Sympathetically evoked Ca2+ signaling in arterial smooth muscle1
Introduction
The autonomic nervous system controls the activity of smooth muscles in the cardiovascular-renal system, gastrointestinal system, and urogenital system. Afferent and efferent (sensory) nerves release a ‘cocktail’ of neurotransmitters that control the contractile and trophic state of the smooth muscle cells[1]. These neurotransmitters activate several cell signaling systems, including the ubiquitous intracellular Ca2+ signaling systems[2]. Yet, neurogenic Ca2+ signaling in smooth muscle (that elicited by neurally released transmitters, as opposed to exogenously applied receptor agonists) has been investigated so far only in a few types of arteries, the vas deferens, the bladder and the uterus. Until now, this remarkable state of affairs reflected the difficulty of observing intracellular (Ca2+) in the muscle cells of intact organs in which nerves can be stimulated. Our intent in this article is to review what is known about the changes in intracellular (Ca2+) that are elicited by sympathetic nerves, and to identify the important remaining questions.
Many factors are involved in regulating arterial diameter including the central and peripheral nervous systems, endothelial cells, circulating hormones, locally released substances, blood pressure, blood flow, and intrinsic mechanisms of smooth muscle. In arteries that contribute significantly to total peripheral resistance, such as mesenteric small arteries[3,4] (which control blood flow to the intestine), control of smooth muscle contraction by the sympathetic nervous system is of major importance. This control is complex, involving multiple neurotransmitters and receptors, as well as complex patterns of nerve fiber activity. It is known that at least 3 different sympathetic neurotransmitters [ATP, norepinephrine (NE), and neuropeptide Y (NPY)] are released from sympathetic varicosities, and that their effects vary with the pattern of nerve fiber activity. Actions of the transmitters to elicit Ca2+ signaling in smooth muscle are believed to be synergistic, although detailed information is lacking. Finally, the probability of transmitter release at a single sympathetic nerve terminal is low (~0.05) and the pattern of sympathetic nerve activity in vivo is complex, consisting of single action potentials (AP) or bursts of AP superimposed on a background of tonic activity[5,6].
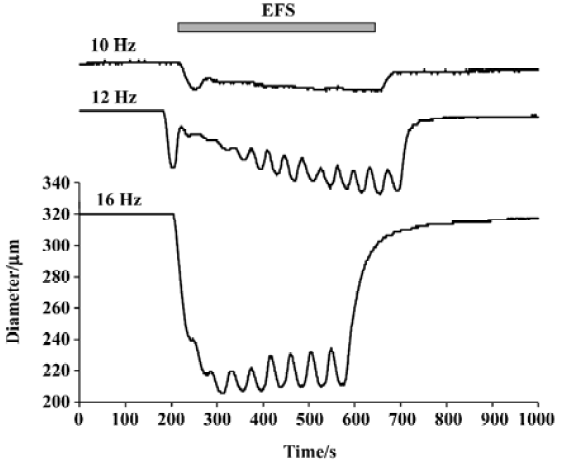
All 3 sympathetic cotransmitters, NE, ATP, and NPY contribute to sympathetically mediated vasoconstriction. Stimulation of the nerves supplying the rat mesenteric arterial bed (the tissue with which we are mainly concerned in this review) elicits an increase in perfusion pressure that can be blocked completely only by the combined administration of prazosin, suramin and BIBP 3226 ((R)-N2-(diphenacetyl)-N-(4-hydroxyphenyl) methyl)-D-arginineamide) (a selective α1-adrenoceptor antagonist, a non-selective antagonist of all P2X receptor subtypes, and a selective antagonist of the NPY receptor subtype, Y1), respectively[7]. Neurogenic contractions of isolated mesenteric small arteries are typically bi-phasic (Figure 1), with the small, initial transient component being attributed to purinergic (P2X) receptors, and the later, slow, large component being attributed to neurally released NE. However, the relative importance of each type of receptor depends on the size and type of the artery, as well as on the pattern of sympathetic nerve activity. In mesenteric arteries, the purinergic component of the contraction is relatively large in very small arteries (compared to the α1-receptor-mediated component), and the purinergic component predominates during brief bursts of sympathetic nerve fiber activity[8]. Indeed, it has recently been concluded that, “all three cotransmitters contribute significantly to vascular responses and their contribution varies markedly with impulse numbers”[9]. The varying contribution of the transmitters under different conditions is the result of both pre- and post-synaptic factors.
Our goal in this review is to summarize recent new information on sympathetic neuromuscular transmission and the resultant Ca2+ signaling in the smooth muscle cells of small arteries during neurogenic contractions. Ca2+ signaling during neurogenic contractions activated by trains of sympathetic nerve fiber action potentials is in fact significantly different from that elicited by the simple application of exogenous neurotransmitters (both ATP and NE) to isolated arteries (or single isolated smooth muscle cells). We end by identifying important questions remaining in our understanding of sympathetic neuromuscular transmission and the physiological regulation of arterial smooth muscle contraction by the sympathetic nervous system.
Sympathetic nerves in isolated small arteries
The perivascular nerve fibers present in rat mesenteric small arteries are of 2 major types: sympathetic and ‘sensory’. The latter, which will not be discussed further here, are the so-called non-adrenergic, non-cholinergic (NANC) or ‘sensory’ nerves, whose cell bodies are located in the dorsal root ganglia. The perivascular sympathetic nerves that remain with isolated arteries consist of the neuroeffector system, that is, the post-ganglionic fibers (cell bodies located in paravertebral ganglia) and their nodal areas of axoplasmic specialization, that is, varicosities and synaptic junctions with the smooth muscle. Experimentally, the perivascular nerves of an isolated artery can easily be electrically stimulated to release transmitters. The function of NANC nerves may be blocked through the use of capsaicin[10], thus permitting selective activation of the sympathetic nerves.
We next review very briefly the salient features of current concepts on the pre-synaptic and post-synaptic mechanisms that are involved in sympathetic neuromuscular transmission at such junctions, particularly those involved in the differential release of sympathetic cotransmitters. A major physiological phenomenon to be explained is that the effects of ATP are predominant at low frequencies of sympathetic nerve fiber activity, while those of NE are predominant at high frequencies.
