Interleukin-1 receptor antagonist intervenes in signaling between different types of synoviocytes in rats with adjuvant arthritis1
Introduction
Rheumatoid arthritis (RA) is a serious medical problem that affects approximately 1% of the world抯 population. |Although palliative treatments (nonsteroidal anti-inflammatory drugs; NSAIDs) are widely prescribed, there are currently only a few treatments that can modify the progression of the disease (disease-modifying antirheumatic drugs; DMARDs). A major obstacle to the development of treatment strategies is that the disease mechanisms remain largely unknown, but clues may come from studying experimental models of arthritis[1,2].
RA is autoimmune in nature and is characterized by chronic inflammation of the synovial tissues in multiple joints, which leads to joint destruction. The characteristic pathological findings of RA synovitis are aberrant proliferation of synoviocytes. It has been reported that the majority of rheumatoid synovial cells, regardless of morphology, consist of 3 different cell types: fibroblast-like synoviocytes (FLS), dendritic-like synoviocytes (DLS) and macrophage-like synoviocytes (MLS)[3]. There are few reports about the relationships and interactions between different types of synoviocytes.
IL-1 mediates bone resorption, cartilage destruction and inflammation in RA. IL-1 receptor antagonist (IL-1ra) selectively inhibits IL-1 by competing for the IL-1 receptor on all surfaces of the synovium. Treatment of rheumatoid patients with IL-1ra leads to an improvement in different clinical and biological parameters and to a reduction in the radiological signs of joint erosion. Encouraging results have also been reported in experimental animal models of arthritis through using other strategies designed to block the effects of IL-1[4,5]. However, the effects of IL-1ra on different types of synoviocytes in vivo are poorly understood.
Adjuvant arthritis (AA) in rats is an experimental model that shares some features with human RA, including swelling, cartilage degradation and loss of joint function[6]. One of the most important features of AA is chronic synovitis, including inflammatory cell infiltration, pannus formation, destruction of cartilage, and bone erosion[7].
The goal of the present study was to investigate the mechanism of IL-1ra in the treatment of AA. We investigated the effects and mechanisms of IL-1ra on the ultrastructure of synoviocytes and pro-inflammatory cytokine production by MLS in AA rats. In addition, we also wanted to investigate mitogen-activated protein kinase (MAPK) phosphorylation and cell proliferation in FLS induced by the culture supernatants of MLS in AA rats treated with IL-1ra.
Materials and methods
Animals Male Sprague-Dawley (SD) rats (180±20 g) and C57BL/6J mice (20±2 g) were purchased from Shanghai BK Experimental Animal Center (Grade II, Certificate N
Materials and reagents Rabbit anti-JNK1/2, rabbit anti-phospho-JNK1/2 (pThr183/pTyr185), rabbit anti-p38 kinase, rabbit anti-phospho-p38 kinase (pThr180/pTyr182), rabbit anti-ERK-1/2 and rabbit anti-phospho-ERK1/2 (pThr202/pTyr 204) antibodies were purchased from Sigma (St Louis, MO, USA). SuperSignal West Femto Maximum Sensitivity Substrate was obtained from Pierce (Rockford, IL, USA). The 125I-TNF-α radioimmunoassay (RIA) kit was the product of Beijing Biotinge Biomedicine Company (Beijing, China). The 125I-PGE2 RIA kit was the product of Suzhou Medical College (Suzhou, China). Bacillus-Calmette-Guerin (BCG) vaccine was obtained from Shanghai Biochemical Factory (Shanghai, China). Collagenase type IA, trypsin, lipopolysaccharide (LPS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H tetrazolium bromide (MTT) and all chemicals with analytical purity were purchased from Sigma. The RPMI-1640 medium from Gibco (CA, USA; pH=7.2) was supplemented with 100 U/mL penicillin, 100 g/L streptomycin, 2 mmol/L L-glutamine, 5×10-5 mol/L 2-mercaptoethanol and 25 mmol/L N-2 hydroxyethyl-piperazine-N-2-ethanesulfonic acid (HEPES).
Drugs IL-1ra (Amgen, Thousand Oaks, CA, batch number 20030701) occurs as white crystals or crystalline powder. The compound was dissolved in phosphate buffered saline (PBS; Pierce, Rockford IL).
