Induction of collateral artery growth and improvement of post-infarct heart function by hepatocyte growth factor gene transfer1
Introduction
With recent progress in cardiac catheterization and surgical techniques, mortality from acute myocardial infarction (AMI) has decreased, while the morbidity associated with congestive heart failure occurring in the later period is gradually increasing. Immediate angiography and PTCA of “infarct-related” vessels could save the lives of patients, but ischemia/reperfusion injury would further aggravate ischemic myocardium, which would induce cardiomyocyte apoptosis, resulting in the occurrence of inevitable heart failure[1,2]. Despite advances in medical and surgical treatment for heart failure, there has been no meaningful change in the overall death rates[3]. Therefore, new therapeutic strategies based on genes and proteins have been proposed. Because of its potential angiogenic, anti-apoptotic, anti-fibrotic and anti-inflammatory benefits, hepatocyte growth factor (HGF) has been the subject of increasing attention in ischemic heart disease[1,4,5]. In the present study, we used adenovirus5-mediated human hepatocyte growth factor (Ad5-HGF) for the treatment of swine myocardium, to evaluate the therapeutic potential of HGF for postinfarction heart failure.
Gene transfer via infarct-related vessels is the usual option considered. However, the infarct-related vessels cannot be opened in some patients, so the second aim of the present study was to establish the results of Ad5-HGF transference via non-infarct-related vessels.
Materials and methods
Animals and drugs This study was approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University. Twelve young male Suzhong swine, weighing 30±5 kg (Jiangsu Academy of Agricultural Sciences) were randomly divided into 2 groups (n=6 in each group). The following equipment and reagents were purchased from the indicated companies: enzyme-linked immunosorbent board (Costar), enzyme-scale equipment (Bio-Rad), single photon emission computerized tomography equipment (SPECT; ECAM+; Siemens, Germany), electrocardio-monitor (PD-1200; Zoll, USA), 99mTcO4 (Jiangsu Institute of Nuclear Medicine), Ad5-HGF and null-Ad5 (Chinese Academy of Military Medical Sciences), mouse monoclonal antibody against human HGF (R&D), recombinant human HGF (Peprotech EC), mouse monoclonal antibody against human α-smooth muscle actin (α-SMA) and SP immunodetection kit (Zhongshan Reagent Company, Beijing).
Myocardial infarction model Swine were anesthetized with a combination of ketamine (50 mg/kg) and xylazine (10 mg/kg). The animals were intubated with a cuffed endotracheal tube and ventilated with 100% oxygen to maintain PaCO2 between 35 and 45 mm Hg. AMI was induced by ligating the left anterior descending coronary artery (LAD). Electrocardiography was used to monitor heart rate, rhythm, and ST-segment changes during the surgical procedure. Seven weeks after LAD ligation, all animals were killed.
Intracoronary gene transfer Four weeks after LAD ligation, swine were treated with Ad5-HGF (4×109 pfu/mL, 1 mL) or null-Ad5 (1 mL) via the right coronary artery. All the infusions were carried out through cardiac catheterization.