Synaptic vesicles in sympathetic varicosities
Varicosities on the sympathetic perivascular nerves in rat mesenteric small arteries contain several different types of synaptic vesicles, probably containing different proportions of NE, ATP and NPY. These vesicles may have different origins (cell body vs formation at synapse) and mechanisms of exocytosis (including regulation by varicosity Ca2+). Rat mesenteric arteries adrenergic nerve terminals contain 3 types of vesicles: large dense-cored vesicles (LDCV; ~100 nm diameter), small dense core vesicles (SDCV; ~50 nm diameter) and small clear vesicles (SCV; ~50 nm diameter)[11–13]. LDCV comprises 5% of the total vesicles in a varicosity, with the remainder being small vesicles (SV)[10]. The majority of the SV have (88%) dense cores (SDCV); the remainder without, SCV[10]. NE and ATP are thought to be stored in all vesicle types, but in different proportions. It is generally agreed that the ATP and NE are released from different nerve terminal stores[12,14] although the type of vesicles involved is not generally agreed upon. The exact vesicular origin of the released transmitters remains to be elucidated and may be species and tissue specific.
Post junctional receptors
Post-synaptic receptors for ATP Receptors for purine (ATP, ADP) and pyrimidine (UTP, UDP) nucleotides are presently divided into 2 families: ionotropic P2X receptors (7 cloned subtypes) and metabotropic P2Y receptors (6 cloned subtypes)[15]. In the cardiovascular system, P2X receptors are expressed predominantly on smooth muscle (but are present on endothelial cells), and P2Y receptors are predominantly expressed on endothelial cells (also present on smooth muscle cells). P1 receptors will not be considered here except as they may be activated by adenosine produced by endothelial nucleotidase activity[16,17]. Neurally-released ATP activated P2X receptors (ligand-gated ion channels) on smooth muscle to produce the excitatory junction potentials, and activated P2Y receptors on endothelium (G protein-coupled receptors) to produce endothelium dependent hyperpolarizing factor. In rat mesenteric arteries, the predominant P2X receptor is P2X1[18].
Neurally-released ATP binds to both P2X and P2Y receptors. While ‘overflow’ of ATP may be quantified chemically, only the effect of ATP on P2X receptors is detectable with electrical and optical methods. There is no doubt that neurally-released ATP produces the excitatory junction currents (EJCs), junctional Ca2+ transients (jCaT) and in vas deferens, the neuroeffector Ca2+ transients (NCT). jCaT and NCT are post-junctional changes in Ca2+ of smooth muscle cells in response to neurally-released ATP. In mouse vas deferens, EJC are of 2 types: (1) large and fast; and (2) small and slow. The 2 types are differentially affected by external (Ca2+) and osmotic pressure[12] and sensitivity to heptanol[19]. Considering the work of these authors and the ‘dual vesicle’ hypothesis of Stjarne[12], it may be speculated that the ‘large and fast’ EJC arise from the release of the ‘big quanta’ type of SV, and that the ‘small and slow’ EJC arise from the ‘small quanta’ type of SV. jCaT and NCT would arise from the ‘big quanta’ SV; the extent to which work in the smooth muscle of vas deferens may apply to that of arteries is not known.
Post-synaptic receptors for NE The second sympathetic cotransmitter, NE, binds to adrenoceptors, the classification and function of which have been reviewed recently[20]. At least 9 subtypes of vascular adrenoceptors have been identified[21]: α1A, α1B, α1D, α2A/D, α2B, α2C, β1, β2, and β3. There is also believed to be a low affinity (to prazosin) state of α1A-adrenoceptors, known as the α1L-adrenoceptors, and they are found in various vascular beds[22]. A useful heuristic generalization is that α1-adrenoceptors are located post-junctionally on vascular smooth muscle and have a primary role in controlling arterial tone, particularly in small resistance arteries. This accounts for the predominant use of the synthetic α1-adrenoceptor agonist, phenylephrine (PE), in the majority of experimental studies. Exogenous activation of adrenoceptors using PE and the use of an α1B-knockout mouse has permitted the conclusion that α1D is most important in conduit arteries, while α1A is most important in small arteries and α1B was reported to have a minor contribution[23]. Recent studies using nerve-evoked contraction, also in small arteries isolated from α1D-knockout mouse, have shown the importance of α1A-adrenoceptors as the predominant subtype and α1D having a small, but significant role[24]. Pre-junctionally, transmitter release at sympathetic varicosities (neuromuscular junctions) is importantly regulated by α2A/D and α2C. Endothelial cells have at least 5 subtypes of adrenoceptors: α2A/D, α2C, β1, β2, and β3. Adrenergic signaling mechanisms in arterial smooth muscle have been reviewed recently[25], with the emphasis on Ca2+ activation of contraction and Ca2+-sensitizing mechanisms activated by bath-applied phenylephrine.
Neurally-released NE has been detected classically by chemical methods in the effluent of neurally stimulated arteries (‘overflow experiments’) or by amperometric methods, in which the oxidation of NE on the surface of a carbon fiber electrode is detected as an electrical current. Most significantly, the release of single quanta of NE has been detected from the surface of rat mesenteric small arteries through the use of carbon fiber microelectrodes (CFmE). These microelectrodes are 7 μm in diameter and are believed to detect the release of NE quanta from a distance of 8 μm[14]. Furthermore, spontaneous oxidation currents (SOC) were also recorded. These authors speculated that the SOC arose from NE released from large dense-cored vesicles (LDV). An important observation was that α-latrotoxin increased the frequency of SOC about 4-fold, but increased the frequency of spontaneous excitatory junction potentials (which monitor packeted or quantal ATP release) by 30-fold. This observation supports the suggestion that SEJP (spontaneous excitatory junction potentials activated by released ATP) and SOC (NE release) occur through different synaptic vesicles, under these conditions.
In most vascular beds, α1-adrenoceptors play an important role in vasoconstriction. The relative importance of the different α1-adrenoceptor subtypes in regulation of peripheral resistance and systemic arterial blood pressure is not clear, as the contribution of different subtypes to vasoconstriction differs with the mode of activation and species[26]. For example, studies in rat mesenteric small arteries using exogenous agonists have revealed the role of α1A-[27,28] or α1B-[29] or α1L-adrenoceptors[30]. In contrast, nerve-evoked contractions in rat mesenteric arteries were predominantly mediated by α1A-adrenoceptors[27,28]. Further, studies in mesenteric small arteries using α1B-adrenoceptor knockout mouse have revealed the predominance of α1A-adrenoceptors in vasoconstriction to phenylephrine[23] and nerve-evoked contractions showed the predominance of α1B-adreno-ceptors[31]. Therefore, it is important to study in vivo preparations to understand the clinical relevance of the α1-adrenoceptor subtypes involved in vasoconstriction.
