Engagement of PSGL-1 enhances β2-integrin-involved adhesion of neutrophils to recombinant ICAM-11
Introduction
Inflammation is a multiple-step process initiated by the adhesion of leukocytes to the activated endothelial cells. The recruitment of leukocytes from the flowing bloodstream is a rapid event that requires special mechanisms to establish the contact of leukocyte with endothelium. Selectins represent a class of cell adhesion molecules that are specialized for this purpose[1]. P-selectin glycoprotein ligand 1 (PSGL-1), the best characterized ligand of the selectins, has been demonstrated to mediate the adhesion of leukocytes to all three selectins in vivo[2–5].
PSGL-1 is a highly extended mucin expressed on microvilli of almost all types of leukocytes. In addition to its direct role in the capture of leukocytes from the bloodstream, PSGL-1 has also been demonstrated to function as a signal-transduced receptor and initiate a series of intracellular signal events during the activation of leukocytes, including activation of β2-integrin[6–8], tyrosine-protein phosphorylation[9,10], secretion of cytokines[9] and transcriptional activation[11]. Our previous work suggests that PSGL-1-mediated signals induce the alteration of F-actin-based cytoskeleton in neutro-phils, and non-receptor tyrosine kinase c-Abl may be involved in the process[12]. A recent study showed that PSGL-1 stimulated lymphocyte function-associated antigen (LFA)-1-mediated Th1 cell binding to intercellular adhesion molecule-1 (ICAM-1)[13].
In the present work, we demonstrated that the engagement of PSGL-1 with mAb or P-selectin induced the increased expression of β2-integrin and enhanced the adhesion of neutrophils to the recombinant ICAM-1. TS1/18, the monoclonal antibody against CD18, impaired the adhesion of the PSGL-1-engaged neutrophils to the ICAM-1, suggesting the contribution of β2-integrins to the adhesion of neutrophils to ICAM-1 is caused by the engagement of PSGL-1. Moreover, cytochalasin B (an inhibitor for the assembly of F-actin-based cytoskeleton) largely blocked the increased expression of β2-integrin and the adhesion of PSGL-1-engaged neutrophils to ICAM-1, which implies an important role of the F-actin-based cytoskeleton. Our combined results suggest that the PSGL-1-transduced signal can enhance β2-integrin-involved adhesion of neutrophils to the recombinant ICAM-1 through the increase of β2-integrin expression, and this process depends on the dynamics of the F-actin-based cytoskeleton.
Materials and methods
Recombinant proteins, antibodies and reagents The recombinant human P-selectin (ADP3) and ICAM-1 (ADP4) were purchased from R&D Systems (Minneapolis, MN, USA).
PL2 (the non-blocking mAb against PSGL-1, mouse IgG1, sc-18856) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). W6/32 (the anti-HLA-A,B,C mAb, mouse IgG1, 14-9983) was purchased from Ebioscience (San Diego, CA, USA). 9E1 (the mAb recognizing the extra-cellular domain of recombinant human P-selectin, mouse IgG1, BBA30) was purchased from R&D Systems. TS1/18 (the mAb against CD18, which can block the ligation of β2-integrin with ICAM-1, mouse IgG1, MA1810) was purchased from Pierce Biotechnology (Rockford, IL, USA). Fluorescein isothiocynate (FITC)-conjugated β2-integrins antibody IB4 was purchased from Ancell Corporation (Bayport, MN, USA) (167-040). Dextran T-500 used for neutrophil isolation was purchased from Pharmacia (Uppsala, Sweden). Cytochalasin B (an inhibitor for assembly of F-actin) was purchased from Sigma (Saint Louis, MO, USA).