Induction and treatment of AA Freund抯 complete adjuvant (FCA) was prepared by suspending heat-killed BCG in liquid paraffin at 10 mg/mL. AA was induced by a single intradermal injection of 100μL FCA into the left hind metatarsal footpads of rats. The onset and severity of disease were monitored daily and evaluated by 2 observers who were blinded to the treatment. At d 0, 5, 9, 13, 17 and 21 after immunization, the right hind paw volume was determined by using a MK-550 volume meter (Muromachi Kikai, Tokyo, Japan). Paw swelling (mL) was calculated by subtracting the paw volume at d 0 from that at d 5, 9, 13, 17 and 21. Our data indicated that paw swelling occurred on d 13 after immunization. Before the onset of arthritis, animals were divided into 5 groups randomly. On d 14 after immunization, rats were given an intracutaneous injection of IL-1ra (2.5, 10, 40 mg/kg, 3 times per day) from d 14 to d 21. For the controls and AA models, rats were given an equal volume of vehicle.
Ultrastructure of synoviocytes Rats were killed on d 22 after immunization. Synovial tissues were fixed with 4% paraformaldehyde solution overnight and were washed with PBS after fixing with 1% osmic acid for 2 h. After being embedded in an Epon/Araldite (Sigma, Saint Louis, USA) mixture and stained with uranyl acetate and lead citrate, the synoviocytes were observed under a 1230-type transmission electron microscope (Electron, Yokohama, Kanagawa, Japan) and photographed.
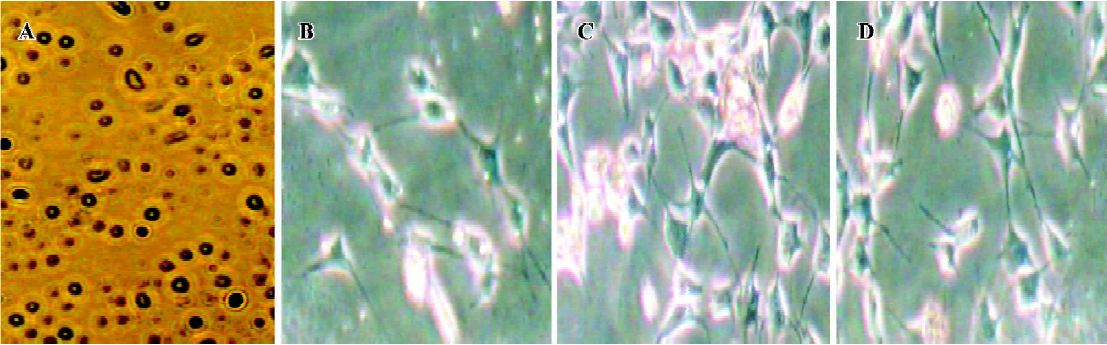
Culture of MLS Synoviocytes from hind paws were excised and dispersed with sequential incubation with 0.4 mg/mL collagenase and 0.25 mg/mL trypsin. In brief, small minced synovial membranes were digested in RPMI-1640 medium containing 5% fetal bovine serum (FBS; Sigma) and 0.4 mg/mL collagenase type IA at 37 °C, at 5% CO2. Two hours later, adherent cells were discarded and non-adherent tissues were incubated in serum-starved RPMI-1640 medium containing 0.25 mg/mL trypsin for 1 h. After incubation, the tissue was pipetted through sterile 108-mm2 nylon mesh into a sterile centrifuge tube. Cells were then washed 3 times with RPMI-1640 plus 5% FBS and added to 20 mL flat-bottomed culture bottles (Sumitomo Bakelite, Tokyo, Japan) and incubated with RPMI-1640 plus 10% FBS at 37 °C, 5% CO2, for 24 h. After cells became adherent, the cultures were replaced and synoviocytes were resuspended in RPMI-1640 medium containing 10% heat-inactivated FBS. The round-shaped cells (Figure 1A) were cultured with 5 mg/L LPS in 24-well flat-bottomed culture plates (Sumitomo Bakelite) at a final concentration of 1?106 cells/1 mL per well. After incubation at 37 °C in a 5% CO2 atmosphere for 48 h, the supernatant containing IL-1, tumor necrosis factor alpha (TNF-α) and prostaglandin E2 (PGE2) was collected and stored at -20 °C.
