Biosynthesis of imipramine glucuronide and characterization of imipramine glucuronidation catalyzed by recombinant UGT1A41
Introduction
In humans, the metabolism of a number of tertiary amine-containing pharmacological agents to quaternary ammonium-linked glucuronides, catalyzed by uridine 5-diphospho-glucuronosyltransferases (UGT), represents a unique and important metabolic pathway for these compounds[1]. UGT1A4 is an important enzyme for the formation of quaternary ammonium-linked glucuronides, and recombinant UGT1A4 glucuronidates tertiary amine substrates such as imipramine, cyproheptadine, tripelennamine, and chlorpromazine[2].
Imipramine is a tricyclic antidepressant that is used for treating the symptoms of major depression. It is the probe substrate of UGT1A4, and is used as a competitor in studies of drugs metabolized by UGT1A4[3–5] . Kinetic studies of imipramine glucuronidation in vivo and via human liver microsomes have been previously carried out[3,6,7].
The baculovirus insect cell system is an efficient expression system for the production of recombinant UGT. A series of UGT produced using this system have been used to screen drug metabolism in vitro. The kinetics of imipramine N+-glucuronidation via UGT1A4 have not yet been studied in detail. Therefore, we used a convenient and efficient method to characterize the activity of recombinant UGT1A4 and to study the imipramine N+-glucuronidation profile in vitro.
Materials and methods
Chemicals and reagents The bacmid and the vectors used to produce the recombinant UGT were purchased from Invitrogen (Beijing, China). The sf9 cell strain was a gift from Hainan Yangshengtang Pharmaceutical Co (China). The imipramine hydrochloride (pure powder) was a gift from Siwei Pharmaceutical Co (China). The β-glucuronidase enzyme from Escherichia coli, uridine 5-diphosphoglucuronic acid (UDPGA), alamethicin, saccharolactone and p-nitrophenol were purchased from Sigma (St Louis, MO, USA). The Discovery DSC-18 cartridges (500 mg, 3 mL) were supplied by Supelco (Bellefonte, PA, USA). All other chemicals and solvents were of analytical grade or high performance liquid chromatography (HPLC) grade and were obtained from standard commercial sources.
Chromatography Chromatographic analysis was performed on a Shimadzu LC-10ATvp system (Kyoto, Japan), equipped with an LC-10AT pump, an SCL-10A system controller, and an SPD-10A UV detector. A Diamonsil C18 column (5 µm particle size, 200 mm×4.6 mm) was used in this assay. The mobile phase consisted of 31% acetonitrile and 69% 0.01 mol/L KH2PO4 in distilled water, with the pH being adjusted to 2.5 by adding phosphoric acid dropwise. The mobile phase was filtered via a 0.45 µm membrane and degassed. The flow rate was set at 1 mL/min and the elution solution was monitored at 254 nm with a UV absorption detector.
Preparation of recombinant UGT1A4 homogenate Recombinant UGT1A4 homogenate was prepared as described elsewhere[8]. Briefly, sf9 cells infected with recombinant baculovirus were subjected to 3 rounds of freeze-thawing, resuspended in phosphate-buffered saline (1×PBS; pH 7.4) and sonicated with 5 s bursts, allowing at least 1 min on ice between bursts. Then the protein concentration of the homogenate was determined using Lowry’s method, with bovine serum albumin as a standard.
Enzymatic activity assay Because the activity of recombinant UGT1A4 was highest at pH 8.4 within the range from 6.0 to 9.0 in this experiment, imipramine was added as a substrate into 100 µL of the incubation solution, which contained 50 mmol/L Tris-HCl (pH 8.4), 10 mmol/L MgCl2, 8.5 mmol/L saccharolactone, 0.02 g/L alamethicin and 2 g/L cell homogenate containing UGT1A4. It is known that the production of imipramine glucuronide is linear for protein concentrations ranging from 0.5 to 2 g/L. After pre-incubation at 37 °C for 5 min, 0.5 µmol UDPGA was added as a cofactor to start the reaction. The enzymatic reaction was stopped by adding 70 µL methanol and 30 µL internal standard (0.02 g/L p-nitrophenol in ethanol) at the designated time at 37 °C in a shaking water bath. The incubation solution was centrifuged at 16 000×g for 20 min. A 20 µL aliquot of the supernatant was analyzed by reversed phase (RP)-HPLC.
