Arterial baroreflex function does not influence telomere length in kidney of rats1
Introduction
Telomeres, the specialized DNA-protein structures at the ends of eukaryotic chromosomes, are essential for the maintenance of genomic stability and integrity by preventing the recognition of chromosomal ends as double-stranded DNA breaks. In humans, telomeres are typically 10 kb in length. Because of the end-replication problem, telomeres may erode by 50–200 bp during each cycle of cell division in most somatic cells. Once telomeres reduce beyond a critical length, growth arrest or senescence is initiated thus arresting or limiting the replicative potential of cells[1,2]. This may cause aging and age-related diseases[3]. It is well known that many pathological changes seen in the development and progression of hypertension are reminiscent of those seen during the biological aging process. Hamet et al reported that shorter telomeres were found in the kidney of spontaneously hypertensive rats (SHR) at all ages examined, suggesting that kidney cells from these animals are subjected to increased turnover, potentially leading to accelerated aging[4]. Some studies showed that white blood cell (WBC) telomere length was inversely correlated with pulse pressure, an index of aortic stiffness that increases with age[5]. However, the relationship between the telomere length and other hemodynamic parameters is not clear, especially those involved in blood pressure regulation.
Arterial baroreflex (ABR) is very important in the regulation of cardiovascular function. It is well known that ABR function, often shown as baroreflex sensitivity (BRS), is impaired in cardiovascular diseases[6]. Recently, it was found that an impaired BRS was associated with a poor prognosis for myocardial infarction, heart failure, hypertension, and atherosclerosis[7–11]. Impaired BRS was also found to be related to reduced survival duration in aconitine-induced arrhythmia and endotoxin-induced shock in rats[12,13].
As both BRS and the telomere length decrease with age and change in some cardiovascular diseases, it seems that there is a relationship that exists between ABR function and the telomere length. It is known that the autonomic nervous system not only regulates cardiovascular system, respiratory system, and digestive system etc, but also is involved in the inflammation and immunological reactions. We speculated that telomere length might be modulated by the autonomic nervous system. Therefore, the present work was designed to elucidate the possible relationship between ABR function and telomere length by using stroke-prone SHR (SHR-SP) and sinoaortic denervated (SAD) rats. SHR-SP possess not only an elevated blood pressure, but also a lower BRS. Sinoaortic denervation destroys baroreflex at baroreceptor and afferent nerve levels.
Materials and methods
Animals SHR-SP rats of both sexes were provided by the animal center at our institute, 2nd Military Medical University. Male Sprague-Dawley (SD) rats at the age of 10 weeks were provided by SIPPR/BK Lab (Shanghai, China). All rats were housed with controlled temperature (23–25 °C) and circadian cycle (lights on 08:00–20:00) with standard rat chow and tap water ad libitum. All surgical and experimental procedures were in accordance with institutional animal care guidelines.
Blood pressure measurement Systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart period [HP, HP=60×1000/heart rate (beat/min)] of rats were continuously recorded using a technique described in previous studies[14,15]. Rats were anesthetized with a combination of ketamine (40 mg/kg) and diazepam (6 mg/kg). A floating polyethylene catheter was inserted into the lower abdominal aorta through the left femoral artery for BP measurement, while another catheter was placed into the left femoral vein for intravenous injection. The catheters were exteriorized through the interscapular skin. After a 2-d recovery period, the animals were placed in individual cylindrical cages containing food and water for BP recording. The aortic catheter was connected to a BP transducer via a rotating swivel that allowed the animals to move freely in the cage. After 4-h habituation, the BP signal was digitized by a microcomputer. SBP, DBP and HP value from every heartbeat were determined on line. The mean value and standard deviation of these parameters during a period of 4 h (13:00–17:00) for each rat were calculated. The standard deviation of all values obtained during 4 h was denoted as the quantitative parameter of variability, ie, systolic blood pressure variability (SBPV), diastolic blood pressure variability (DBPV), and heart period variability (HPV) for each rat.