RIA of TNFα and PGE2 One hundred microliters of supernatant containing TNF-α and PGE2 were measured according to the procedures outlined in the TNF-α and PGE2 125I RIA kit.
Analysis of IL-1 activity IL-1 activity was measured by using the ConA-induced thymocyte proliferation in C57BL/6J mice assay. Fifty microliters of a thymocyte suspension (5×109 cells/L) taken from C57BL/6J mice were distributed over a flat-bottomed 96-well microtiter plate (Sumitomo Bakelite). Then, 100 μL supernatant samples of MLS containing IL-1 and 50μL ConA (with a final concentration of 5 mg/L) were added and incubated. IL-1 activity was measured by using a thymocytes proliferation assay. Briefly, the cells were incubated at 37 °C in a 5% CO2 atmosphere for 48 h. MTT (5 g/L, 10μL) was added to each well. After being incubated at 37 °C for an additional 2 h, the cells were centrifuged at 760×g for 10 min and all the supernatants were discarded. Formazan crystals were dissolved in 120μL isobutanol (containing 0.04 mol/L HCl) and the absorbance was read at 570 nm using an EJ301 (Bio-Tek Instruments, Winooski, Vermont) enzyme-linked immunosorbent assay (ELISA) Microwell reader. The results are presented as the average absorbance (A) and expressed as the mean of triplicate samples.
FLS culture and protein sample preparation Synovial tissues were isolated from hind paws by removing the skin, muscle, fatty tissues, bone, and tendons, then were homogenized to yield approximately 1 mg protein/mL for the detection of MAPK phosphorylation. For other samples, synovial tissues obtained from the hind paws of AA rats were minced and digested by collagenase type IA for 3 h, filtered, extensively washed, and then cultured in RPMI-1640 medium (pH 7.2) containing 20% heat-inactivated FBS at 37 °C in a humidified atmosphere of 5% CO2. At confluence, adherent cells were trypsinized, split in a 1:3 ratio, and recultured in medium. The spindle-shaped cells were used from passages 3 through to 9 in these experiments, during which time they were a homogeneous population of FLS (Figure 1B-1D). After that, cells were washed with PBS, distributed in 20 mL flat-bottomed culture bottles at a concentration of 2?106 cells per bottle and kept in 2 mL serum-starved RPMI-1640 medium (containing 0.5% FBS) for 24 h. Then 1 mL supernatant of MLS was added to each well and the plates were incubated for 30 min (for measurement of MAPK phos-phorylation). When the reaction was stopped, cell cultures were washed twice with PBS, monolayers were detached from the plastic by using a tissue scraper, and cells were suspended in PBS (pH 7.4). Samples were sedimented for 10 min at 3000×g (4°C) and the pellets were then suspended in 10 mL lysis buffer (20 mmol/L HEPES, 2 mmol/L MgCl2, 1 mmol/L EDTA, 2 mmol/L DTT, pH 7.4) followed by homogenization in a glass-Teflon Potter homogenizer at 4°C. The homogenate was centrifuged at 3000×g for 4 min and the resulting supernatants spun at 20 000×g for 30 min. Supernatants obtained were diluted into 1 mg protein/mL for measurement of MAPK phosphorylation. Protein content was determined with bovine serum albumin (BSA) as a standard according to the Bradford method[8].
Western blot analysis Protein samples (20 μg/lane) from FLS were run on 12% sodium dodecylsulfate (SDS)-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride (PVDF) microporous membranes (Millipore, Beijing, China) at 50 mA in transfer buffer containing 25 mmol/L Tris-base, 192 mmol/L glycine, 20% methanol. Western blot analysis was performed. Briefly, blots were blocked with PBS saline plus 0.05% Tween 20 and 5% BSA for 1 h and then were incubated with appropriate antibodies at 4°C overnight. The membranes were washed 4‒6 times and incubated with horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature. The proteins were visualized by chemiluminescence using hydrogen peroxide and luminol as a substrate using Kodak X-AR film. All the experiments reported in the present study were performed 3 times and the results were reproducible.