Neutrophil isolation Neutrophils were isolated by standard procedures after sedimentation of erythrocytes by dextran T-500 and centrifugation of leukocytes over Ficoll-Hypaque gradients as described in our previous work[12]. After lysis of contaminating erythrocytes with hypotonic saline (0.17 mol/L Tris, 0.16 mol/L NH4Cl, pH 7.2), the neutrophils were washed and re-suspended in cold PBS containing 0.1% bovine serum albumin (BSA). The isolated neutrophils were adjusted at a concentration of 0.5×106 cells/mL and stored at 4 ºC until use. More than 95% of the isolated cells were polymorphonuclear leukocytes, and viability was determined to be 98% by Trypan blue exclusion.
Engagement of PSGL-1 Preceding the interaction with ICAM-1 monolayer, the isolated neutrophils were ligated with PL2, an mAb against PSGL-1 (or control antibody W6/32) at a concentration of 10 µg/mL at 4 ºC for 20 min. In other experiments, the isolated neutrophils were subjected to flow on the P-selectin/ICAM-1 monolayer to encounter and engage with P-selectin.
Monolayer preparation To prepare the ICAM-1 monolayer, the petri dishes were coated with 3 µg/mL recombinant ICAM-1 overnight and then blocked with 1% BSA at 37 ºC for 2 h, and in control groups, recombinant ICAM-1 was substituted with BSA. In other experiments, the petri dishes were simultaneously coated with 3 µg/mL of each recombinant P-selectin and ICAM-1 overnight and then blocked with 1% BSA at 37 ºC for 2 h.
Cell adhesion assay The adhesion of PSGL-1-engaged neutrophils to the recombinant ICAM-1 monolayer was detected by using a parallel-plate flow chamber (GlycoTech, Rockville, MD, USA). Briefly, the neutrophils were washed twice and re-suspended in RPMI 1640 medium. At a shear stress of 1.2 dyn/cm2, cells were infused over the ICAM-1 (or P-selectin/ICAM-1) monolayer via a syringe pump[14,15]. Interactions of neutrophils with monolayer were allowed by stopping the flow at different times, after which the flow was carried out again at a shear stress of 1.2 dyn/cm2. The adherent cells could then be counted by using an inverted microscope (Olympus Optical, Tokyo, Japan) equipped with a camera (Panasonic, Yokohama, Japan) connected to a VCR and a TV monitor. Each data represented the percentage of the number of remaining cells visible when flow was re-infused to that of all halted cells after flow was stopped in 10 fields of view under a ×10 objective.
For antibody blocking experiments, neutrophils (or antibody-engaged neutrophils) were treated with TS1/18 at a concentration of 10 µg/mL at 4 ºC for 15 min preceding being infused on the monolayer. In other experiments the P-selectin/ICAM-1 monolayer was blocked with 9E1, the mAb recognizing the extra-cellular domain of recombinant human P-selectin, at a concentration of 10 µg/mL at 4 ºC for 15 min to block the engagement of PSGL-1 with P-selectin.
For inhibition experiments, the isolated neutrophils were treated with 20 µmol/L cytochalasin B at 4 ºC for 30 min preceding the engagement of PSGL-1 and the interaction with the ICAM-1 (or P-selectin/ICAM-1) monolayer. The control cells were treated with the equivalent volume of dimethyl sulfoxide (DMSO).
Fluorescence assay To examine the expression of β2-integrins on the surface of neutrophils, cells (1×106/mL) were treated with (or without) PL2 (10 µg/mL) at 37 ºC for 30 min, then washed with PBS twice before being fixed with 4% paraformaldehyde for 20 min at room temperature. The expression of CD18 was visualized by staining with FITC-conjugated β2-integrins antibody IB4 (10 µg/mL) for 30 min. The levels of surface expression of β2-integrins were detected with Fluorescence Reader Gemini EM (Molecular Device Corporation, Sunnyvale, CA, USA). The well was set with an equal number of cells, without antibody treatment and FITC-labeled CD18 antibody IB4 staining as blank, the data of all sample wells were normalized by the value of blank. The data indicate the OD values of the detector’s absorbance of the fluorescence that samples emit, and each data represents the mean±SD of three independent experiments and the significant difference from the control was determined by ANOVA (P<0.01).