Preparation and purification of imipramine N+-glucuronide The volume of incubation solution was amplified to 100 mL and the incubation concentration of imipramine was 2 mmol/L. Twofold the volume of CHCl3 was added after 2 h incubation. After the mixture was vortexed, the protein was precipitated by centrifugation and the remaining imipramine was extracted with CHCl3. The aqueous fraction was transferred into a fresh tube and added to twofold the volume of CHCl3 again. The procedure was repeated to completely detach the remaining imipramine, then this was confirmed with HPLC. Solid-phase extraction was used to purify the imipramine N+-glucuronide[9–11]. The aqueous phase was loaded onto Discovery DSC-18 cartridges. Each cartridge was then washed with 6 mL deionized water twice, followed by 3 mL 30% methanol and 70% methanol, respectively. Finally the imipramine N+-glucuronide was eluted by 2 mL methanol. The final elution solution was vacuum dried, and the residue was reconstituted with 900 µL deionized water; this solution was then used as the standard stock solution for the imipramine N+-glucuronide.
Characterization of imipramine N+-glucuronide by LC-MS The HPLC-mass spectrometer (MS) (Esquire, Bruker, Germany) was equipped with an electrospray mass spectrometric detector. The same column was used, and the mobile phase consisted of acetonitrile/acetic acid solution (31/69, v/v; pH 4.5) with a flow rate of 0.4 mL/min[12].
Quantification of imipramine N+-glucuronide by hydrolysis with β-glucuronidase Four microliters of the imipramine N+-glucuronide solution and 4 µL of the β-glucuronidase solution (4000 U/mL) were added into 192 µL of PBS (pH 5.0)[11]. After incubation at 37 °C for 24 h, 40 µL of p-nitrophenol solution (0.1 g/L in methanol) and 160 µL methanol were added into the solution. Three replicate samples and three control samples without incubation were examined. The solutions were vortexed for 20 s and centrifuged at 16 000×g for 20 min. A 20 µL aliquot was injected onto the column. The imipramine N+-glucuronide was completely hydrolyzed to imipramine and the content of imipramine was assayed accurately by using the HPLC method described herein. The peak area ratio of imipramine in the hydrolytes versus p-nitrophenol was compared using the calibration curve prepared using imipramine.
Results
Characterization of imipramine N+-glucuronide by RP-HPLC and LC-MS Chromatographic separation of imipramine N+-glucuronide, p-nitrophenol and imipramine was excellent, with no interfering peaks from the cell homogenate. Typical chromatograms of the incubate are shown in Figure 1. Chromatograms of control samples, including the incubate solutions without enzyme or substrate, also indicated that there were also no interfering peaks (data not shown).

The peak at m/z 281 corresponded to protonated imipra- mine (Figure 2A). The API-electrospray (API-ES) mass spectrum of a peak typically formed by incubation of imipramine with the recombinant UGT1A4 is shown in Figure 2B. The [M+ H] + ionic peak at m/z 457 corresponded to imipramine N+-glucuronide.
Preparation of the calibration curve of imipramine A standard stock solution of imipramine hydrochloride was prepared by dissolving pure imipramine hydrochloride in deionized water to a concentration of 10 mmol/L. A series of standard solutions (5, 10, 25, 50, 75, and 100 µmol/L) were prepared by diluting the stock solution with PBS (pH 5.0). The calibration curve was constructed according to the procedure described in the Materials and Methods section. We found that imipramine concentrations were linearly related to imipramine versus p-nitrophenol area ratios over the range studied, with a correlation coefficient of >0.999. The equation obtained by using least squares linear regression was: y =0.039x– 0.0728. The limit of detection (LOD) and the limit of quantitation (LOQ) were measured by carrying out stepwise dilutions of the samples at known concentrations. The results indicated that LOD was 0.05 µmol/L and LOQ was 0.5 µmol/L [Relative Standard Deviation (RSD<7%); n=5] for this assay.
Quantification of imipramine N+-glucuronide by hydrolysis Imipramine N+-glucuronide was hydrolyzed thoroughly by β-glucuronidase after 24 h incubation (Figure 3). According to the calibration curve for imipramine, the area ratios for imipramine after hydrolysis versus p-nitrophenol were used to calculate the concentration of the imipramine that was transformed from imipramine N+-glucuronide by hydrolysis. The concentration of the stock imipramine N+-glucuronide solution was calculated as being 1.37 mmol/L.