FLS proliferation assay AA FLS were isolated according to the method outlined earlier and were cultured with 0.5% FBS-RPMI-1640 for 24 h. Then cells were resuspended in 100 mL 10% FBS-RPMI-1640 medium containing 10 mg/L LPS and 100 mL supernatant of MLS at a concentration of 2×1010 cell/L in 96-well flat-bottomed culture plates. The cultures were incubated at 37°C, 5% CO2 for 48 h. A 10 mL sample of MTT (5 g/L) was added to each well, and the plates were oscillated for 1 min on an oscillator and incubated at 37°C for 2 h and 5% CO2. After incubation, the cultures were centrifuged (760×g, 10 min). The supernatants were aspirated, 120μg of isopropanol (containing 0.04 mol/L HCl) was added to each well and the mixture was oscillated for 30 s again. Absorbance (A) was measured on an EJ301 ELISA Microwell Reader at 570 nm. The results are presented as the average absorbance and expressed as the mean of triplicate samples.
Statistical analysis Data are expressed as mean±SED. Analysis of variance and the t-test were used to determine significant differences between groups. P values less than 0.05 were considered to be significant.
Results
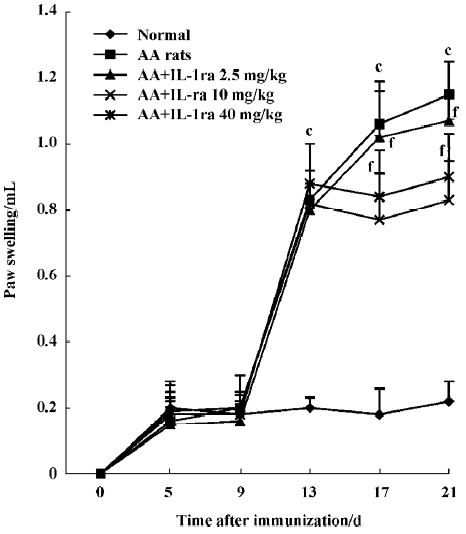
Effects of IL-1ra on secondary inflammatory reaction Inflammatory polyarthritis was induced in all immunized rats. The paw swelling occurred on d 13 after immunization. Treatment with IL-1ra (10 and 40 mg/kg per d, ic, d 14‒21) diminished the swelling of the right hind paw measured on d 17 and d 21 after immunization (P<0.01, Figure 2).
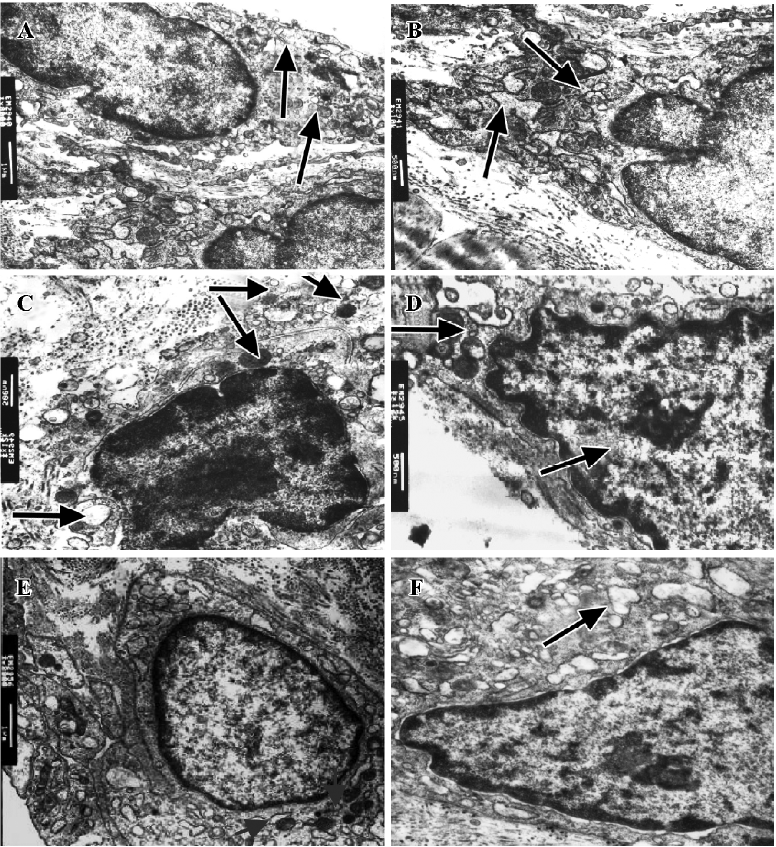
Effect of IL-1ra on ultrastructure of synoviocytes Intracellular changes were observed in AA rats. In type A synoviocytes we observed reduction and curling of Golgi bodies, swelling of mitochondria with decreased ridges and increased vacuoles, and in type B synoviocytes we observed accumulation of extracellular matrix and collagen, and dilation of rough endoplasmic reticulum (RER). IL-1ra alleviated these changes to some extent (Figure 3).