BRS determination To determine the function of ABR in conscious rats the widely accepted method, which was originally designed for humans, is derived from that of Smyth et al[16]. The principle of this method is to measure the prolongation of HP in response to an elevation of BP. This method was used in conscious rats with some modifications[17]. A bolus injection of phenylephrine was used to induce an elevation of SBP. The dose of phenylephrine was adjusted to raise SBP between 20–40 mmHg. HP was plotted against SBP for linear regression analysis and the slope of SBP–HP was expressed as BRS (ms/mmHg). As a delay existed (approximately 1 s) between the stimulus and response, the slopes were calculated by computer with 1 to 10 beats of shift for linear regression analysis and the slope with the highest correlation coefficient was used as BRS. A correlation analysis with 5 beats of shift, for example, meant that values of HP6/SBP1, HP7/SBP2, HP8/SBP3, …, were used.
Sinoaortic denervation Sinoaortic denervation, an interruption of ABR by destroying the afferent fibers of baro-receptors, was performed as described in previous studies[11,13]. Male SD rats at 10 weeks of age were anesthetized with a combination of ketamine (50 mg/kg, ip) and diazepam (5 mg/kg, ip) and were then medicated with atropine sulfate (0.5 mg/kg, ip) and procaine benzylpenicillin (60 000 U, im). After a midline neck incision and bilateral isolation of the neck muscles, aortic baroreceptor denervation was carried out bilaterally by cutting the superior laryngeal nerves near the vagi, removing the superior cervical ganglia, including a small section of the sympathetic trunk, and sectioning aortic depressor nerves. The carotid sinus baroreceptors were denervated bilaterally by stripping the carotid bifurcation and its branches, followed by the application of 10% phenol (in 95% ethanol) to the external, internal and common carotid arteries and the occipital artery. Sham operation was performed with the midline neck incision and bilateral isolation of the neck muscles. After operation, rats were maintained under normal rearing conditions. The completeness of SAD was assessed by intravenous injection of phenylephrine (2–5 g/kg) via the left femoral vein. If phenylephrine induced an increase of SBP by 50 mmHg with less than 20 beats/min decrease in heart rate, the SAD was considered complete. In this experiment, only completed SAD rats were used. Sham-operated animals exhibited a decrease of 60–100 beats/min in heart rate.
Measurements of the terminal restriction fragment (TRF) length Telomere length was evaluated in the kidneys by measuring the mean length of the TRF. After hemodynamic monitoring, animals were weighed and anaesthetized as described above. The peritoneal cavity was immediately opened. The right kidney was excised and rinsed in physiological saline for DNA extracting. DNA was isolated from the kidney with Wizard genomic kits (Promega, USA), followed by digestion of 10 µg DNA with restriction enzymes Hinf I (25 U) (Promega) at 37 ºC overnight. DNA samples (10 µg each) and DNA ladders (1 kb DNA ladder plus λDNA/Hind III fragments; Promega) were resolved on a 0.7% agarose gel (25 cm×20 cm) at 30 V. Duplicates from the same samples were resolved on different gels. After 30 h, the gels were depurinated for 30 min in 0.25 mol/L HCl, denatured 40 min in 0.5 mol/L NaOH/1.5 mol/L NaCl, and neutralized for 30 min in 0.5 mol/L Tris, pH 8/1.5 mol/L NaCl. The DNA was transferred for 18 h to a nylon membrane, positively charged (Roche, USA), according to the manufacturer’s protocol. The membranes were then hybridized at 65ºC with the telomeric probe [digoxigenin 3'-end labeled 5'-(TTAGGG)3) overnight in 5×SSC 0.1% Sarkosyl, 0.02% SDS, and 1% blocking reagent (Roche)]. The membranes were washed 3 times at room temperature in 2×SSC, 0.1% SDS for 15 min and once in 2×SSC for 15 min. The probe was detected by the digoxigenin luminescent detection procedure (Roche) and exposed on X-ray film. The length of TRF was calculated as TRF=ΣODi/Σ(ODi/MWi), where ODi was optical density at a given position in the lane and MWi was molecular weight at that position[2].
Experimental protocols
SHR-SP study Experiments were performed in both male (n=10) and female (n=23) SHR-SP at the age of 24 weeks and in female SHR-SP at 40 weeks of age (n=12). Blood pressure was recorded during a period of 4 h and BRS was measured with the methods mentioned above. After BRS measurement, TRF length was evaluated in the kidney by Southern blotting.
SAD study Male SD rats were used for SAD operation at the age of 10 weeks. After operation, the animals were divided into four groups and reared for 4 or 35 weeks (n=8 in each group): 4 weeks after SAD operation, 4 weeks after sham operation, 35 weeks after SAD operation and 35 weeks after sham operation. BP was recorded for 4 h; the determination of BRS and TRF length then followed.
