): 5'-GTC GGT GTG AAC GGA TTT GG-3' and GAPDH (–): 5'-GCT CCT GGA AGA TGG TGA TGG-3'. PCR products in 10 µL were separated by electrophoresis on a 2% agarose gel containing ethidium bromide and photographed. The optical density of the bands was determined by an image analysis system (Bio-Rad, Hercules, California, USA). The PCR product sizes of CysLT1, CysLT2 receptors and GAPDH were162 bp, 180 bp and 223 bp, respectively (Figure 1A). CysLT1 and CysLT2 receptor mRNA levels were normalized to the level of GAPDH mRNA.
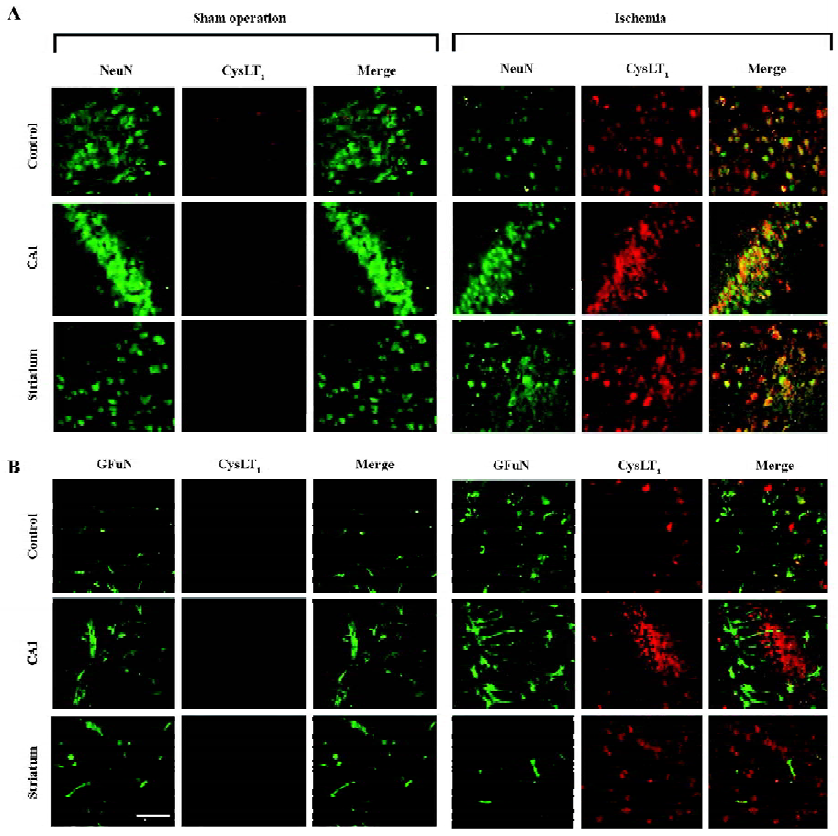
Double immunofluorescence To visualize the localization of the CysLT1 receptor, double immunofluorescence was employed. Briefly, after blocking non-specific binding of IgG with 5% normal goat serum for 2 h at room temperature, the brain sections were incubated overnight at 4 °C with a mixture of rabbit polyclonal anti-human CysLT1 receptor antibody (1:150, Cayman Chemicals, Ann Arbor, MI, USA) and mouse monoclonal antibody against neuronal nuclei (NeuN; 1:200; Chemicon, Temecula, California, USA), a specific marker of neurons, or against a glial fibrillary acidic protein (GFAP; 1:800; Chemicon, USA), a specific marker of astrocytes. Then, the sections were incubated with the mixture of fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG and Cy3-conjugated goat anti-mouse IgG (1:100; Chemicon, USA).