Results
Engagement of PSGL-1 enhanced the adhesion of neutrophils to recombinant ICAM-1 We set up an in vitro flow system to investigate the contribution of signals transduced by PSGL-1 to the activation of neutrophils and the mechanism by which the interaction of PSGL-1 with P-selectin enhances the adhesion of neutrophils to ICAM-1 expressed on the surface of activated endothelial cells during inflammation.
First, recombinant ICAM-1 was coated as a monolayer on the petri dish bottom. After engagement with (or without) antibodies, the neutrophils were infused in the flow system at a shear stress of 1.2 dyn/cm2. The adhesion of neutrophils to the recombinant ICAM-1 was allowed for different times. The percentage of adherent cells for those engaged with PL2 was significantly higher compared with that of resting, Mo-IgG-treated or W6/32-treated neutrophils (Figure 1A). The extreme lack of adhesion for those differently treated neutrophils to BSA-coated petri dishes implied the specificity of the interaction of engagement-activated neutrophils to the ICAM-1 monolayer (Figure 1B).
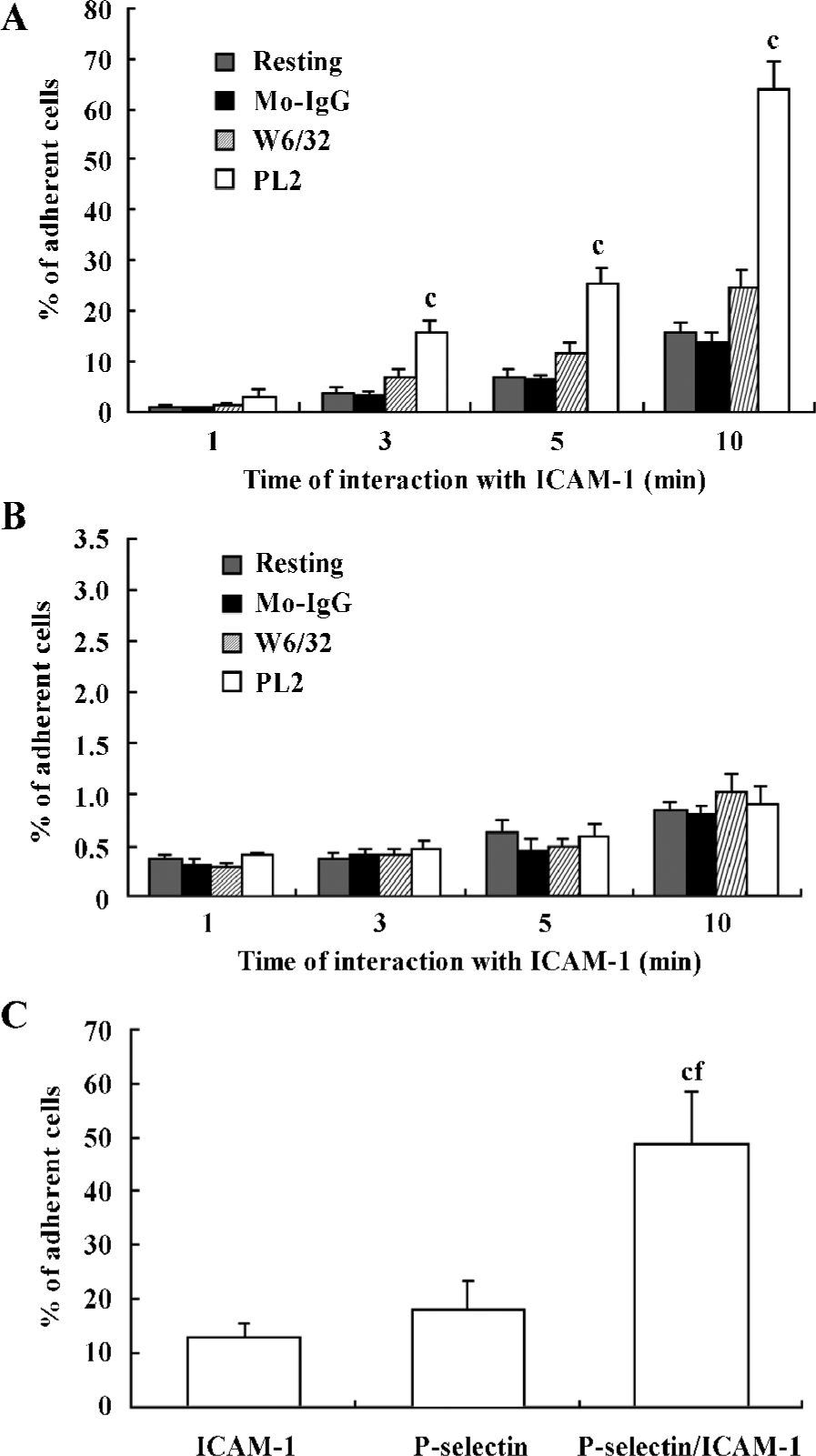