Statistical analysis All data were expressed as mean± SD. The means of each group were evaluated by two-tailed Student’s unpaired t-test. The relationships between two variables were assessed by linear regression analysis. Differences were considered significant when P<0.05.
Results
Hemodynamics and TRF length in SHR-SP The general findings of hemodynamics and TRF length in SHR-SP are shown in Table 1. It was found that SBP, DBP, HP, SBPV were significantly (P<0.01) higher in male than in female SHR-SP at the age of 24 weeks. In female rats, SBP, HP, SBPV, HPV were significantly (P<0.05) higher in the group of rats at 40 weeks of age than those at 24 weeks of age. TRF length was significantly (P<0.05) longer in female SHR-SP than age-matched male SHR-SP. Furthermore, it was found that TRF length was significantly (P<0.01) shortened at 40 weeks compared to 24 weeks in female SHR-SP (Table 1, Figures 1,2).
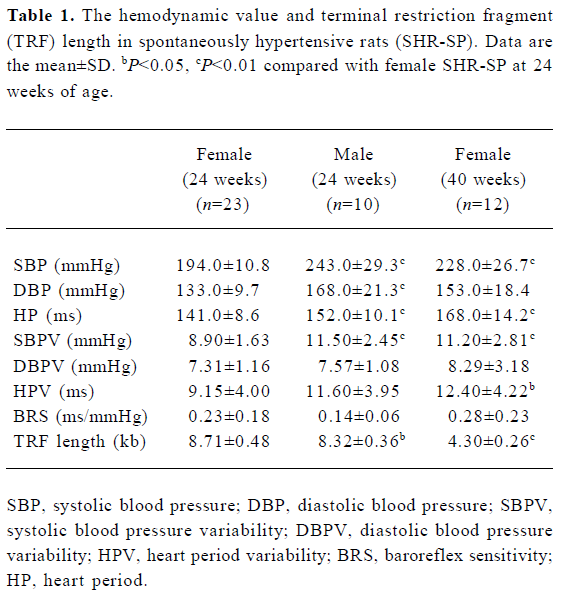
Full table
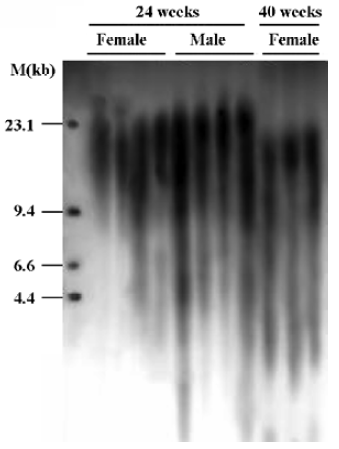
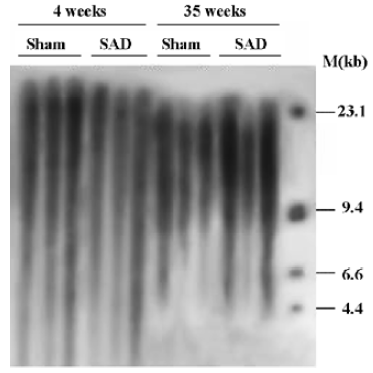
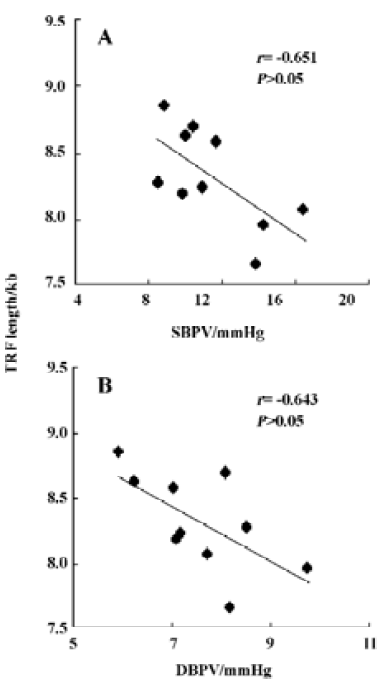
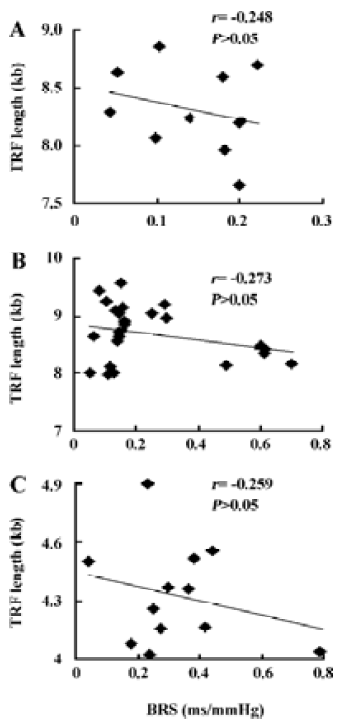
Table 2 shows the correlation between hemodynamic values and TRF in SHR-SP. TRF length was negatively correlated with SBPV (r=-0.65, P<0.05; Figure 4A) and DBPV (r=-0.64, P<0.05; Figure 4B) in male SHR-SP at the age of 24 weeks. No other significant correlations were observed in any subgroups (Figure 3).