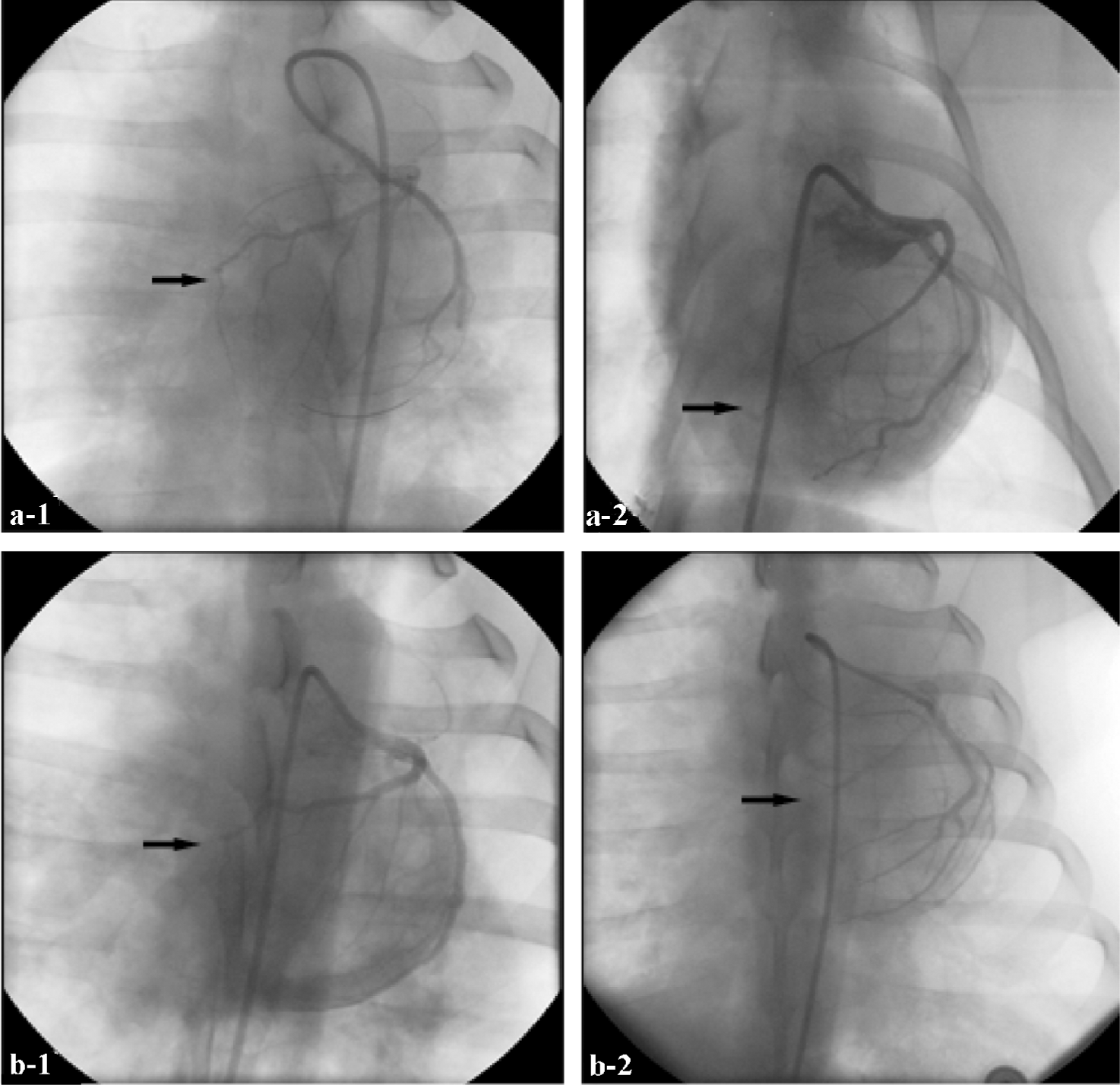
Evaluation of collateral artery growth Four and 7 weeks after ligation, collateral artery growth was evaluated by coronary angiography (Rentrop). Grades of collateral filling from the contralateral vessel were: 0, none; 1, filling of side branches of the artery to be dilated via collateral channels without visualization of the epicardial segment; 2, partial filling of the epicardial segment via collateral channels; 3, complete filling of the epicardial segment of the artery being dilated via collateral channels[6].
Heart function Heart function and cardiac perfusion were evaluated by 99mTc-MIBI gated cardiac perfusion imaging.