To confirm the specificity of the polyclonal rabbit anti-human CysLT1 receptor antibody used in the double immunofluorescence of the mouse brain tissue, we collected protein samples of cultured human neuroblastma cell line SK-N-SH cells (purchased from the Institute of Cell Biology, Chinese Academy of Sciences, Shanghai, China) as well as a normal mouse lung and cerebral cortex. Protein samples (50 μg) were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes then reacted with the rabbit polyclonal anti-human CysLT1 receptor antibody (1:2000), and with the peroxidase-conjugated goat anti-rabbit IgG (1:2000) after repeated washing. Finally the protein bands were visualized by enhanced chemiluminescence. The result showed that a band was close to 43 kDa in the mouse cerebral cortex, the same as that in the SK-N-SH cells (Figure 1B). This was consistent with previously published results using the same polyclonal antibody[27,28]. Thus, the antibody was usable for immunohistochemistry of mouse brain tissue.
Statistical analysis Data are presented as mean±SD. Differences between groups were analyzed by one-way ANOVA (Student-Newman-Keuls) using the SPSS software package (Version 10.0 for Windows; SPSS, Chicago, Illinois, USA). P<0.05 was considered statistically significant.
Results
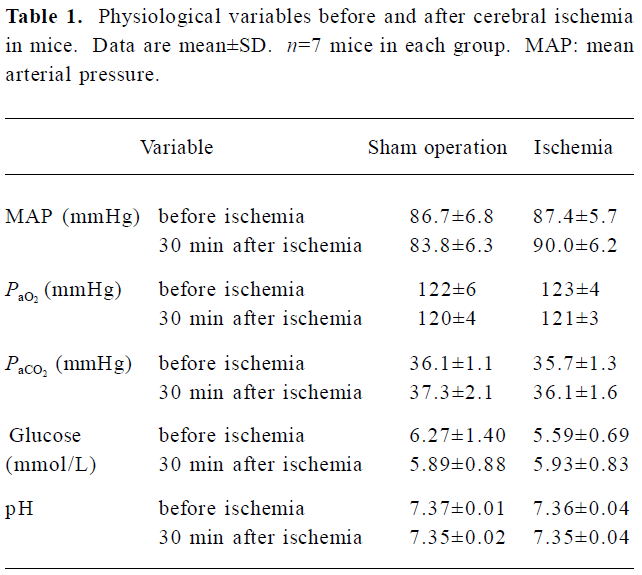
Physiological variables There was no significant difference in mean arterial blood pressure, PaO2, PaCO2, arterial blood pH, and blood glucose between 30 min before and 30 min after ischemia (Table 1).
Full table
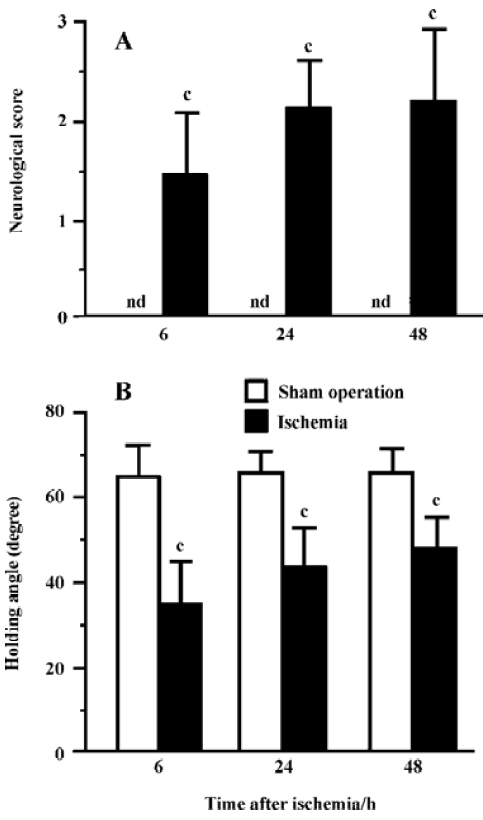
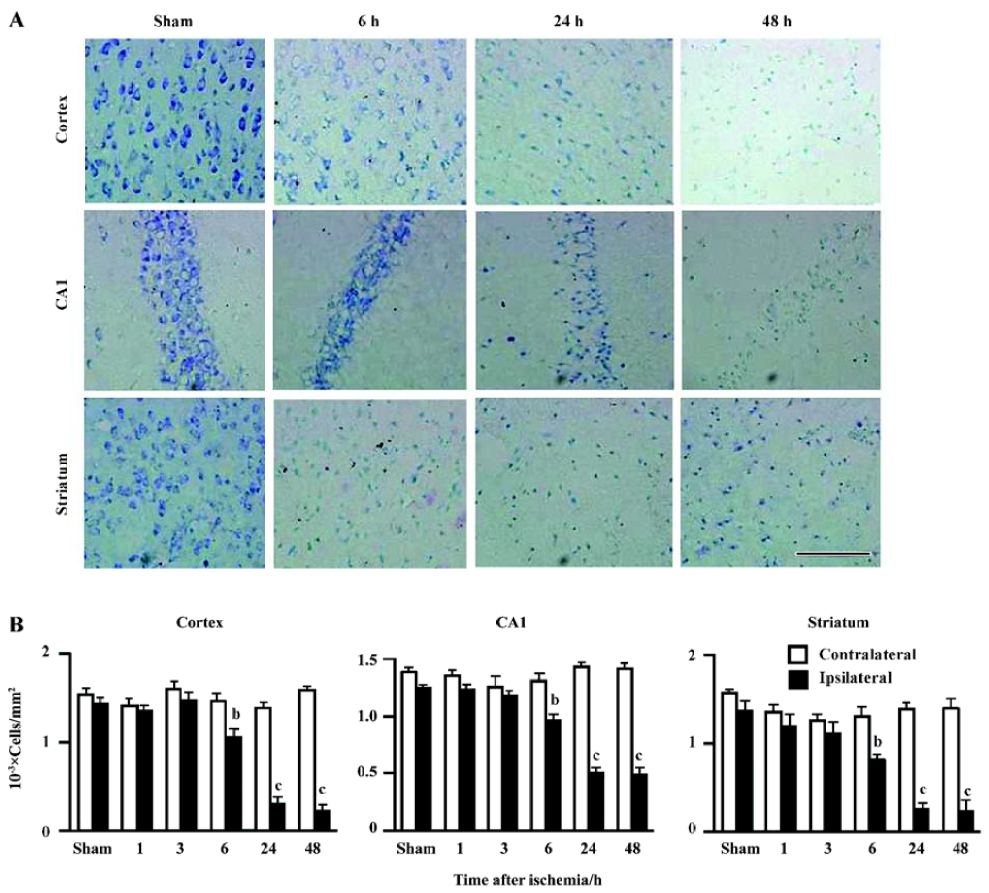