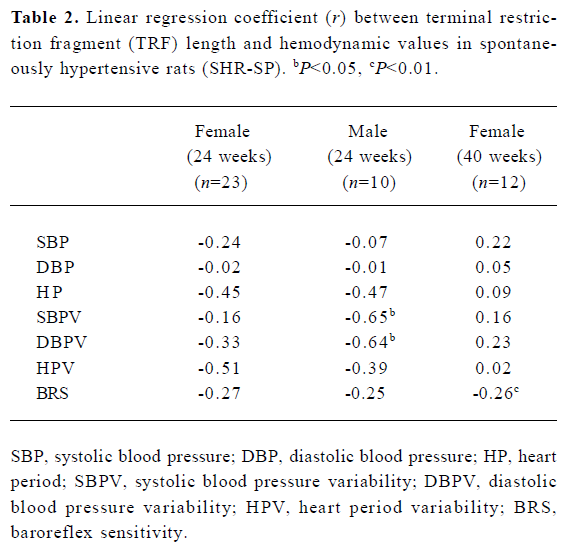
Full table
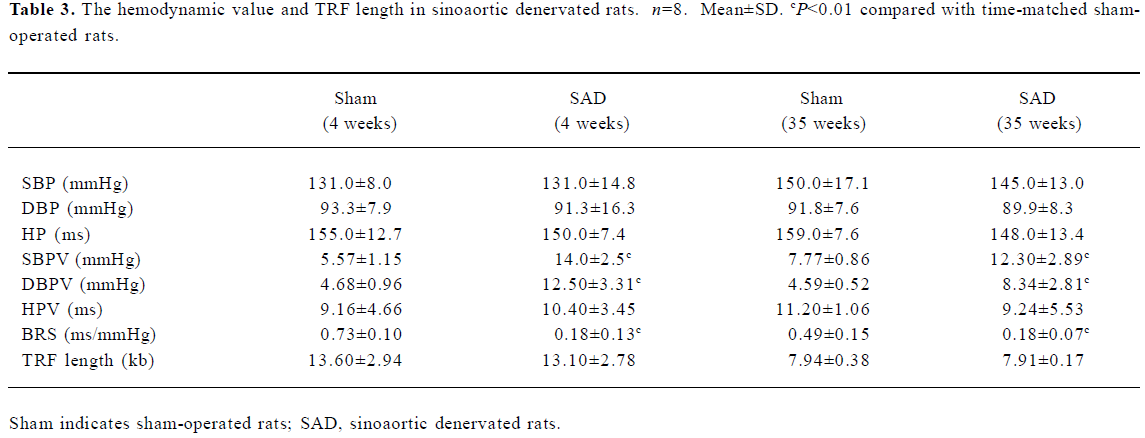
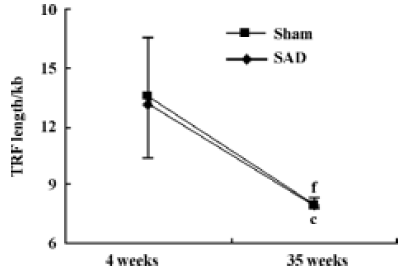
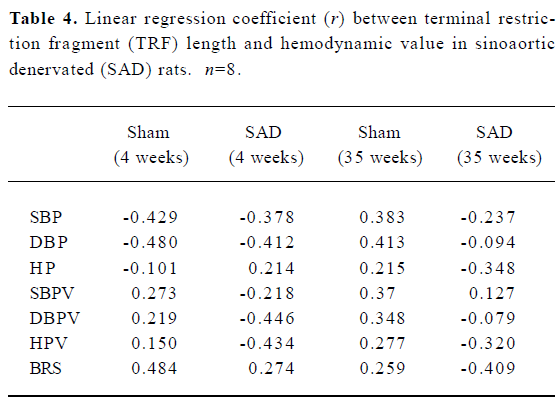
Hemodynamics and TRF length in SAD rats Table 3 shows that SBP, DBP and HP were similar in SAD rats and sham-operated rats. However, SBPV and DBPV were significantly (P<0.01) higher in SAD rats than in time-matched sham-operated rats. As expected the BRS values were significantly (P<0.01) lower in SAD rats compared to time-matched sham-rats. However, TRF length in rats 35 weeks after operation was significantly (P<0.01) shorter than in rats 4 weeks after operation in both sham-operated and SAD rats (Figure 5). There was no significant correlation between hemodynamics and TRF length in any SAD subgroups (Table 4).