Post-synaptic receptors for NPY The third sympathetic cotransmitter, NPY, is believed to enhance the effects of both ATP and NE, by acting on post-junctional NPY-Y1 receptors[32]. Five distinct NPY receptors (Y1, Y2, Y4, Y5, and Y6) have been cloned[33]. Y1 receptors are believed to be the major type present post-junctionally in the cardiovascular system and to mediate the response to NPY, although the possible involvement of pre- and post-junctional Y2 receptors has been suggested. NPY receptors act via pertussis toxin-sensitive G proteins. A major effect of their activation is the inhibition of adenylyl cyclase. NPY shifts agonist dose-response curves to the right[34]. It has been suggested that NPY activates mesenteric small arteries through 2 different mechanisms: activation of non-selective cation channels and consequent Ca2+ entry, and the inhibition of the hyperpolarization produced by cAMP[35] (cyclic adenosine monophosphate).
Differential release of ATP and NE There is good evidence that ATP is the predominant sympathetic effector in response to ‘single’ action potentials, while at high frequencies of activation, NE is the predominant sympathetic effector. This may result from the pre- and post-synaptic mechanisms discussed earlier. Low frequency electrical field stimulation (EFS; eg 1 Hz) elicits relatively small, brief, phasic contraction of rat mesenteric arteries, whereas higher frequency EFS (eg 16 Hz) elicits larger, sustained contractions. The irreversible adrenergic antagonist, phenoxy-benzamine, abolished the response to bath-applied norepinephrine, but reduced the response to a single nerve stimulus by only 20%[36]. Conversely, the stable ATP analogue, α,β-methylene ATP (which desensitizes P2X receptors), reduced the response to a single pulse by 70%, while reducing the contraction to high frequency stimulation only 10%. Similarly, in the rabbit ear artery, it has been concluded that ‘short pulse bursts at low frequency favor the prazosin-resistant (purinergic) component of the response’[37].
Post-synaptic Ca2+ signaling
Recent work has revealed in detail the Ca2+ signals in smooth muscle cells during sympathetically-mediated neurogenic contractions of small arteries[38] and vas deferens[39]. There are differences between these 2 tissues (which have very different functions), and we will focus here on what is known about sympathetic neuromuscular transmission and neurogenic contractions of vascular smooth muscle. The goals of the current research in this area are to attribute different types of post-synaptic Ca2+ signals to the activation of specific receptors, channels, and organelles, and to determine to what extent the different post-junctional Ca2+ signals actually activate contraction. Here, we summarize the recent studies in which Ca2+ signals, attributable to both neurally-released ATP and neurally released NE, have been observed.
jCaT: post-synaptic Ca2+ signals activated by neurally released ATP Neurally-released ATP activates a specific, localized, Ca2+ transient in arterial smooth muscle cells[40] that has been termed a ‘jCaT’, for junctional Ca2+ transient. It was shown that these Ca transients arise in the vicinity of perivascular nerves[38] or even directly beneath single visualized nerve fibers[40]. The confocal images of jCaT in Figure 2 were obtained in pressurized (70 mmHg) rat mesenteric small arteries subjected to EFS. Low frequency, low voltage stimulation (0.67 Hz, 0.2 ms) excited nerve fibers without causing an appreciable contraction. This was referred to this as ‘sub-threshold’ EFS, as it is sub-threshold for muscle contraction. Thus, in these experiments, motion did not occur and the characteristics of the jCaT could be studied in detail. Figure 2 illustrates the basic appearance of jCaT in line-scan images and demonstrates that: (1) nerve fibers are being excited by each EFS pulse; (2) a jCaT occurs nearly simultaneously with an EFS pulse; (3) jCaT occur near nerve fibers; and (4) jCaT are events of very low probability.
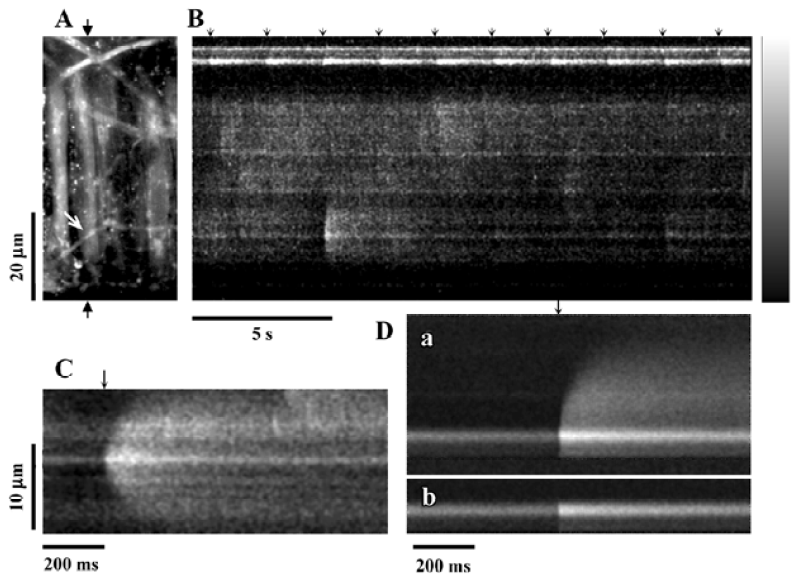
Spatio-temporal characteristics of jCaT JCaT are larger in spatial spread and last longer than spontaneous Ca2+ sparks. JCaT always occur with brief latency to the EFS pulse. The spatio-temporal differences between the jCaT and the sparks are obvious in published records[40]: the jCaT is larger in space, lasts longer, and occurs at the time of the stimulus pulse. These characteristics and their pharmacology are how jCaT are distinguished from sparks. The vast majority of jCaT occurred within 12 ms (4 scan lines) after the stimulus pulse. The evidence linking the occurrence of jCaT to the stimulus seems unequivocal. The spatial full-width-at-half-maximum (FWHM) for jCaT is 4.8 µm, and the time taken to fall to half-amplitude, t1/2, (from the peak) is 145 ms. The means of these distributions are quite different from those of sparks in smooth muscle (sparks: t1/2, 48–56 ms; FWHM, 2.4 µm)[41]. Some jCaT occurred before the stimulus, and some much after; we hypothesize that these are associated with spontaneous neurotransmitter release.