Preparation of the calibration curve of imipramine N+-glucuronide Imipramine N+-glucuronide was spiked into the incubation solution containing the inactivated UGT1A4 to make a series of standard concentrations (1.24, 2.26, 4.52, 9.04, 13.70, 18.22, and 22.74 µmol/L). Then the samples were mixed with 70 µL methanol and 30 µL internal standard (0.02 mg/mL p-nitrophenol). After removal of the protein by centrifugation at 16 000×g for 20 min, a 20 µL aliquot of supernatant was injected into HPLC for analysis. The results indicated that the imipramine N+-glucuronide concentrations were linearly related to the imipramine N+-glucuronide versus p-nitrophenol area ratios over the range studied, with a correlation coefficient of >0.999. The equation obtained by least squares linear regression was y=0.1942x–0.0562. The LOD and the LOQ were measured by carrying out stepwise dilutions of the samples at known concentrations. The results indicated that LOD was 0.04 µmol/L and LOQ was 0.4 µmol/L (RSD <7%; n=5) for this assay.
Recovery and precision A series of hydrolysis solutions, spiked with various amounts of imipramine, were processed according to the procedure described in the Materials and Methods section. The peak area ratios of imipramine and the internal standard were compared with the calibration curve of imipramine. The results of the recovery assay are shown in Table 1. Another series of solutions containing the inactivated recombinant UGT1A4, added with various amounts of imipramine N+-glucuronide, were processed as described in the Materials and Methods section. The peak area ratios of imipramine N+-glucuronide versus the internal standard were compared with the calibration curve of imipramine N+-glucuronide. The result of the recovery assay is shown in Table 2. For these two series of assays, intra-assay variability was determined by analyzing 5 replicate samples, and inter-assay variability was determined by analyzing samples on 5 separate days.

Full table

Full table
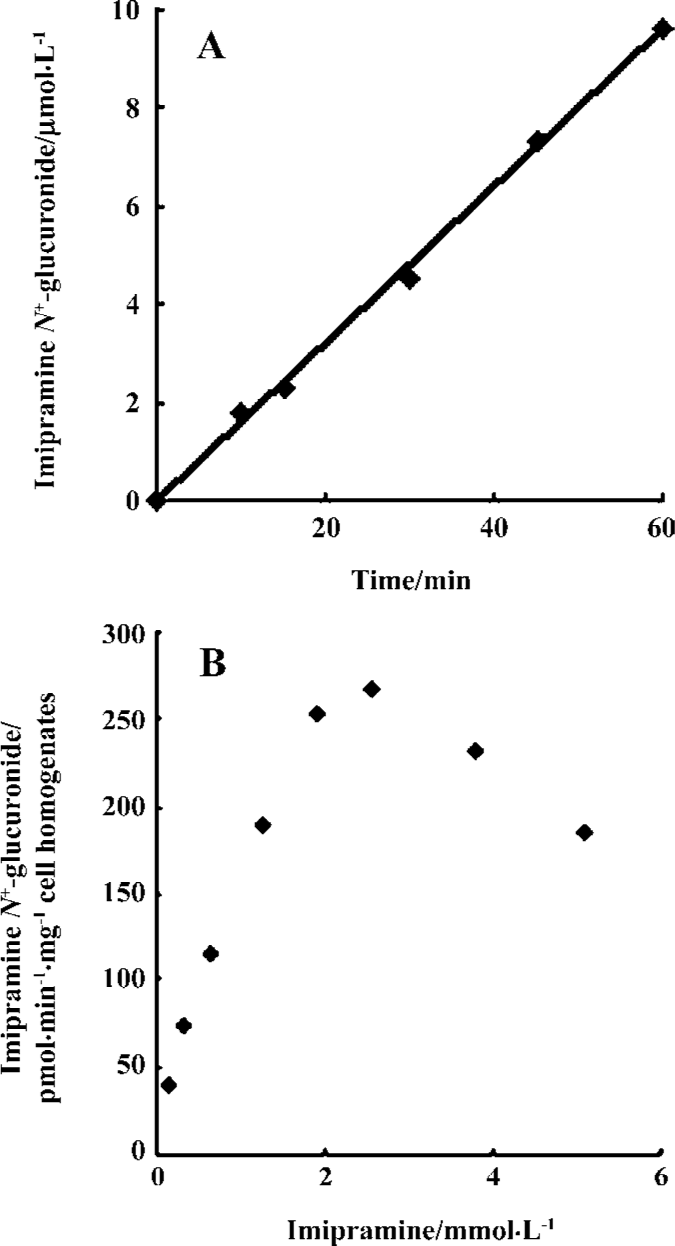
Enzymatic activity assay of the recombinant UGT1A4 Imipramine as substrate was added into 0.1 mL incubation solutions containing recombinant UGT1A4. For the time-course experiment, the samples were incubated at a concentration of 0.50 mmol/L imipramine at 37 °C for 10, 15, 30, 45, or 60 min. For the dose-course experiment, the samples were incubated for 30 min at a series of concentrations that ranged from 0.16 mmol/L to 5.09 mmol/L. Three replicate samples were examined for every time point and concentration. Then the assays were performed according to the procedure described earlier. Finally, the metabolite was detected and quantified according to the calibration curve of imipramine N+-glucuronide. It was demonstrated that imipramine N+-glucuronide was produced linearly over the time range studied (Figure 4A). The metabolite decreased when the substrate concentration was more than 2 mmol/L (Figure 4B); the parameters were calculated according to the formula V=Vmax· S/(Km+S+S2/Ki) by using MATLAB software. The value of Km was 1.39±0.09 mmol/L, Ki was 6.24±0.45 mmol/L and Vmax was 453.81±32.12 pmol/min per mg cell protein (n=3).