Second, the isolated neutrophils were infused by flow on the petri dish bottom, which was coated with P-selectin, ICAM-1, or both P-selectin and ICAM-1 as a monolayer. The neutrophils were infused in the flow system and the adhesion of neutrophils to the monolayer was allowed for 10 min. As shown in Figure 1C, the percentage of neutrophils adherent to P-selectin/ICAM-1 monolayer was significantly increased compared with that of neutrophils adherent to ICAM-1 or P-selectin monolayer alone, which suggested that the presence of P-selectin enhances the interaction of neutrophils with the recombinant ICAM-1 (Figure 1C).
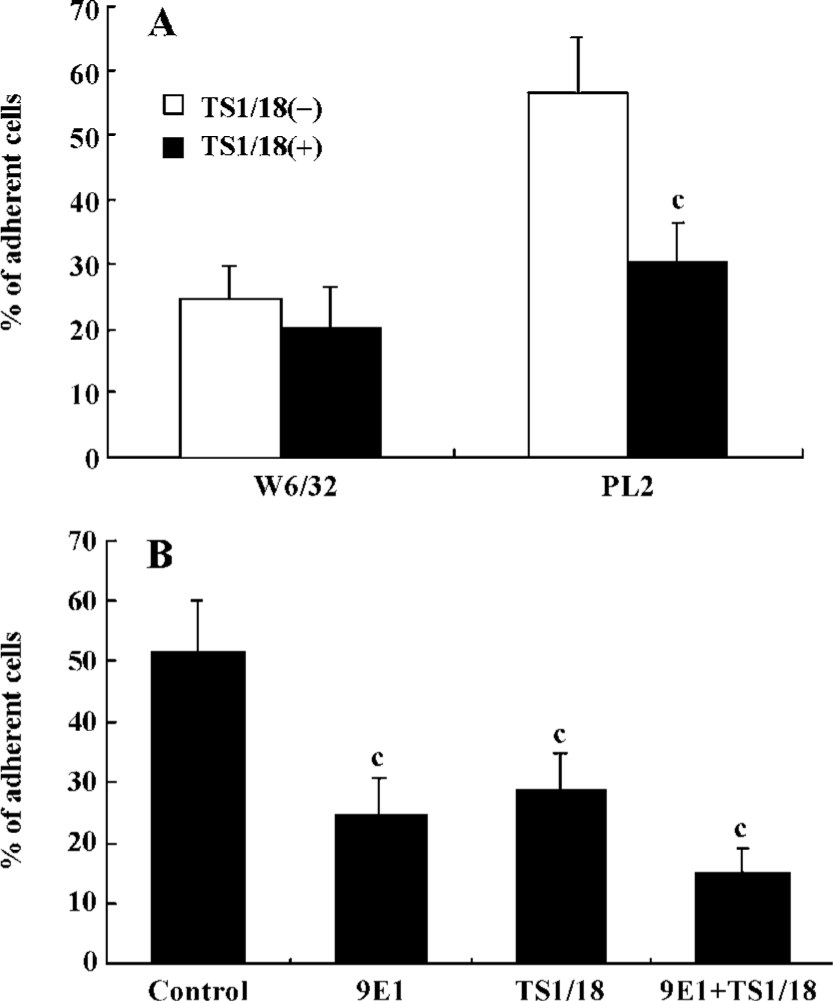
β2-Integrins were involved in the adhesion of PSGL-1-engaged neutrophils to recombinant ICAM-1 The leukocyte-specific β2-integrins regulate the migration of leukocytes and the initiation of immune responses through binding to ICAM-1, -2 or -3[16,17]. In order to investigate the role of β2-integrins in the adhesion of PSGL-1-engaged neutrophils to the recombinant ICAM-1 monolayer, we performed antibody-blocking experiments by using antibodies TS1/18 and 9E1, respectively, against CD18 and P-selectin (both antibodies recognize the extra-cellular ligand-binding domains of CD18 and P-selectin). The results showed that the increased percentage of adherent neutrophils induced by PL2 antibody-cross-linking was abolished by the incubation of neutrophils with TS1/18, the mAb against