Pharmacology of jCaT Pharmacological studies of jCaT[40] have provided strong evidence that they arise from the activation of purinergic receptors. JCaT persist, apparently unchanged, in the presence of capsaicin, and are thus not dependent on sensory nerves. They are completely absent in the presence of the purinergic receptor blocker, suramin (300 µmol/L). They persist in the presence of α1-adrenergic blocker prazosin (10 µmol/L), sufficient to block neurogenic adrenergic responses completely. They are also largely unaffected by ryanodine (30 µmol/L), while Ca2+ sparks are abolished. Although we favor the hypothesis that jCaT are due to ATP and P2X1 receptors, further studies are required, particularly to be sure that it is the P2X1 receptor subtype. NPY is also reported to activate non-specific cation channels[36] and thus it could contribute to jCaT. This possibility could be tested using specific Y1 receptor antagonists (BIBP 3226).
In our recent study[42] using P2X1 knockout animals[43], we clearly showed that jCaT represent Ca2+ that enter vascular smooth muscle cells through P2X1 receptors activated by neurally-released ATP. The P2X1 knockout models also showed the importance of the involvement of ATP-mediated P2X1 activation in the initial rapid component of the nerve-evoked contraction[42].
Ca2+ waves: post-synaptic Ca2+ signals activated by α1-adrenergic agonists The Ca2+ signaling elicited by adrenergic agonists has been studied mainly through the use of exogenous, bath-applied synthetic catecholamines such as the α1-adrenoceptor specific agonist, PE. An early study was that of Zang and colleagues[44], in which it was shown that PE elicited asynchronous propagating Ca2+ waves in rat mesenteric arteries. The basis of the dose-response to PE was increasing recruitment of individual smooth muscle cells to produce Ca2+ waves, and to produce them at higher and higher frequencies. As expected, asynchronous propagating Ca2+ waves seem also to be the response elicited by neurally released NE. This is discussed in more detail later in relation to the activation of contraction by neurally released NE, during neurogenic contractions of small arteries.
Neurogenic contractions of small arteries
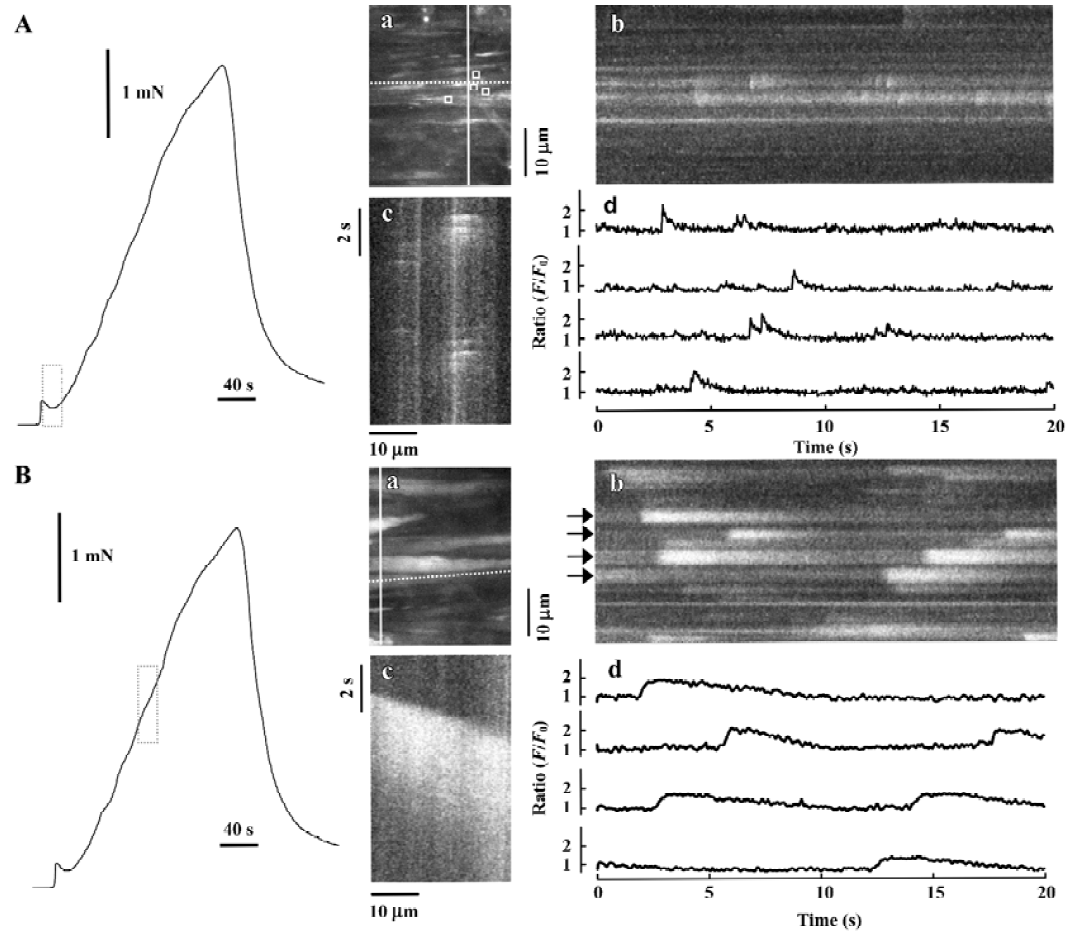
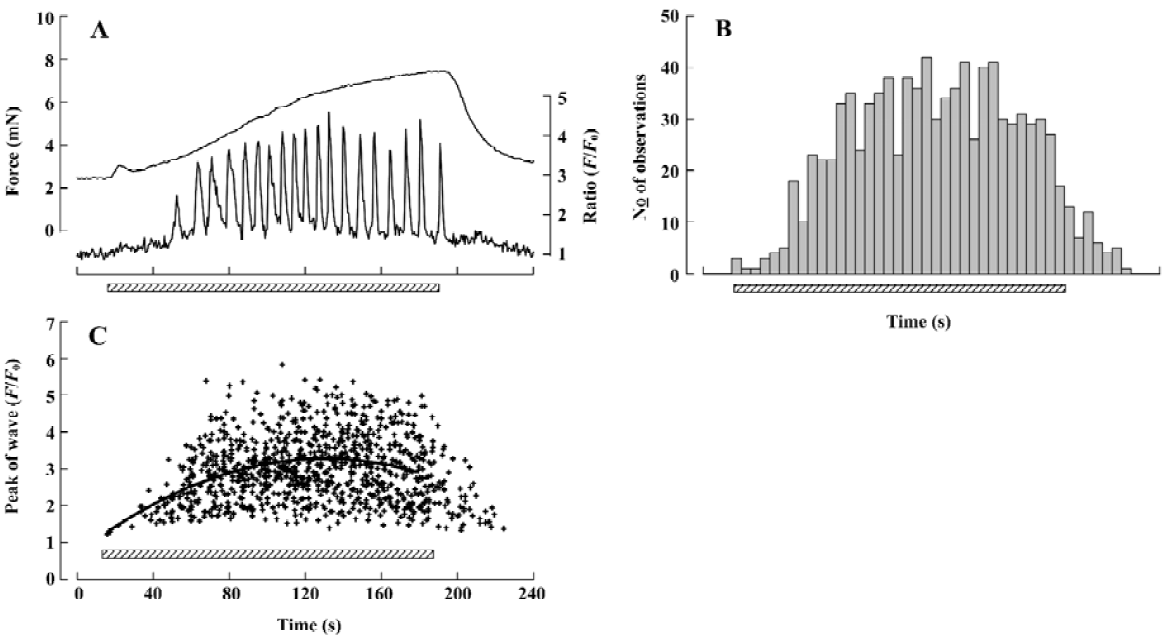
The detailed studies on jCaTs described above were performed in pressurized small arteries that did not contract because the electrical stimulation was ‘sub-threshold’ for contraction. In this section, we review the studies in which jCaT and Ca2+ waves have been observed during isometric neurogenic contractions. For these studies, the arteries were mounted in a myograph that permitted simultaneous (i) high-speed confocal imaging of fluorescence from individual smooth muscle cells; (ii) electrical stimulation of perivascular nerves; and (iii) recording of isometric tension. Sympathetic neuro-muscular transmission was achieved by EFS (frequency, 10 Hz; pulse voltage, 40 V; pulse duration, 0.2 ms) in the presence of capsaicin and scopolamine (to inhibit ‘sensory’ and cholinergic nerves, respectively). As shown