Discussion
The biotransformation of quaternary ammonium N+-glucuronidation has been characterized in humans for more than 30 drugs and xenobiotics, including antihistamines, antidepressants, and antipsychotics[13]. Glucuronidation is generally considered to be a pathway of detoxification, which commonly transforms lipophilic compounds into hydrophilic metabolites. However, quaternary ammonium N+-glucuronides are suspected to be of toxic, despite a lack of evidence either way. In one experiment concerning the toxicity of amitriptyline N+-glucuronide, after one healthy female volunteer was given an iv infusion of amitriptyline N+-glucuronide (17.5 mg, 38.5 mmol), she became flushed and developed tachycardia 25 min later[14]. In another study, it was found that chlorpromazine, amitriptyline, imipramine, promethazine and cyproheptadine were potent inhibitors of the glucuronidation of testosterone, androsterone, estriol and 1-naphthol, respectively, with steroid activities being more susceptible to inhibition (up to 90%)[15]. This may be connected with the pathogenesis of adverse drug reactions.
It has been reported that the quaternary ammonium N+-glucuronides are mainly catalyzed via UGT1A3 and UGT1A4 in humans[1,2,16]. The sequence of the UGT1A3 protein is 93% identical to that of UGT1A4. However, UGT1A3 has a higher apparent Km than UGT1A4, and the expression level of UGT1A3 is low in human liver[1,16]. So UGT1A4 is considered to contribute greatly to N+-glucuronidation in humans. Further supporting this conclusion is the fact that imipramine was also incubated with recombinant UGT1A3 in the present study, but no metabolite was detected (data not shown).
N+-glucuronidation is a pathway specific to humans, which does not occur in rats. An explanation for this lack in rats is that the corresponding gene of UGT1A4 in rats is a pseudo-gene, and does not code for a full-length UGT protein[16]. Therefore it should be noted that if pharmacokinetic data for the metabolism of these tertiary drugs are obtained from rats, the possible metabolites of N+-glucuronide existing in humans will be ignored.
In a study of imipramine glucuronidation using human liver microsomes, the Eadie-Hofstee plots of imipramine N+-glucuronidation in human liver microsomes were biphasic. Compared with the Km obtained from human liver microsomes, the apparent Km from baculovirus-expressed UGT1A4 was higher than the low affinity Km of 0.70±0.29 mmol/L[3]. This different result may be attributed to the existence of different protein structure in the recombinant and non-recombinant enzymes in humans: the enzymes expressed by baculovirus insect cells have a different glycosylation course from those produced in mammalian cells. In addition, the pH of the incubation solutions used by Nakajima et al in their experiment using human liver microsomes was 7.4[3], lower than the 8.4 used in the present experiment, which may have contributed to the different pharmacokinetics. So the data gathered in vitro should be considered as reference data only for studies in vivo. Figure 4 shows that a high concentration of imipramine inhibits glucuronidation. This is similar to the effects of clozapine, which is reported to inhibit glucuronidation by UGT1A4 at high concentration[17]. Considering that the 2 drugs have a similar tricyclic structure, whether substrates with a similar molecular structure have similar substrate protein interactions in glucuronidation is worth studying further.