CD18 (Figure 2A). Moreover, if the P-selectin/ICAM-1 monolayer and neutrophils were respectively pre-incubated with 9E1 and TS1/18 alone, or pre-incubated with both 9E1 and TS1/18 prior to the neutrophils being infused in the flow system, the percentage of the adherent neutrophils decreased in different degrees compared with that of the control. Especially when the neutrophils interacted with the monolayer were blocked with both 9E1 and TS1/18, the percentage of the adherent neutrophils dropped more than 70% compared with that of the control (from 51.36% to 15.08%) (Figure 2B). The results from the antibody-blocking experiments suggested that β2-integrins were involved in the increased adhesion of neutrophils to the recombinant ICAM-1 caused by the engagement of PSGL-1.
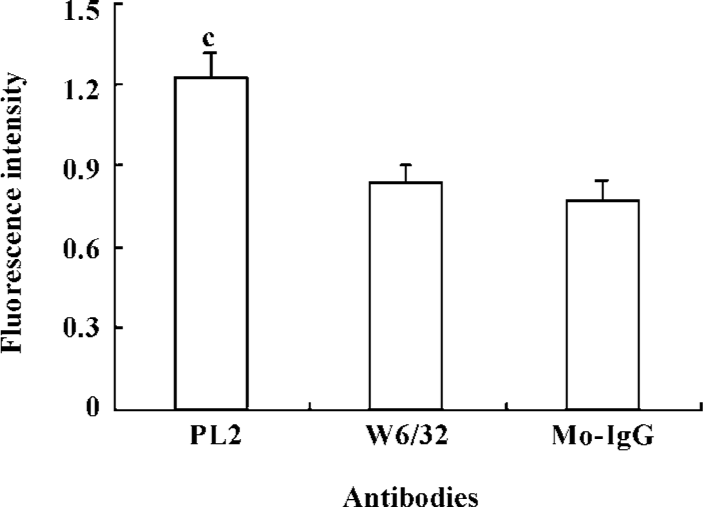
To further understand the contribution of β2-integrins to the adhesion of activated neutrophils to the recombinant ICAM-1, we investigated the alteration of β2-integrin expression. The neutrophils were incubated with PL2 or control IgG at 37 ºC for 30 min, and then fixed and labeled with FITC-conjugated antibody IB4. The result showed that the engagement of PSGL-1 induced an increased expression of β2-integrins on the surface of activated neutrophils (Figure 3).