in Figure 3, during the first 20 s of EFS, force rose to a small peak, then declined, similar to that recorded previously[45]. During this time, jCaT were present at a relatively high frequency. Propagating asynchronous Ca2+ waves, previously associated with bath-applied α1-adrenoceptor agonists, were not initially present. During the next 2.5 min of EFS, force rose slowly, and asynchronous propagating Ca2+ waves appeared. The selective α1-adrenoceptor antagonist, prazosin, abolished both the slowly developing contraction and the Ca2+ waves, but reduced the initial transient contraction by only ~25%.
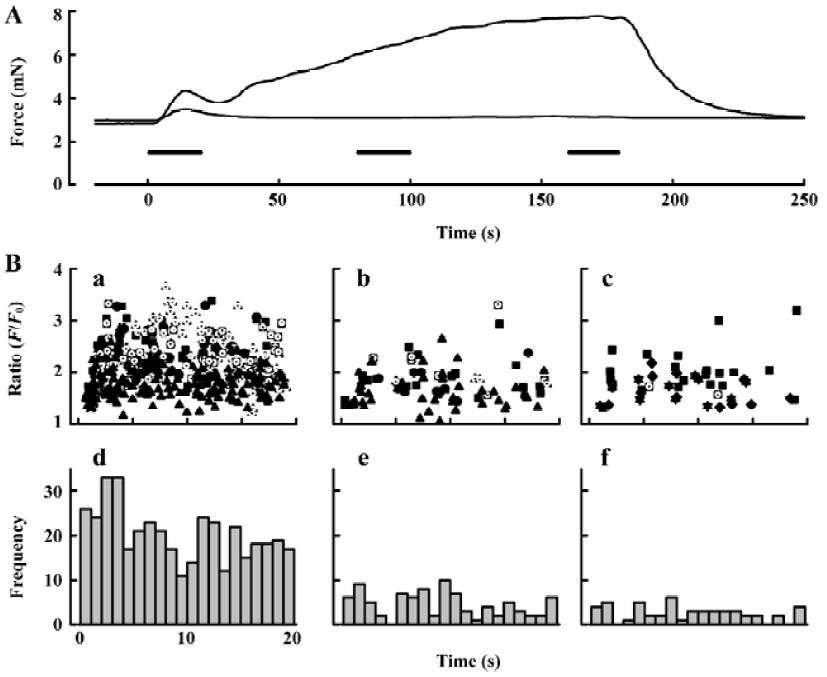
Purinergic component of neurogenic contraction In order to study selectively the arterial contractions generated by neurally-released ATP, arteries were exposed to prazosin (1–10 µmol/L) to block α1-adrenergic receptors (Figure 4). Others[8] have shown that purinergic receptor antagonists, such as suramin, abolish the small contractions that remain in prazosin. We found that after prazosin treatment 73.7%±14.0% (n=7) of the initial transient contraction remained and 5.00%±0.98% (n=5) of the maintained contraction. We then sought to characterize the changes in frequency and amplitude of jCaT that might occur during the EFS, and which activate contraction. Because the jCaT is a local Ca2+ signal, it was not necessarily clear that jCaT would activate contraction effectively. Confocal imaging of Fluo-4 fluorescence at 30 images·s-1 was performed for 3 periods of 20 s in the beginning (0–20 s), middle (80–100 s), and end (160–180 s) of 3 min EFS (periods indicated by bars in Figure 4A). The frequency of jCaT declined markedly during the 3 min of EFS (Figure 4B, iv–vi). In contrast to the frequency, the peak amplitude of the jCaT changed little during 3 min EFS. JCaT occurred in sufficient numbers during the first 20 s of EFS to produce a detectable elevation of average (Ca2+; fluorescence ratio), which paralleled the transient contraction that occurred during this time. On the other hand, jCaT occurred at a very low frequency later in the EFS, when contractile force fell to very low levels. Thus, it seems reasonable to attribute the contractile activation to jCaT, despite their limited spatial extent and frequency.
Adrenergic component of neurogenic contraction Later during neurogenic contractions, asynchronous Ca2+ waves propagated within individual smooth muscle cells of the arterial wall. A representative Ca2+ signal, obtained as the average fluorescence with an area of interest (AOI; 1.35 mm square) within a single smooth muscle cell is shown in Figure 5A (lower trace, right hand scale). For this data, images were obtained at 2 s-1; a rate which is too slow to resolve the jCaT[40] generated during the initial purinergic component. For the analysis of the adrenergic component of the experiment illustrated in Figure 5, 74 individual smooth muscle cells were identified and an AOI placed on each. In these 74 cells, 809 Ca2+ waves were detected and the time of onset and peak amplitude of each was determined, and the data presented as a histogram.