Ischemic injury The neurological deficit score was increased (Figure 2A) and the holding angle in the inclined board test was reduced (Figure 2B) 6, 24, and 48 h after MCAO. In the cortex, the hippocampal CA1 region and the striatum of the ischemic hemisphere, no apparent change was observed, and the number of neuron-appearing cells was unchanged at 1 and 3 h after MCAO. The Nissl bodies in the neurons disappeared and the neurons shrunk at 6 h after MCAO; most of neurons were destroyed and left vacuoles at 24 and 48 h after MCAO (Figure 3A). The number of neuron-appearing cells in these regions was significantly reduced at 6, 24, and 48 h after MCAO (Figure 3B). As the control, the cell number in the contralateral non-ischemic hemispheres had no significant change within 48 h after MCAO.
Temporal expressions of CysLT1 and CysLT2 receptor mRNA The expression of CysLT1 receptor mRNA in the ischemic hemisphere initially increased at 1 (1.9-fold) and 3 h (1.6-fold), partially recovered at 6 h, and largely increased again 24 (4.5-fold) and 48 h (3.8-fold) after MCAO (Figure 4A,C). Similarly, the expression of CysLT2 receptor mRNA in the ischemic hemisphere significantly increased at 1 h (2.2-fold), partially recovered at 3–6 h, then largely increased again 24 (5.3-fold) and 48 h (3-fold) after MCAO (Figure 4B,4D).
CysLT1 receptor localization Double immunofluorescence staining showed that the CysLT1 receptor was localized in the NeuN-positive neurons in the cortex, the hippocampal CA1 region and the striatum of the ischemic hemispheres at 24 h after MCAO (right panels in Figure 5A); almost none were detected in the hemisphere from the sham-operated mice (left panels in Figure 5A). On the other hand, the CysLT1 receptor was not localized in GFAP-positive astrocytes in these regions in both the sham-operated and ischemic hemispheres (Figure 5B).