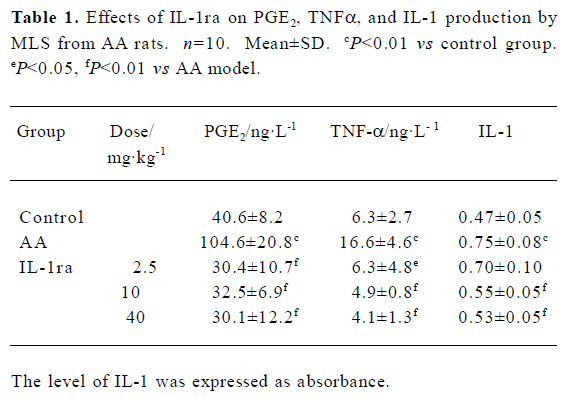
Effects of IL-1ra on cytokine production by MLS from AA rats MLS from AA rats stimulated by LPS produced more cytokines, including IL-1, PGE2, and TNF-α, compared with the controls. The administration of IL-1ra (10 and 40 mg/kg, ic, d 14‒21) decreased the production of IL-1, PGE2, and TNF-α stimulated by LPS in MLS from AA (P<0.01). IL-1ra (2.5 mg/kg) also decreased the production of PGE2 (P<0.01) and TNF-α (P<0.05) stimulated by MLS (Table 1).
Full table
Effects of IL-1ra on phosphorylation of JNK, ERK and p38 MAPK in the synovial membrane of AA rats JNK, ERK, and p38 phosphorylation increased in the synovial membrane of AA rats. IL-1ra (40 and 10 mg/kg, ic, d 14?21) inhibited the phosphorylation of MAPK, whereas IL-1ra (2.5 mg/kg) had no significant effect on MAPK phosphorylation in the synovial membrane of AA rats (Figure 4).
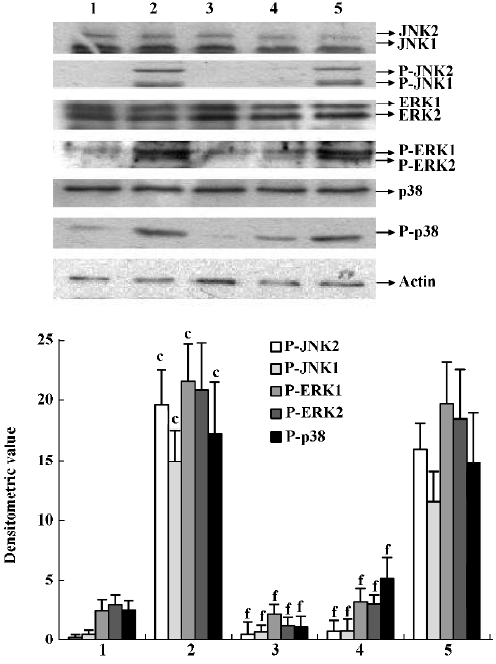
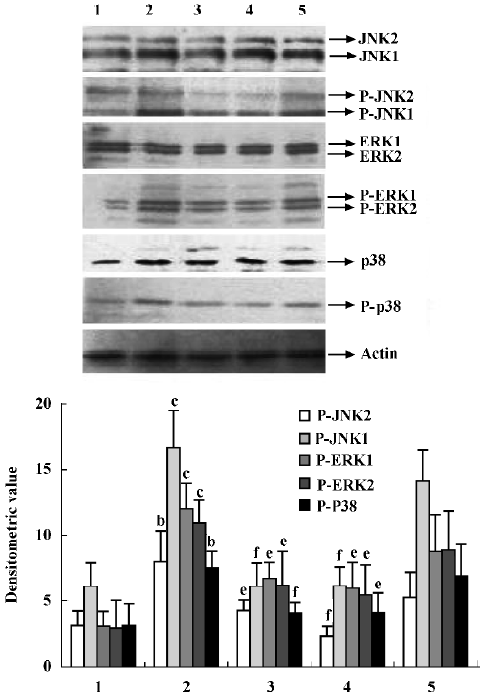
Effects of supernatant of MLS from AA rats treated with IL-1ra on JNK, ERK and p38 phosphorylation in AA FLS Supernatant of MLS from AA rats induced more phosphorylation of MAPK, including JNK, ERK, and p38, in AA FLS than that from control rats. The supernatant of MLS from AA rats treated with IL-1ra (40 and 10 mg/kg, ic, d 14‒21) induced less phosphorylation of MAPK in AA FLS than that from AA rats (Figure 5).