Different roles of ryanodine (RyR) and inositol(1,4,5)-trisphosphate receptors (InsP3R) in neurogenic contractions The role of the sarcoplasmic reticulum (SR) and its Ca2+-release channels (RyR, (Ins(1,4,5)P3R) of individual smooth muscle cells of the arterial wall and in these Ca2+ signals is not completely known. Zang et al[44] used confocal laser scanning microscopy and Fluo-4 to visualize Ca2+ transients within individual smooth muscle cells of rat resistance arteries during α1-adrenoceptor activation. They noticed that in the presence of PE, caffeine also elicited a massive release of Ca2+, at a time when Ca2+waves had died away completely, or when further responses to PE would have been much diminished. This result shows that caffeine-sensitive Ca2+ stores are not depleted of Ca2+ in the presence of PE and further indicates that the α1-agonist-releasable Ca2+ store and the caffeine releasable Ca2+ store are different[44]. More recent experiments by our group (Lamont and Wier, 2004)[46] showed that Ins(1,4,5)P3R are essential for adrenergically-induced asynchronous Ca2+ waves and the associated steady vasoconstriction, but RyR are not appreciably opened during adrenergic activation (because PE did not facilitate the development of the effects of ryanodine). Also Ins(1,4,5)P3R are not essential for Ca2+ sparks. This provides an explanation of the fact that adrenergic stimulation decreases the frequency of Ca2+ sparks (previously reported) while simultaneously increasing the frequency of asynchronous propagating Ca2+ waves; different SR Ca2+-release channels are involved[46].
Both the contraction and the underlying Ca2+ signals during the adrenergic component of the neurogenic isometric contraction are also distinctly different from those occurring during externally applied α1-adrenoceptor agonist (typically PE). After the initial purinergic component, the adrenergic component of the neurogenic contractions, even at maximally effective EFS, rises much more slowly than does the contraction in response to bath-applied α1-adrenoceptor agonist. Maximally effective concentrations of exogenous PE elicit an initial synchronous release of Ca2+, followed by asynchronous propagating Ca2+ waves, both in veins[45] and in the arteries[34,44]. Thus, the initial rapid rise in force in response to externally applied PE appears to be generated by a synchronous release of Ca2+ from intracellular stores. This does not occur during neurogenic contractions, possibly because neuronally released NA does not initially reach the uniformly high levels that are achieved rapidly after external application.
Influence of myogenic tone
Another significant complication that arises from arteries pressurized at 70 mmHg above room temperature is the development of tone. Earlier studies (eg Mauban et al[47]), were at room temperature, which is not conductive for the development of myogenic tone. The only previous study in which spatially resolved imaging was performed in mesenteric small arteries at 37 ºC[48] was complicated by the development of adrenoceptor-mediated vasomotion, preventing clear observation of Ca2+ signaling in individual smooth muscle. However, mouse mesenteric small arteries at 32 ºC showed the development of myogenic tone and also rarely resulted in vasomotion on stimulation by PE. In such arteries, PE elicited only a spatially uniform increase in Ca2+ with little or no Ca2+ waves, suggesting that the rise in Ca2+i leads to the development of myogenic tone and also inactivation of IP3 receptors[49].
Conclusion
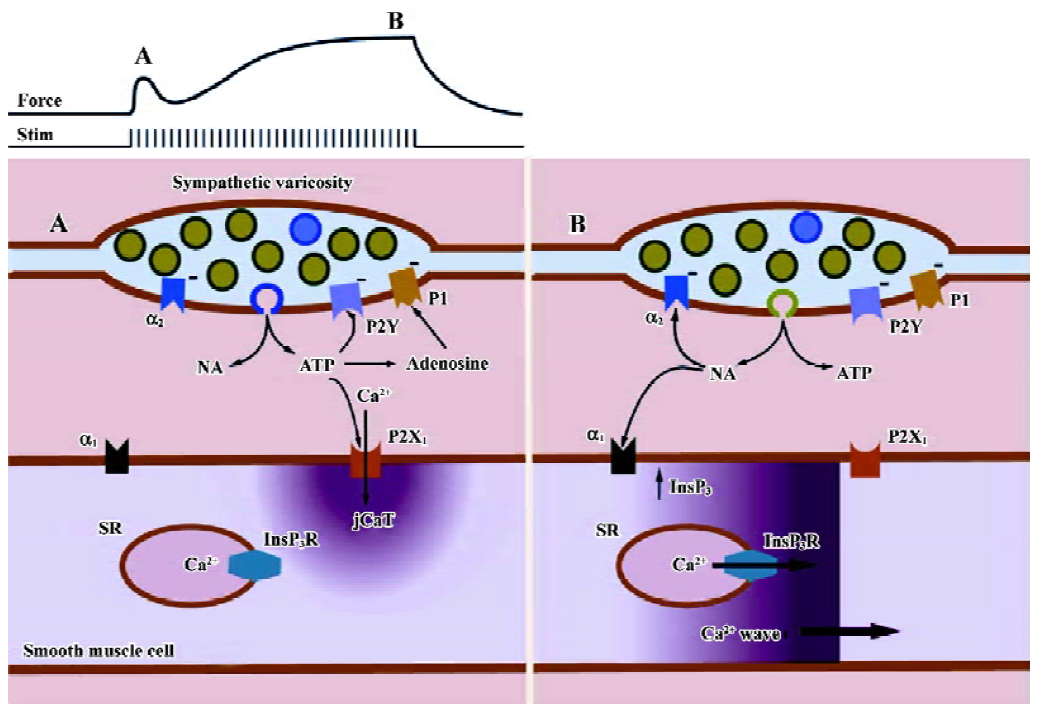
The confocal microscope, along with other new technologies has provided much new information on the physiology and pharmacology of sympathetically evoked Ca2+ signaling in arterial smooth muscle. We have previously advanced a scheme to explain the Ca2+ signals and isometric contraction elicited by electrical field stimulation of perivascular sympathetic nerves of a rat mesenteric small artery (Figure 6). Early during a train of nerve fiber action potentials, smooth muscle contraction is activated mainly by jCaT induced by neurally released ATP. JCaT are localized to the post-junctional region, and arise from Ca2+ that has entered via P2X1 receptors. At this time, sympathetic varicosities may release mainly synaptic vesicles that contain a relatively high concentration of ATP (the relatively few ‘big’ quanta proposed by Stjarne[12]). Later during a train of nerve fiber action potentials, jCaT are rare, and contraction is activated by Ca2+ waves that arise from SR. Ca2+ release from SR is activated by InsP3, produced after the binding of NE to α1-adrenoceptors. At this time, sympathetic varicosities may release small synaptic vesicles (the more numerous ‘small’ quanta, green; Figure 6) that contain a relatively high concentration of NE. We stress that the mechanisms accounting for differential release of ATP and NE are quite speculative at this time.