Discussion
In the present study, the most important finding is that both the expressions of CysLT1 and CysLT2 receptor mRNA in the ischemic brain are upregulated in the acute phase of focal cerebral ischemia. The increased expressions are temporally related to neuronal injury, including neurological deficits and regional neuron loss. These results suggest that both CysLT1 and CysLT2 receptors may be involved in acute ischemic neuronal injury.
Because neuronal injury occurs initially in the acute phase after cerebral ischemia, in this study, we focus on the temporal changes in CysLT1 and CysLT2 receptor mRNA as well as the neuronal injury within 48 h after focal cerebral ischemia. Neurological deficits and neuron loss appeared from 6 to 48 h (Figures 2, 3). Both CysLT1 and CysLT2 receptor mRNA were initially increased at 1 h, and largely increased at 24 and 48 h after ischemia with peaks at 24 h (Figure 4). We can not explain why both the receptor mRNA expressions temporally increased, however, the initial increase might be induced by excitotoxicity that occurs at minute to hour after cerebral ischemia, and might mediate the initiation of delayed responses (hour to day), such as neuronal injury and inflammation[29]. As supporting evidence, we found that microinjection of N-methyl-D-aspartate (an exogenous excitatory acid) into the mouse cortex induced CysLT1 receptor expression (unpublished observations). On the other hand, the delayed increase of the 2 receptor mRNA expressions (24 and 48 h after MCAO) might be induced in response to secondary inflammation, and might mediate late responses like neuronal injury. The possible mediation was suggested by a close relationship between the increased expressions and the neuronal injury 24 and 48 h after MCAO in the present study. Of course, ischemic neuronal injury is caused by more complex systems, not by the CysLT1 or CysLT2 receptor alone.
The novel finding is that the expression of CysLT2 receptor mRNA is upregulated after focal cerebral ischemia. However, because neither selective antagonists nor specific antibodies (for rats and mice) are available for studying the CysLT2 receptor, we can not explore more properties of this receptor in mice or rats with cerebral ischemia. In peripheral tissues, the CysLT2 receptor mediates endothelial and macrophage activation, mast cell interleukin-8 secretion, and fibrosis[30], but it is not known how the CysLT2 receptor regulates the central nervous function. Recently, we showed that the CysLT2 receptor expression increased in neuron-appearing cells as detected by immunohistochemical staining in human brain specimens from patients with traumatic brain injury[24], but the implication is still unclear. We also found that PC12 cells transfected with the CysLT2 receptor were more sensitive than those transfected with the CysLT1 receptor to the in vitro ischemic-like injury induced by oxygen-glucose deprivation[31], suggesting that the CysLT2 receptor may play a more important modulating role in ischemic neuronal injury.
Another important finding is that the CysLT1 receptor is localized in the neurons in the ischemic brain and may mediate neuronal injury as confirmed by CysLT1 receptor antagonism. For detecting CysLT1 receptor localization, we performed double immunofluorescence using a polyclonal rabbit anti-human CysLT1 receptor antibody, which was also specific to mouse brain tissue (Figure 1B). Much less CysLT1 receptor is expressed in the normal brain from the sham-operated mice, but it was highly expressed in the neurons in the ischemic cortex, the hippocampal CA1 region and the striatum 24 h after focal cerebral ischemia. Furthermore, the CysLT1 receptor was less expressed in astrocytes. A similar pattern of CysLT1 receptor localization was also found in the rat brain after transient focal cerebral ischemia in our recent study (unpublished observations). In that study, we found that the CysLT1 receptor was primarily expressed in neurons, not in astrocytes, in the cortex 24 h after transient focal cerebral ischemia, while it was expressed in the astrocytes, not in neurons, in the boundary zone adjacent to the ischemic core 14 d after ischemia. The present finding further indicated the localization of the CysLT1 receptor in the striatum and the hippocampal CA1 region in addition to the cortex in a mouse model of permanent focal cerebral ischemia without reperfusion. Both the results from the focal ischemic mice and rats indicate that the CysLT1 receptor may mediate neuron injury in the acute phase of ischemia. This mediation has been suggested by the neuroprotective effect of selective CysLT1 receptor antagonists as we previously reported[17–20], although the antagonists may have other non-specific effects attenuating ischemic injury. The CysLT1 receptor regulates vascular leak, bronchoconstriction, dendritic cell maturation and migration, macrophage activation, hematopoietic progenitor cell recruitment, eosinophil and mast cell cytokine secretion, and smooth muscle proliferation in peripheral tissues[30]. Our finding indicates a role of the CysLT1 receptor in ischemic neuronal injury.