Full table
Full table
Discussion
The major findings of the present work may be summarized as follows. (1) TRF length was found shorter in: a) male SHR-SP compared with age-matched female SHR-SP; b) female SHR-SP at 40 weeks of age compared with those 24 weeks of age; c) rats 35 weeks after operation compared with rats 4 weeks after operation in both sham-operated and SAD rats. (2) Arterial baroreflex function did not relate to the TRF length in rats.
The SHR-SP model was derived from SHR rats with higher propensity for developing stroke. SHR-SP rats usually died of stroke at an average age of 9 months for males and 13 months for females. Obviously a gender difference exists in the life span in SHR-SP. It is well documented that blood pressure is higher in male SHR-SP than in female SHR-SP[18]. In the present study, the difference in sexes was demons-trated. The raised blood pressure level may significantly contribute to the shorter life span in male SHR-SP. Further-more, it has been well documented that the TRF length is related to life span in many observations. In the present work we compared the TRF length in male and female SHR-SP. Our results show that TRF was shorter in males than in females. This gender difference in the TRF length may also contribute to the gender difference in life span in SHR-SP. Although a similar result has been reported by stindl R[19], we have found a gender difference in the TRF length in SHR-SP for the first time. Previous studies have found that males had shorter telomeres than females in rats in all organs analyzed except the brain[20]. Higher telomerase activity, the major factor determining telomere length, was found in females when compared with males in human and Wistar rat studies[21]. Estrogen can enhance telomerase activity through several pathways that can inhibit the rate of telomere attrition[22–24]. It is thought that the cumulative free reactive oxygen species (ROS) and chronic inflammation, may be important initiators of the cell senescence and involved in the loss of telomere[25,26]. Interestingly, levels of ROS are lower in women than men[27], perhaps because of the ability of estrogen to curtail ROS production and enhance ROS scavenging and detoxification[28,29].
It is well known that telomere length decreases with age and the length is an indicator of replicative history and replicative potential of somatic cells. In our study we found that TRF length decreased with age in female SHR-SP, sham-operated and SAD rats. Because the mean life-span of male SHR-SP is less than 40 weeks, we did not compare the TRF length at different ages in male SHR-SP. In cultured somatic cells from humans, telomeres undergo attrition with each cycle of cellular replication until a critical telomere length is attained, at which point cells experience replicative senescence[30,31]. In contrast, most somatic cell lines, except in Mus musculus and domesticated chickens[32], have low or undetectable telomerase, a ribonucleoprotein capable of elongating telomeres de novo[33]. Oxidative stress is at the center of the free radical theory of aging, which proposes that degenerative senescence is largely the result of the cumulative effect of oxidative end products. ROS could accelerate the rate of telomere attrition in different cell types[25,26].
It is known that the telomere length is decreased in hypertension and other cardiovascular diseases. In examining the relationship between hemodynamic parameters and the telomere length, it was reported that pulse pressure (the difference between SBP and DBP) affected the telomere length[5]. However, there is no evidence to show SBP levels affecting telomere length. In the present work, no significant correlation was detected between SBP level and the telomere length in SHR-SP or in SAD rats. It may be interesting to observe the relationship between SBP and telomere length in different hypertensive animal models.