JCaT are distinct from Ca2+ transients activated in isolated venous myocytes by exogenously applied ATP[50]. Previous studies of the effects of ATP on small arteries utilized spatially averaged measurements of Ca2+ [51] and we can not determine therefore whether jCaT might be produced by bath-applied ATP or not. In rat mesenteric small arteries similar to those used here, low (0.01–1 mmol/L) concentrations of exogenous ATP caused ‘global’ Ca2+ transients that seemed to involve Ca2+ influx through channels sensitive to nifedipine and the putative blocker of receptor operated channels, SKF 96365[52], whereas higher concentrations (1–3 mmol/L) caused a release of Ca2+ from intracellular stores (Ca2+ transients were elicited by high ATP in the absence of external Ca2+). When applied to isolated venous myocytes, low concentrations of ATP (0.1 µmol/L) induced rather uniform increases in Ca2+ (which started from the edges of the cell), and at higher concentrations (1 µmol/L), propagating Ca2+ waves[50].
In the arteries studied here in the presence of prazosin, no propagating Ca2+ waves were ever observed during EFS. We interpret this to mean that neurally-released ATP does not evoke significant release of Ca2+ from intracellular stores, a result in agreement with previous pharmacological studies on rat mesenteric small arteries[7]. We speculate that the differences between the effects of bath-applied ATP and neurally-released ATP are due to a markedly different spatio-temporal pattern of ATP on the smooth muscle cell in the 2 cases.
Both the contraction and the underlying Ca2+ signals during the adrenergic component of the neurogenic isometric contraction are also distinctly different from those occurring during externally applied α1-adrenoceptor agonist (typically phenylephrine, PE). After the initial purinergic component, the adrenergic component of the neurogenic contractions, even at maximally effective EFS, rises much more slowly than the contraction in response to bath-applied α1-adrenoceptor agonist. Maximally effective concentrations of exogenous PE elicit an initial synchronous release of Ca2+, followed by asynchronous propagating Ca2+ waves, both in veins[53] and arteries[44,47]. Thus, the initial rapid rise in force in response to externally applied PE appears to be generated by a synchronous release of Ca2+ from intracellular stores. This does not occur during neurogenic contractions, possibly because neurally-released NE does not initially reach the uniformly high levels that are achieved rapidly after external application.
Acknowledgement
We gratefully acknowledge the assistance of Becky SAUNDERS in the preparation of this manuscript.
References
- Todorov LD, Mihaylova-Todorova ST, Bjur RA, Westfall DP. Differential cotransmission in sympathetic nerves: role of frequency of stimulation and prejunctional autoreceptors. J Pharmacol Exp Ther 1999;290:241-66.
- Cheng H, Wei S, Verkhratsky A. Calcium signaling in physiology and pathophysiology. Acta Pharmacol Sin 2006;27:767-72.
- Christensen KL, Mulvany MLJ. Mesenteric arcade arteries contribute substantially to vascular resistance in conscious rats. J Vas Res 1993;30:73-9.
- Fenger-Gron J, Mulvany MJ, Christensen KL. Intestinal blood flow is controlled by both feed arteries and microcirculatory resistance vessels in freely moving rats. J Physiol 1997;498:215-24.
- Johnson CD, Gilbey MP. Focally recorded single sympathetic postganglionic neuronal activity supplying rat lateral tail vein. J Physiol 1998;508:575-85.
- Johnson CD, Gilbey MP. Effects of aortic nerve stimulation on discharges of sympathetic neurons innervating rat tail artery and vein. Am J Physiol 1998;275:R942-9.
- Donoso MV, Steiner M, Huidobro-Toro JB. BIBP 3226, suramin and prazosin identify neuropeptide Y, adenosine 5'-triphosphate and noradrenaline as sympathetic cotransmitters in the rat mesenteric bed. J Pharmocol Exp Ther 1997;282:691-8.
- Gitterman DP, Evans RJ. Nerve evoked P2X receptor contractions of rat mesenteric arteries; dependence on vessel size and lack of role of L-type calcium channels and calcium induced calcium release. Br J Pharmacol 2001;132:1201-8.
- Bradley E, Law A, Bell D, Johnson CD. Effects of varying impulse number on cotransmitter contributions to sympathetic vasoconstriction in rat tail artery. Am J Physiol 2003;284:H2007-14.
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev 1991;43:143-201.
- Tranzer JP. New aspects of the localisation of catecholamine in adrenergic neurons. Frontiers Catecholamine Res 1973; 453–8.
- Stjarne L. Novel dual ‘small’ vesicle model of ATP- and noradrenaline-mediated sympathetic neuromuscular transmission. Auton Neurosci 2001;87:16-36.
- Blair DH, Lin YQ, Bennett MR. Differential sensitivity to calcium and osmotic pressure of fast and slow ATP currents at sympathetic varicosities in mouse vas deferens. Auton Neurosci 2003;105:45-52.
- Brock JA, Dunn WR, Boyd NSF, Wong DKY. Spontaneous release of large packets of noradrenaline from sympathetic nerve terminals in rat mesenteric arteries in vivo. Br J Pharmacol 2000;131:1507-11.
- Ralevic V. The involvement of smooth muscle P2X receptors in the prolonged vasorelaxation response to purine nucleotides in the rat mesenteric arterial bed. Br J Pharmacol 2002;35:1988-94.
- North RA, Surprenant A. Pharmacology of cloned P2X receptors. Ann Rev Pharmacol Toxicol 2000;40:563-80.
- Fredholm BB, Abbracchio MP, Burnstock G, Dubyak GR, Harden TK, Jacobson KA, et al. Towards a revised nomenclature for P1 and P2 receptors. Trends Pharmacol Sci 1997;18:79-82.
- Lewis CJ, Evans RJ. Comparison of P2X receptors in rat mesen-teric, basilar and septal (coronary) arteries. J Auton Nerv Sys 2000;81:69-74.
- Manchanda R, Venkateswarlu K. Quantal evoked depolarizations underlying the excitatory junction potential of the guinea-pig isolated vas deferens. J Physiol 1999;520:527-37.
- Guimaraes S, Moura D. Vascular adrenoceptors: An update. Pharmacol Rev 2001;53:319-56.
- Alexander SPH, Peters JA. Receptor and ion channel nomencla-tures. Trends Pharmacol Sci 1998.Suppl:1-98.