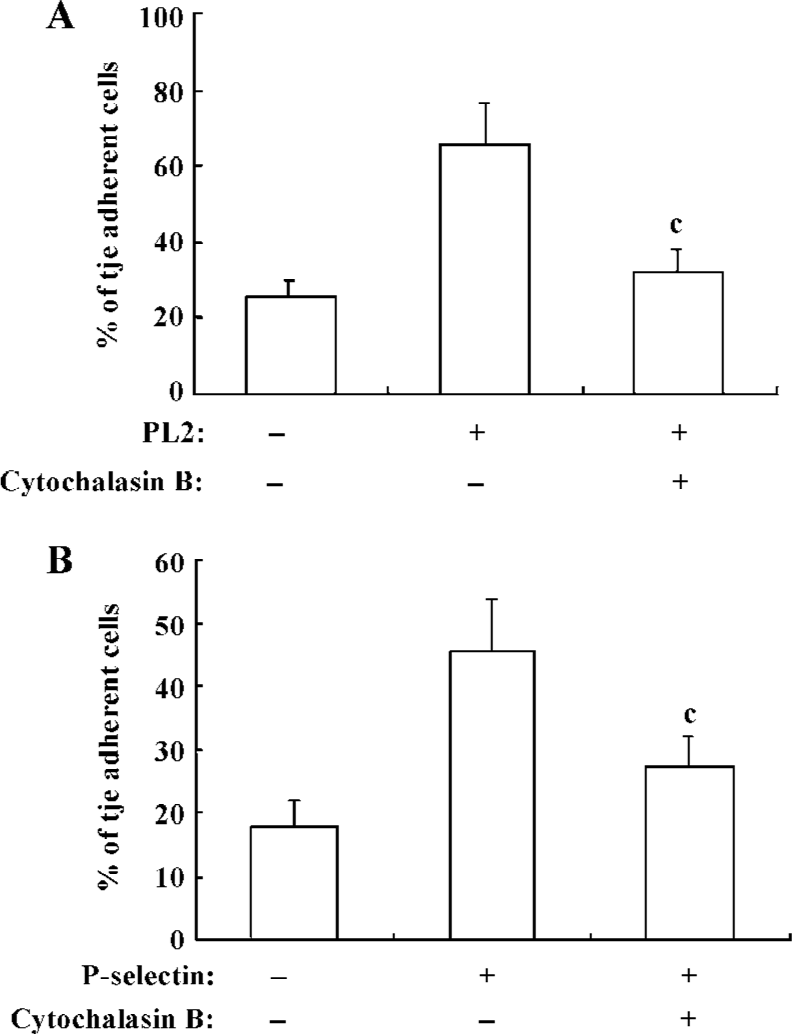
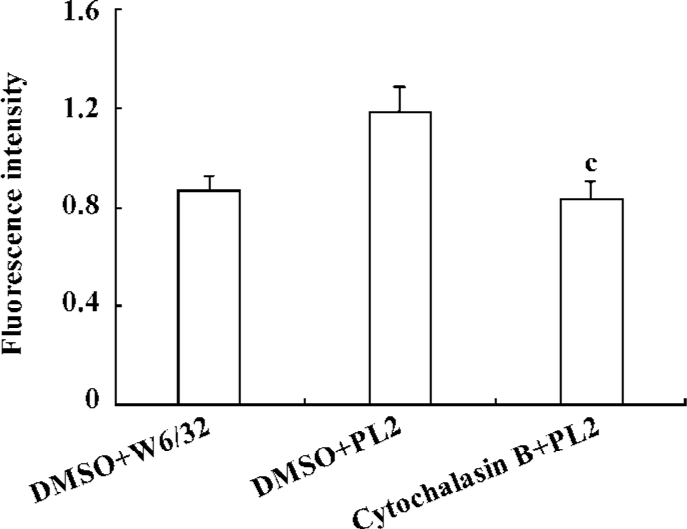
β2-Integrin-involved adhesion of neutrophils to recombinant ICAM-1 depends on the dynamics of F-actin-based cytoskeleton Because the cytoskeleton often plays an important role in the transduction of extra-cellular signals, we further explored the significance of the dynamics of the cytoskeleton to the PSGL-1-initiated and β2-integrin-involved adhesion of neutrophils to the recombinant ICAM-1. We pre-incubated the isolated neutrophils with cytochalasin B, the cells were then engaged with mAb and infused to the ICAM-1 monolayer, or the inhibitor-treated cells were infused to the P-selectin/ICAM-1 monolayer. Consequently, the adhesion of PSGL-1-engaged neutrophils to recombinant ICAM-1 was significantly suppressed, suggesting that the PSGL-1-initiated adhesion of neutrophils to the recombinant ICAM-1 depend on the dynamics of the cytoskeleton (Figure 4A and 4B). The exposure of neutrophils to cytochalasin B also resulted in a dramatic blocking of the increased expression of β2-integrins (Figure 5), implying the significance of the dynamics of the F-actin-based cytoskeleton to the β2-integrin-mediated adhesion of neutrophils to ICAM-1.