In addition, it was reported that the rate of telomere loss as a function of donor age was greater in the intimal DNA of iliac arteries compared to that of the internal thoracic arteries. This was attributed to the effect of shear stress on the telomere length[34]. The shear stress is greater in arteries with bifurcation than other arteries.
The present work showed that TRF length was inversely correlated with SBPV and DBPV in male SHR-SP. That is to say that, higher BPV is associated with shorter TRF length. As this relationship was found only in male SHR-SP, its importance and possible significance remain unknown. Furthermore, as all animals do not age at the same speed, the ones which age more rapidly (ie, have a lower TRF) are also likely to have more rigid blood vessels and thus some degree of high BPV. This could be fully independent of telomere length. In SAD rats, the BPV was significantly higher than sham-operated rats, but no difference in telomere length was found.
ABR is a very important mechanism in maintaining stable blood pressure. It is decreased with age and is impaired in hypertension[35,36]. SAD is a commonly used method to interrupt the arterial baroreflex arc for baroreflex functional studies. It has been found that BPV is markedly increased, however, 24-hour average BP levels were normal in SAD animals, including rats, rabbits, monkeys, and dogs[37,38]. It is accepted that baroreflex dysfunction is not the cause of hypertension. However, baroreflex function can predict end-organ damage in hypertension, such as myocardial damage, renal lesion, and arterial remodeling[39]. Telomeres are situated at the ends of linear chromosomes and protect them from degradation as well as end-to-end fusions, and replicative senescence is associated with telomere erosion. Concerning the relationship between arterial baroreflex function and the TRF length, no significant correlations were found between BRS and the TRF length in any sub-groups of SHR-SP. Furthermore, completely destroying the ABR arc did not change the TRF length. These results indicated that baroreflex function did not influence the TRF length in rats.
In conclusion, the present study demonstrated that the TRF length was shorter in males than in females in age-matched SHR-SP, and in aged female SHR-SP. In both sham-operated rats and SAD rats, the TRF length was shorter in older rats than in younger rats. In SHR-SP, the TRF length did not relate to BRS. In addition, SAD did not affect the TRF length. These results demonstrated that baroreflex function did not influence the TRF length at the current experimental setting.
References
- Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, et al. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J 1992;11:1921-9.
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature 1990;345:458-60.
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003;361:393-5.
- Hamet P, Thorin-Trescases N, Moreau P, Dumas P, Tea BS, de Blois D, et al. Excess growth and apoptosis: is hypertension a case of accelerated aging of cardiovascular cells? Hypertension 2001;37:760-6.
- Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, Aviv A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension 2000;36:195-200.
- Su DF, Miao CY. Arterial baroreflex function in conscious rats. Acta Pharmacol Sin 2002;23:673-9.
- La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 1998;351:478-84.
- Mortara A, La Rovere MT, Pinna GD, Prpa A, Maestri R, Febo O, et al. Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation 1997;96:3450-8.
- Tao X, Zhang SH, Shen FM, Su DF. High-level apoptosis is persistent in myocardiocytes of sinoaortic-denervated rats. J Hypertens 2004;22:557-63.
- Su DF, Miao CY. Reduction of blood pressure variability: a new strategy for the treatment of hypertension. Trends Pharmacol Sci 2005;26:388-90.
- Cai GJ, Miao CY, Xie HH, Lu LH, Su DF. Arterial baroreflex dysfunction promotes atherosclerosis in rats. Atherosclerosis 2005;183:41-7.
- Shu H, Yi-Ming W, Xu LP, Miao CY, Su DF. Increased susceptibility of ventricular arrhythmias to aconitine in anaesthetized rats is attributed to the inhibition of baroreflex. Clin Exp Pharmacol Physiol 2004;31:249-53.
- Shen FM, Guan YF, Xie HH, Su DF. Arterial baroreflex function determines the survival time in lipopolysaccharide-induced shock in rats. Shock 2004;21:556-60.
- Shen FM, Xie HH, Ling G, Xu LP, Su DF. Synergistic effects of atenolol and amlodipine for lowering and stabilizing blood pressure in 2K1C renovascular hypertensive rats. Acta Pharmacol Sin 2005;26:1303-8.