In summary, the present study shows that CysLT1 and CysLT2 receptors are upregulated in acute neuronal injury after focal cerebral ischemia; The CysLT1 receptor is localized in neurons after ischemia. Since inflammation is one of the post-ischemic events in the brain[29] and pro-inflammatory CysLT are produced in the brain after cerebral ischemia[1–3], it is necessary to clarify the modulating roles of CysLT1 and CysLT2 receptors in cerebral ischemia. Our findings of CysLT1 and CysLT2 receptor expression will contribute to understanding the mechanisms of cerebral ischemic injuries, including neuronal injury, and to developing new therapeutics for the treatment of ischemic stroke.
Acknowledgement
We thank Prof Zhong CHEN, Department of Pharmacology, School of Medicine, Zhejiang University, for critically reading and commenting on this manuscript.
References
- Ohtsuki T, Matsumoto M, Hayashi Y, Yamamoto K, Kitagawa K, Ogawa S, et al. Reperfusion induces 5-lipoxygenase translocation and leukotriene C4 production in ischemic brain. Am J Physiol 1995;268:H1249-57.
- Ciceri P, Rabuffetti M, Monopoli A, Nicosia S. Production of leukotrienes in a model of focal cerebral ischaemia in the rat. Br J Pharmacol 2001;133:1323-9.
- Zhang RL, Lu CZ, Ren HM, Xiao BG. Metabolic changes of arachidonic acid after cerebral ischemia-reperfusion in diabetic rats. Exp Neurol 2003;184:746-52.
- Dhillon HS, Dose JM, Prasad MR. Regional generation of leukotriene C4 after experimental brain injury in anesthetized rats. J Neurotrauma 1996;13:781-9.
- Black KL, Hoff JT, McGillicuddy JE, Gebarski SS. Increased leukotriene C4 and vasogenic edema surrounding brain tumors in humans. Ann Neurol 1986;19:592-5.
- Simmet T, Tippler B. Cysteinyl-leukotriene production during limbic seizures triggered by kainic acid. Brain Res 1990;515:79-86.
- Rao AM, Hatcher JF, Kindy MS, Dempsey RJ. Arachidonic acid and leukotriene C4: role in transient cerebral ischemia of gerbils. Neurochem Res 1999;24:1225-32.
- Shishido Y, Furushiro M, Hashimoto S, Yokokura T. Effect of nordihydroguaiaretic acid on behavioral impairment and neuronal cell death after forebrain ischemia. Pharmacol Biochem Behav 2001;69:469-74.
- Lynch KR, O’Neill GP, Liu Q, Im DS, Sawyer N, Metters KM, et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature 1999;399:789-93.
- Sarau HM, Ames RS, Chambers J, Ellis C, Elshourbagy N, Foley JJ, et al. Identification, molecular cloning, expression, and characterization of a cysteinyl leukotriene receptor. Mol Pharmacol 1999;56:657-63.
- Heise CE, O’Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS, . Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem 2000; 275: 30 531–6.
- Nothacker HP, Wang Z, Zhu Y, Reinscheid RK, Lin SH, Civelli O. Molecular cloning and characterization of a second human cysteinyl leukotriene receptor: discovery of a subtype selective agonist. Mol Pharmacol 2000;58:1601-8.
- Ogasawara H, Ishii S, Yokomizo T, Kakinuma T, Komine M, Tamaki K, . Characterization of mouse cysteinyl leukotriene receptors mCysLT1 and mCysLT2: differential pharmacological properties and tissue distribution. J Biol Chem 2002; 277: 18 763–8.