Discussion
During the rolling adhesion of leukocytes on the activated endothelial cells, PSGL-1 functions not only as an anchor to arrest the leukocytes through the interaction with P-selectin, but also as a signal receptor to transduce the extra-cellular signals and participate in the activation of leukocytes[18,19]. ICAM-1 is an Ig-like cell adhesion molecule expressed by several cell types, including leukocytes and endothelial cells after stimulation with cytokines such as IL-1, TNF-α or IFN-γ[20]. Within the vascular system, ICAM-1 expressed on the surface of activated endothelial cells interacts with integrins expressed by leukocytes to achieve the firm adhesion of leukocytes on the endothelium.
CD18 integrins are leukocyte-specific, and it is still controversial as to what mechanism CD18 integrins rely on to enhance the adhesion of activated leukocytes to ICAM-1. It may be the extroversion of the β2-integrin from the intracellular vesicles[21], increased affinity of β2-integrin due to the conformational change of extra-cellular structure, de novo synthesis or simply the cluster of the molecules on the surface of leukocytes[17].
In the present study, we addressed the implication of leukocyte-specific β2-integrins in the adhesion of neutrophils to endothelial cells induced by the engagement of PSGL-1 with P-selectin during inflammation. We established an in vitro adhesion system by using a parallel-plate flow chamber. The incubation with TS1/18 abolished the increased adhesion of neutrophils caused by the engagement of PSGL-1. Meanwhile, fluorescence assay revealed that the engagement of PSGL-1 led to an increase of β2-integrin expression on the activated neutrophils, which suggested the involvement of CD18 integrins in the adhesion process. Additionally, the pre-incubation of neutrophils with cytochalasin B not only significantly decreased the percentage of the adherent cells, but also blocked an increase in the level of CD18 on the PSGL-1-engaged neutrophils, which suggested that the dynamics of cytoskeleton is essential for the β2-integrin-mediated adhesion of PSGL-1-engaged neutrophils to ICAM-1.
It is well known that PSGL-1-transduced signals can enhance β2-integrin-mediated adhesion of activated neutrophils to ICAM-1. Our present study revealed that increased expression of β2-integrin contributed to this adhesion enhancement. Our previous study demonstrated engagement of PSGL-1 with antibodies led to the association of PSGL-1 with F-actin-based cytoskeleton[12], and the present study further elaborated that PSGL-1-transduced signals depended on the dynamic of F-actin-based cytoskeleton to increase the expression of CD18 integrin and β2-integrin-mediated adhesion.
References
- Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev 1999;79:181-213.
- Norman KE, Katopodis AG, Thoma G, Kolbinger F, Hicks AE, Cotter MJ, et al. P-selectin glycoprotein ligand-1 supports rolling on E- and P-selectin in vivo. Blood 2000;96:3585-91.
- Hirata T, Furie BC, Furie B. P. -, E-, and L-selectin mediate migration of activated CD8+ T lymphocytes into inflamed skin. J Immunol 2002;169:4307-13.