In conclusion, a simple and effective RP-HPLC method was established for determining the concentration of imipramine and imipramine N+-glucuronide, and characterizing the activity of recombinant UGT1A4. The kinetics of imipramine N+-glucuronidation using recombinant enzymes are different from those noted using human liver microsomes. For unique quaternary ammonium N+-glucuronidation in humans, there is potential for more tertiary drugs to be screened by using the recombinant enzyme, which may reveal some valuable information.
References
- Green MD, Tephly TR. Glucuronidation of amine substrates by purified and expressed UDP-glucuronosyltransferase proteins. Drug Metab Dispos 1998;26:860-7.
- Green MD, Bishop WP, Tephly TR. Expressed human UGT1.4 protein catalyzes the formation of quaternary ammonium-linked glucuronides. Drug Metab Dispos 1995;23:299-302.
- Nakajima M, Tanaka E, Kobayashi T, Ohashi N, Kume T, Yokoi T. Imipramine N-glucuronidation in human liver microsomes: biphasic kinetics and characterization of UDP-glucuronosyltrans-ferase isoforms. Drug Metab Dispos 2002;30:636-42.
- Guengerich FP, Parikh A, Johnson EF, Richardson TH, von Wachenfeldt C, Cosme J, et al. Heterologous expression of human drug-metabolizing enzymes. Drug Metab Dispos 1997;25:1234-41.
- Kuehl GE, Murphy SE. N-glucuronidation of nicotine and cotinine by human liver microsomes and heterologously expressed UDP-glucuronosyltransferases. Drug Metab Dispos 2003;31:1361-8.
- Coughtrie MW, Sharp S. Glucuronidation of imipramine in rabbit and human liver microsomes: assay conditions and interaction with other tertiary amine drugs. Biochem Pharmacol 1991;42:1497-501.
- Nielsen KK, Brosen K. High-performance liquid chromatography of imipramine and six metabolites in human plasma and urine. J Chromatogr 1993;612:87-94.
- Qian M, Chen S, Li X, Zeng S. Cloning and expression of human UDP-glucuronosyltransferase 1A4 in Bac-to-Bac system. Bio-chem Biophys Res Commun 2004;319:386-92.
- Tugnait M, Ghauri FY, Wilson ID, Nicholson JK. NMR-monitored solid-phase extraction of phenolphthalein glucuronide on phenylboronic acid and C18 bonded phases. J Pharm Biomed Anal 1991;9:895-9.
- Luan LJ, Shao Q, Zhang XH, Zeng S. Effects of microsome enzyme induced by phenobarbarbital on the stereoselectivity of recemic propranolol glucuronidation metabolism. Zhejiang Da Xue Xue Bao Yi Xue Ban 2004;33:7-10.
- Yu LS, Luan LJ, Shao Q, Zeng S. Direct determination of S-(-)- and R-(+)-propranolol glucuronide in rat hepatic microsomes by RP-HPLC. Biomed Chromatogr 2004;18:833-7.
- Kowalczyk I, Hawes EM, McKay G. Stability and enzymatic hydrolysis of quaternary ammonium-linked glucuronide metabolites of drugs with an aliphatic tertiary amine-implications for analysis. J Pharm Biomed Anal 2000;22:803-11.
- Hawes EM. N+-glucuronidation, a common pathway in human metabolism of drugs with a tertiary amine group. Drug Metab Dispos 1998;26:830-7.
- Breyer-Pfaff U, Becher B, Nusser E, Nill K, Baier-Weber B, Zaunbrecher D, et al. Quaternary N-glucuronides of 10-hydroxylated amitriptyline metabolites in human urine. Xenobiotica 1990;20:727-38.
- Sharp S, Mak LY, Smith DJ, Coughtrie MW. Inhibition of human and rabbit liver steroid and xenobiotic UDP-glucuronosyl-transferases by tertiary amine drugs implications for adverse drug reactions. Xenobiotica 1992;22:13-25.
- Green MD, King CD, Mojarrabi B, Mackenzie PI, Tephly TR. Glucuronidation of amines and other xenobiotics catalyzed by expressed human UDP-glucuronosyltransferase 1A3. Drug Metab Dispos 1998;26:507-12.
- Mori A, Maruo Y, Iwai M, Sato H, Takeuchi Y. UDP-glucuronosyl-transferase 1A4 polymorphisms in a Japanese population and kinetics of clozapine glucuronidation. Drug Metab Dispos 2005;33:672-5.