Effects of supernatant of MLS from AA rats treated with IL-1ra on FLS proliferation Compared with supernatant of MLS from control rats, the supernatant of MLS from AA rats increased cell proliferation in AA FLS. The supernatant of MLS from AA rats treated with IL-1ra (40 or 10 mg/kg, ic, d 14‒21) decreased cell proliferation in AA FLS more than that from AA rats (Table 2).
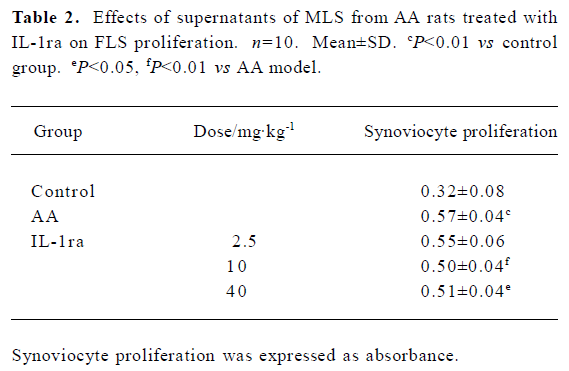
Full table
Discussion
In the present study, we found that IL-1ra inhibited second inflammatory reactions and modulated the ultrastructure of synoviocytes in AA rats. Furthermore, we showed that IL-1ra modulated the production of pro-inflammatory mediators by MLS and inhibited the phosphorylation of MAPK in FLS.
The characteristic pathological findings of RA synovitis are aberrant proliferation of synovial lining cells, neova-scularization by small vessels, accumulation of inflammatory cells in the synovium, and subsequent degradation of cartilage matrix. In particular, this cartilage degradation has long been explained by the actions of extracellularly secreted matrix metalloproteinases released from synoviocytes and chondrocytes stimulated by several cytokines, including IL-1 and TNF-α[9,10]. It has been reported that the majority of rheumatoid synovial cells consist of 3 different cell types: spindle-shaped FLS (Thy-1+), dendritic-like synoviocytes (DLS), and large, round MLS (CD68+). FLS is a major part of the invasive pannus, a vascular and fibrous granulation tissue arising from the joint recesses and extending onto the surface of cartilage. Activation of FLS in vitro generates several functional responses that may considerably contribute to joint pathology in RA; that is, the production of matrix components, soluble mediators, or matrix-degrading enzymes[3,11]. There are 2 types of synoviocytes, divided on the basis of their ultrastructure. Type A synoviocytes are derived from MLS and type B synoviocytes derive from FLS. Golgi bodies in type A synoviocytes mainly take part in secretory functions, whereas the mitochondrion is the energy provider for cell metabolism. In type B synoviocytes, however, the rough endoplasmic reticulum is where proteins are processed and secreted. We also found that the morphology of type A synoviocytes and type B synoviocytes from rats with AA was changed, accompanying an increase in the level of IL-1, TNF-α, and PGE2 produced by synoviocytes in these rats[12]. In the present study we showed that the secretion and metabolism of synoviocytes from AA rats became hyperfunctional and IL-1ra amended the morphological changes in synoviocytes. It is well known that the activation of monocytes/macrophages plays a critical role in inducing inflammatory responses, including AA. Macrophages and macrophage-like synoviocytes stimulated by LPS or other inflammatory factors release large quantities of various proinflammatory cytokines including TNF, IL-1, PGE[12-14]. p38 and ERK play important roles in LPS-induced signaling in macrophages[15]. Results from the present study also showed that MLS in vitro produced excessive amounts of pro-inflammatory cytokines, including IL-1, TNF-α, and PGE2 stimulated by LPS, and that those cytokines could be inhibited by IL-1ra.