- Xie HH, Shen FM, Miao CY, Su DF. Blood pressure, baroreflex sensitivity, and end organ damage in hybrid offspring of spontaneously hypertensive rats and Sprague-Dawley rats. Acta Pharmacol Sin 2005;26:1049-56.
- Smyth HS, Sleight P, Pickering GW. Reflex regulation of arterial pressure during sleep in man: a quantitative method of assessing baroreflex sensitivity. Circ Res 1969;24:109-21.
- Xie HH, Miao CY, Jiang YY, Su DF. Synergism of atenolol and nitrendipine on hemodynamic amelioration and organ protection in hypertensive rats. J Hypertens 2005;23:193-201.
- Masineni SN, Chander PN, Singh GD, Powers CA, Stier CT Jr. Male gender and not the severity of hypertension is associated with end-organ damage in aged stroke-prone spontaneously hypertensive rats. Am J Hypertens 2005;18:878-84.
- Stindl R. Tying it all together: telomeres, sexual size dimorphism and the gender gap in life expectancy. Med Hypotheses 2004;62:151-4.
- Cherif H, Tarry JL, Ozanne SE, Hales CN. Ageing and telomeres: a study into organ- and gender-specific telomere shortening. Nucleic Acids Res 2003;31:1576-83.
- Leri A, Malhotra A, Liew CC, Kajstura J, Anversa P. Telomerase activity in rat cardiac myocytes is age and gender dependent. J Mol Cell Cardiol 2000;32:385-90.
- Kyo S, Takakura M, Kanaya T, Zhuo W, Fujimoto K, Nishio Y, et al. Estrogen activates telomerase. Cancer Res 1999;59:5917-21.
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 2000;407:538-41.
- Imanishi T, Hano T, Nishio I. Estrogen reduces endothelial progenitor cell senescence through augmentation of telomerase activity. J Hypertens 2005;23:1699-706.
- von Zglinicki T. Role of oxidative stress in telomere length regulation and replicative senescence. Ann N Y Acad Sci 2000;908:99-110.
- Xu D, Neville R, Finkel T. Homocysteine accelerates endothelial cell senescence. FEBS Lett 2000;470:20-4.
- Lacy F, Kailasam MT, O’Connor DT, Schmid-Schonbein GW, Parmer RJ. Plasma hydrogen peroxide production in human essential hypertension. Role of heredity, gender, and ethnicity. Hypertension 2000;36:878-84.
- Tang M, Subbiah MT. Estrogens protect against hydrogen peroxide and arachidonic acid induced DNA damage. Biochim Biophys Acta 1996;1299:155-9.
- Romer W, Oettel M, Menzenbach B, Droescher P, Schwarz S. Novel estrogens and their radical scavenging effects, iron-chelating and total antioxidative activities: 17 alpha- substituted analogs of delta 9 (11)-dehydro-17 beta estradiol. Steroids 1997;62:688-94.
- Harley CB, Vaziri H, Counter CM, Allsopp RC. The telomere hypothesis of cellular aging. Exp Gerontol 1992;27:375-82.
- Aviv A, Harley CB. How long should telomeres be? Curr Hypertens Rep 2001;3:145-51.
- Venkatesan RN, Price C. Telomerase expression in chickens: constitutive activity in somatic tissues and down-regulation in culture. Proc Natl Acad Sci USA 1998;95:14763-8.
- Prowse KR, Greider CW. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc Natl Acad Sci USA 1995;92:4818-22.
- Chang E, Harley CB. Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci USA 1995;92:11190-4.
- Kornet L, Hoeks AP, Janssen BJ, Houben AJ, De Leeuw PW, Reneman RS. Neural activity of the cardiac baroreflex decreases with age in normotensive and hypertensive subjects. J Hypertens 2005;23:815-23.
- Sleight P. The importance of the autonomic nervous system in health and disease. Aust N Z J Med 1997;27:467-73.
- Norman RA Jr, Coleman TG, Dent AC. Continuous monitoring of arterial pressure indicates sinoaortic denervated rats are not hypertensive. Hypertension 1981;3:119-25.
- Saito M, Terui N, Numao Y, Kumada M. Absence of sustained hypertension in sinoaortic denervated rabbits. Am J Physiol 1986;251:H742-7.
- Shan ZZ, Dai SM, Su DF. Relationship between baroreceptor reflex function and end-organ damage in spontaneously hypertensive rats. Am J Physiol 1999;277:H1200-6.