- Ford AP, Daniels DV, Chang DJ, Gever JR, Jasper JR, Lesnick JD. Pharmacological pleiotropism of the human recombinant α1A-adrenoceptor: implications for α1-adrenoceptor classification. Br J Pharmacol 1997;121:1127-35.
- Daly CJ, Deighan C, McGee A, Mennie D, Ali Z, McBride M, et al. A knockout approach indicates a minor vasoconstrictor role for vascular alpha 1B-adrenoceptors in mouse. Physiol Genomics 2002;9:85-91.
- Zacharia J, Hillier C, Tanoue A, Tsujimoto G, Daly CJ, McGrath JC, et al. Br J Pharmacol 2005;146:679-91.
- Wier WG, Morgan KG. Adrenergic signaling mechanisms in mammalian resistance arteries. Rev Physiol Biochem Pharmacol 2003;150:91-139.
- Vargas HM, Gorman AJ. Vascular alpha-1 adrenergic receptor subtypes in the regulation of arterial pressure. Life Sci 1995;57:2291-308.
- Kong JQ, Taylor DA, Fleming WW. Functional distribution and role of α1-adrenoceptor subtypes in the mesenteric vasculature of the rat. J Pharmacol Exp Ther 1994;268:1153-9.
- Williams T, Clarke D. Characterization of α1-adrenoceptors mediating vasoconstriction to noradrenaline and nerve stimulation in the isolated perfused mesentery of rat. Br J Pharmacol 1995;114:531-6.
- Piascik MT, Hrometz SL, Edelmann SE, Guarino RD, Hadley RW, Brown RD. Immunocytochemical localization of the α1B adrenergic receptor and the contribution of this and the other subtypes to vascular smooth muscle contraction: analysis with selective ligands and antisense oligonucleotides. J Pharmacol Exp Ther 1997;283:854-68.
- Stam WB, Van Der Graaf PH, Saxena PR. Analysis of α1L-adreno-ceptor pharmacology in rat small mesenteric artery. Br J Pharmacol 1999;127:661-70.
- Townsend SA, Jung AS, Gillian Hoe YS, Lefkowitz RY, Khan SA, Lemmon CA. Critical role of α1B-adrenergic receptor at sympathetic neuroeffector junction. Hypertension 2004;44:776-82.
- Westfall TC, Yang CL, Curfman-Falvey M. Neuropeptide -Y- ATP interactions at the vascular sympathetic neuro-effector junc-tion. J Cardiovasc Pharmacol 1995;26:682-7.
- Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, et al. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev 1998;50:143-50.
- Chen H, Fetscher C, Shäfers RF, Wambach G, Philipp T, Michel MC. Effects of noradrenaline and neuropeptide Y on rat mesenteric microvessel contraction. Naunyn-Schmiedeberg’s Arch Pharmacol 1996;353:314-23.
- Prieto D, Buus CL, Mulvany MJ, Nilsson H. Interactions between neuropeptide Y and the adenyl cyclase pathway in rat mesenteric small arteries: role of membrane potential. J Physiol 1997;502:281-92.
- Sjoblom-Widfedt N, Gustafsson H, Nilsson H. Transmitter characteristics of small mesenteric arteries from the rat. Acta Physiol Scand 1990;138:203-12.
- Kennedy C, Saville VL, Burnstock G. The contributions of noradrenaline and ATP to the responses of the rabbit central ear artery to sympathetic nerve stimulation depend on the parameters of stimulation. Eur J Pharmacol 1986;122:291-300.
- Lamont C, Vainorius E, Wier WG. Purinergic and adrenergic Ca2+ transients during neurogenic contractions of rat mesenteric small arteries. J Physiol 2003;549:801-8.
- Brain KL, Cuprian AM, Williams DJ, Cunnane TC. The sources and sequestration of Ca2+ contributing to neuroeffector Ca2+ transients in the mouse vas deferens. J Physiol 2003;553:627-35.
- Lamont C, Wier WG. Evoked and spontaneous purinergic junctional Ca2+ transients (jCaTs) in rat small arteries. Circ Res 2002;91:454-6.
- Jaggar JH, Porter VA, Lederer MR, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol 2000;278:C235-56.
- Lamont C, Vial C, Evans RJ, Wier GW. P2X1 receptors mediate sympathetic post-junctional Ca2+ transients (jCaTs) in mesenteric small arteries. Am J Physiol Heart Circ Physiol 2006;291:H3106-13.
- Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, et al. Reduced vas deferens contraction and male fertility in mice lacking P2X1 receptors. Nature 2000;403:86-9.
- Zang WJ, Balke CW, Wier WG. Graded α1-adrenoceptor activation of arteries involves recruitment of smooth muscle cells to produce ‘all or none’ Ca2+ signals. Cell Calcium 2001;29:327-34.
- Nilsson H, Goldstein M, Nilsson O. Adrenergic innervation and neurogenic response in large and small arteries and veins from the rat. Acta Physiol Scand 1986;126:121-33.
- Lamont C, Wier WG. Different roles of ryanodine receptors and inositol (1,4,5)-trisphosphate receptors in adrenergically stimulated contractions of small arteries. Am J Physiol Heart Circ Physiol 2004;287:H617-25.
- Mauban JRH, Lamont C, Balke CW, Wier WG. Adrenergic stimulation of rat resistance arteries affects Ca2+ sparks, Ca2+ waves, and Ca2+ oscillations. Am J Physiol 2001;280:H2399-405.
- Miriel VA, Mauban JR, Blaustein MP, Wier WG. Local and cellular Ca2+ transients in smooth muscle of pressurized rat resistance arteries during myogenic and agonist stimulation. J Physiol 1999;518:815-24.
- Zacharia J, Zhang J, Wier GW. Calcium signaling in mouse mesenteric small arteries: myogenic tone and adrenergic vasoconstric-tion. FASEB J 2006;20:A1174.
- Mironneau J, Coussin F, Morel JL, Barbot C, Jeyakumar LH, Fleischer S, et al. Calcium signaling through nucleotide receptor P2X1 in rat portal vein myocytes. J Physiol 2001;536:339-50.
- Lagaud GJ, Stoclet JC, Andriantsitohaina R. Calcium handling and purinoceptor subtypes involved in ATP-induced contraction in rat small mesenteric arteries. J Physiol 1996;492:689-703.
- Putney JW. The pharmacology of capacitative calcium entry. Mol Interven 2001;1:84-94.
- Ruehlmann DO, Lee CH, Poburko D, van Breemen C. Asynchronous Ca2+ waves in intact venous smooth muscle. Circ Res 2000;86:E72-9.