In vitro, MLS and FLS are major sources of several pro-inflammatory cytokines, such as IL-1, IL-6, TNF-α, which promote the induction of adhesion molecules and proteinase gene expression, and play a major role in the progression of joint destruction and synovial proliferation[16]. It has been reported that culture supernatants from synoviocytes obtained from patients with RA have mitogenic activity[17]. Recent reports have shown that Ras-MAPK signaling pathways are involved in the activation of FLS and the destruction of bone in arthritic joints induced by IL-1[18]. MAPK activation is detected in synovial tissue from RA. ERK activation is localized around synovial microvessels, JNK activation is localized around and within mononuclear cell infiltrates, and p38 MAPK activation is observed in the synovial lining layer and in synovial endothelial cells[19]. Signaling through stress- and mitogen-activated protein kinase (SAPK/MAPK) pathways in synoviocytes, which is induced by IL-1 and TNF-α, is a typical feature of chronic synovitis in RA[19,20]. However, during signal transduction of IL-1 in RA FLS, tyrosine phosphorylation is increased transiently, and the MAPK cascade is activated in a few minutes[21].
It has been reported that IL-1α, IL-1β, and IL-1ra are present in fresh and cultured synovial cell samples of synovium from RA. However, the IL-1ra:IL-1 ratios ranged from 1.2 to 3.6, which is below the 10‒100-fold excess of IL-1ra needed to inhibit IL-1 bioactivity[22]. Hence the induced endogenous production of IL-1ra, in the presence of RA synovitis, is too low to compete with the high affinity of IL-1 for the cell receptors. Therefore, the presence of IL-1ra should result in very effective prevention of IL-1 signal transduction, particularly in the inflammatory site. In laboratory and animal studies, inhibition of IL-1 by either antibodies to IL-1 or IL-1ra proved beneficial to the outcome[23]. It has been reported that FR167653, an inhibitor of IL-1 and TNF, inhibits the phosphorylation of p38 MAPK[24]. However, there is little information about the effects of IL-1ra on MAPK phosphorylation in the synovial membrane in RA or AA.
In the present study, the phosphorylation of ERK, JNK, and p38 was enhanced not only in the synovial membranes of AA rats but also in AA FLS stimulated by supernatant of MLS from AA rats. Accordingly, the proliferation of FLS activated by supernatant of MLS from AA rats was also enhanced. IL-1ra decreased the phosphorylation of ERK, JNK, and p38 in the synovial membrane of AA rats. In addition, the supernatant of MLS that came from AA rats treated with IL-1ra and contained fewer proinflammatory cytokines induced less MAPK phosphorylation and cell proliferation in AA FLS. The results of the present study indicate that IL-1ra inhibits AA through suppression of the cytokine-signaling pathways between different types of synoviocytes.
In summary, a variety of signals generated by cytokines may play an important role in the development of AA. From the present study, we conclude that IL-1ra exerts an anti-inflammatory effect by modulating the ultrastructure of synoviocytes, decreasing the production of proinflammatory mediators by MLS, and inhibiting the phosphorylation of MAPK in FLS.
References
- Tehranzadeh J, Ashikyan O, Dascalos J, Dennehey C. Advanced imaging of early rheumatoid arthritis. Magn Reson Imaging Clin N Am 2004;12:707-26.
- Hunter D, Allaire S. Combination disease-modifying antirheumatic drug therapy reduced work disability in early rheumatoid arthritis. Arthritis Rheum 2004;50:55-62.
- Zimmermann T, Kunisch E, Pfeiffer R, Hirth A, Stahl HD, Sack U, et al. Isolation and characterization of rheumatoid arthritis synovial fibroblasts from primary culture: primary culture cells markedly differ from fourth-passage cells. Arthritis Res 2001;3:72-6.
- Gabay C, Arend WP. Treatment of rheumatoid arthritis with IL-1 inhibitors. Springer Semin Immunopathol 1998;20:229-46.
- Schiff MH. Role of interleukin 1 and interleukin 1 receptor antagonist in the mediation of rheumatoid arthritis. Ann Rheum Dis 2000;59:103-8.