- Brink C, Dahlen SE, Drazen J, Evans JF, Hay DW, Nicosia S, et al. International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol Rev 2003;55:195-227.
- Maekawa A, Kanaoka Y, Lam BK, Austen KF. Identification in mice of two isoforms of the cysteinyl leukotriene 1 receptor that result from alternative splicing. Proc Natl Acad Sci USA 2001;98:2256-61.
- Zhang WP, Hu H, Zhang L, Ding W, Yao HT, Chen KD, et al. Expression of cysteinyl leukotriene receptor 1 in human traumatic brain injury and brain tumors. Neurosci Lett 2004;363:247-51.
- Zeng LH, Zhang WP, Wang RD, Wang PL, Wei EQ. Protective effect of ONO-1078, a leukotriene antagonist, on focal cerebral ischemia in mice. Acta Pharm Sin 2001;36:148-50.
- Zhang WP, Wei EQ, Mei RH, Zhu CY, Zhao MH. Neuropro-tective effect of ONO-1078, a leukotriene receptor antagonist, on focal cerebral ischemia in rats. Acta Pharmacol Sin 2002;23:871-7.
- Zhang LH, Wei EQ. Neuroprotective effect of ONO-1078, a leukotriene receptor antagonist, on transient global cerebral ischemia in rats. Acta Pharmacol Sin 2003;24:1241-7.
- Yu GL, Wei EQ, Zhang SH, Xu HM, Chu LS, Zhang WP, et al. Montelukast, a cysteinyl leukotriene receptor-1 antagonist, dose- and time-dependently protects against focal cerebral ischemia in mice. Pharmacology 2005;73:31-40.
- Yu GL, Wei EQ, Wang ML, Zhang WP, Zhang SH, Weng JQ, et al. Pranlukast, a cysteinyl leukotriene receptor-1 antagonist, protects against chronic ischemic brain injury and inhibits the glial scar formation in mice. Brain Res 2005;1053:116-25.
- Chu LS, Wei EQ, Yu GL, Fang SH, Zhou Y, Wang ML, et al. Pranlukast reduces neutrophil but not macrophage/microglial accumulation in brain after focal cerebral ischemia in mice. Acta Pharmacol Sin 2006;27:282-8.
- Hui Y, Yang G, Galczenski H, Figueroa DJ, Austin CP, Copeland NG, The murine cysteinyl leukotriene 2 (CysLT2) receptor. cDNA and genomic cloning, alternative splicing, and characterization. J Biol Chem 2001; 276: 47 489–95.
- Hu H, Chen G, Zhang JM, Zhang WP, Zhang L, Ge QF, et al. Distribution of cysteinyl leukotriene receptor 2 in human traumatic brain injury and brain tumors. Acta Pharmacol Sin 2005;26:685-90.
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989;20:84-91.
- Yonemori F, Yamaguchi T, Yamada H, Tamura A. Evaluation of a motor deficit after chronic focal cerebral ischemia in rats. J Cereb Blood Flow Metab 1998;18:1099-106.
- Mazzetti L, Franchi-Micheli S, Nistri S, Quattrone S, Simone R, Ciuffi M, et al. The ACh-induced contraction in rat aortas is mediated by the Cys Lt1 receptor via intracellular calcium mobilization in smooth muscle cells. Br J Pharmacol 2003;138:707-15.
- Ohd JF, Nielsen CK, Campbell J, Landberg G, Lofberg H, Sjolander A. Expression of the leukotriene D4 receptor CysLT1, COX-2, and other cell survival factors in colorectal adenocarcinomas. Gastroenterology 2003;124:57-70.
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci 2003;26:248-54.
- Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol 2004;173:1503-10.
- Sheng WW, Li CT, Zhang WP, Yuan YM, Fang SH, Zhang L, et al. Distinct roles of CysLT1 and CysLT2 receptors in oxygen glucose deprivation-induced PC12 cell death. Biochem Biophys Res Commun 2006;346:19-25.