- Sperandio M, Smith ML, Forlow SB, Olson TS, Xia L, McEver RP, et al. P-selectin glycoprotein ligand-1 mediates L-selectin-dependent leukocyte rolling in venules. J Exp Med 2003;197:1355-63.
- Hicks AE, Nolan SL, Ridger VC, Hellewell PG, Norman KE. Recombinant P-selectin glycoprotein ligand-1 directly inhibits leukocyte rolling by all 3 selectins in vivo: complete inhibition of rolling is not required for anti-inflammatory effect. Blood 2003;101:3249-56.
- Evangelista V, Manarini S, Sideri R, Rotondo S, Martelli N, Piccoli A, et al. Platelet/polymorphonuclear leukocyte interaction: P-selectin triggers protein-tyrosine phosphorylation-dependent CD11b/CD18 adhesion: role of PSGL-1 as a signaling molecule. Blood 1999;93:876-85.
- Simon SI, Hu Y, Vestweber D, Smith CW. Neutrophil tethering on E-selectin activates beta 2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J Immunol 2000;164:4348-58.
- Ma YQ, Plow EF, Geng JG. P-selectin binding to P-selectin glycoprotein ligand-1 induces an intermediate state of alphaMbeta2 activation and acts cooperatively with extracellular stimuli to support maximal adhesion of human neutrophils. Blood 2004;104:2549-56.
- Hidari KI, Weyrich AS, Zimmerman GA, McEver RP. Engagement of P-selectin glycoprotein ligand-1 enhances tyrosine phosphorylation and activates mitogen-activated protein kinases in human neutrophils. J Biol Chem 1997;272:28750-6.
- Haller H, Kunzendorf U, Sacherer K, Lindschau C, Walz G, Distler A, et al. T cell adhesion to P-selectin induces tyrosine phosphorylation of pp125 focal adhesion kinase and other substrates. J Immunol 1997;158:1061-7.
- Urzainqui A, Serrador JM, Viedma F, Yanez-Mo M, Rodriguez A, Corbi AL, et al. ITAM-based interaction of ERM proteins with Syk mediates signaling by the leukocyte adhesion receptor PSGL-1. Immunity 2002;17:401-12.
- Ba X, Chen C, Gao Y, Zeng X. Signaling function of PSGL-1 in neutrophil: tyrosine-phosphorylation-dependent and c-Abl-involved alteration in the F-actin-based cytoskeleton. J Cell Biochem 2005;94:365-73.
- Atarashi K, Hirata T, Matsumoto M, Kanemitsu N, Miyasaka M. Rolling of Th1 cells via P-selectin glycoprotein ligand-1 stimulates LFA-1-mediated cell binding to ICAM-1. J Immunol 2005;174:1424-32.
- Wei M, Tai G, Gao Y, Li N, Huang B, Zhou Y, et al. Modified heparin inhibits P-selectin-mediated cell adhesion of human colon carcinoma cells to immobilized platelets under dynamic flow conditions. J Biol Chem 2004;279:29202-10.
- Gao Y, Li N, Fei R, Chen Z, Zheng S, Zeng X. P-selectin-mediated acute inflammation can be blocked by chemically modified heparin, RO-heparin. Mol Cells 2005;19:350-5.
- Harris ES, McIntyre TM, Prescott SM, Zimmerman GA. The leukocyte integrins. J Biol Chem 2000;275:23409-12.
- van Kooyk Y, Figdor CG. Avidity regulation of integrins: the driving force in leukocyte adhesion. Curr Opin Cell Biol 2000;12:542-7.
- Moore KL. Structure and function of P-selectin glycoprotein ligand-1. Leuk lymphoma 1998;29:1-15.
- McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest 1997;100:485-92.
- van de Stolpe A, van der Saag PT. Intercellular adhesion molecule-1. J Mol Med 1996;74:13-33.
- Berton G, Lowell CA. Integrin signaling in neutrophils and macrophages. Cell Signal 1999;11:621-35.