- Jacobson PB, Morgan SJ, Wilcox DM, Nguyen P, Ratajczak CA, Carlson RP, et al. A new spin on an old model: in vivo evaluation of disease progression by magnetic resonance imaging with respect to standard inflammatory parameters and histopathology in the adjuvant arthritic rat. Arthritis Rheum 1999;42:2060-73.
- Ding CH, Li Q, Xiong ZY, Zhou AW, Jones G, Xu SY. Oral administration of type II collagen suppresses pro-inflammatory mediators production by synoviocytes in rats with adjuvant arthritis. Clin Exp Immunol 2003;132:416-23.
- Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-54.
- Walakovits LA, Moore VL, Bhardwaj N, Gallick GS, Lark MW. Detection of stromelysin and collagenase in synovial fluid from patients with rheumatoid arthritis and posttraumatic knee injury. Arthritis Rheum 1992;35:35-42.
- Langdon C, Leith J, Smith F, Richard CD. Oncostatin M stimulates monocyte chemoattractant protein-1- and interleukin-1-induced matrix metalloproteinase-1 production by human synovial fibroblasts in vitro. Arthritis Rheum 1997;40:2139-46.
- Neidhart M, Seemayer CA, Hummel KM, Michel BA, Gay RE, Gay S. Functional characterization of adherent synovial fluid cells in rheumatoid arthritis: destructive potential and . Arthritis Rheum 2003; 48: 1873-80.
- Dai M, Wei W, Shen YX, Zheng YQ. Glucosides of Chaenomeles speciosa remit rat adjuvant arthritis by inhibiting synoviocyte activities. Acta Pharmacol Sin 2003;24:1161-6.
- Xu SJ, Gao WJ, Cong B, Yao YX, Gu ZY. Effect of lipopolysaccharide on expression and characterization of cholecystokinin receptors in rat pulmonary interstitial macrophages. Acta Pharmacol Sin 2004;25:1347-53.
- Li WD, Ran GX, Teng HL, Lin ZB. Dynamic effects of leflunomide on IL-1, IL-6, and TNF-α activity produced from peritoneal macrophages in adjuvant arthritis rats. Acta Pharmacol Sin 2002;23:752-6.
- Olsson S, Sundler R. The role of lipid rafts in LPS-induced signaling in a macrophage cell line. Mol Immunol 2005.16. [Epub ahead of print].
- Nanki T, Nagasaka K, Hayashida K, Saita Y, Miyasaka N. Chemokines regulate IL-6 and IL-8 production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. J Immunol 2001;167:5381-5.
- Melnyk VO, Shipley GD, Sternfeld MD, Sherman L, Rosenbaum JT. Synoviocytes synthesize, bind, and respond to basic fibroblast growth factor. Arthritis Rheum 1990;33:493-500.
- Yamamoto A, Fukuda A, Seto H. Suppression of arthritic bone destruction by adenovirus-mediated dominant-negative Ras gene transfer to synoviocytes and osteoclasts. Arthritis Rheum 2003;48:2682-92.
- Schett G, Tohidast-Akrad M, Smolen JS, Schmid BJ, Steiner CW, Bitzan P, et al. Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-JUN N-terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthritis Rheum 2000;43:2501-12.
- Foster ML, Halley F, Souness JE. Potential of p38 inhibitors in the treatment of rheumatoid arthritis. Drug News Perspect 2000;13:488-97.
- Lu H, Sun T, Yao L, Zhang Y. Role of protein tyrosine kinase in IL-1 beta induced activation of mitogen-activated protein kinase in fibroblast-like synoviocytes of rheumatoid arthritis. Chin Med J 2000;113:872-6.
- Firestein GS, Boyle DL, Yu C, Paine MM, Whisenand TD, Zvaifler NJ, et al. Synovial interleukin-1 receptor antagonist and interleukin-1 balance in rheumatoid arthritis. Arthritis Rheum 1994;37:644-52.
- Cutolo M. IL-1 Ra: its role in rheumatoid arthritis. Reumatism 2004;56:41-5.
- Yao HW, Yue L, Chen JQ. Effects and mechanisms of FR167653, a dual inhibitor of interleukin-1 and tumor necrosis factor, on adjuvant arthritis in rats. Int Immunopharmacol 2004; 4: 1625-32.