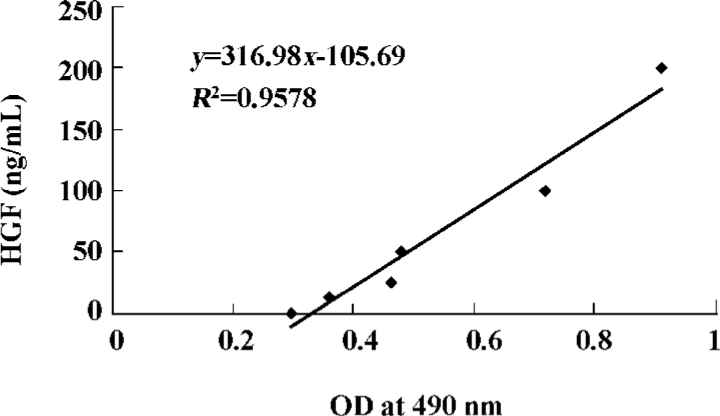
Expression of HGF in local myocardium Expression of HGF in myocardial tissue was evaluated by using enzyme-linked immunosorbent assay (ELISA). Three weeks after gene transfer, 200 mg of myocardium from the transitional zones of each animal (12 pigs) was pulped, and the supernatant was collected. ELISA was carried out using a mouse monoclonal antibody against human HGF, horseradish peroxidase-labeled goat antimouse immunoglobulin, and a spectrophotometric o-phenylenediamine color-developing system. The optical density (OD) was measured at a wavelength of 490 nm (Microplate Manager 450). A standard curve using recombinant human HGF was constructed for calculation of the expression of HGF (Figure 1).
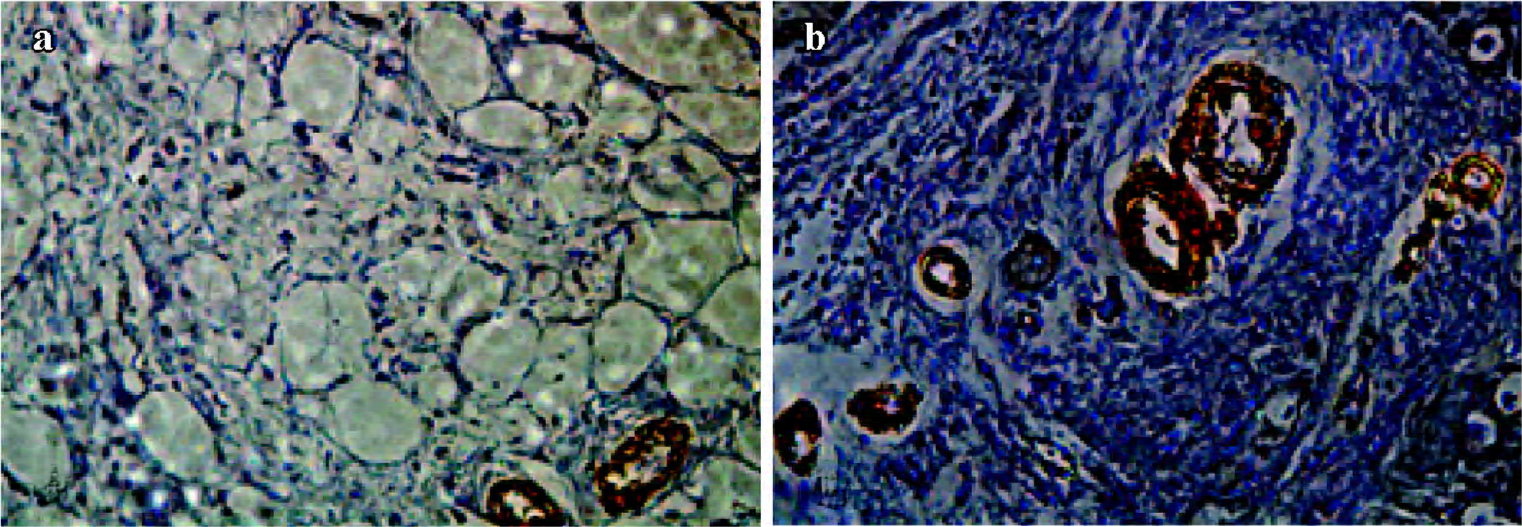
Immunohistochemistry analysis Three weeks after gene transfer, the myocardium samples collected from all animals were fixed in 4% formaldehyde and embedded in paraffin, then cut into 10 µm-thick sections. The sections were stained with hematoxylin and eosin for cell and blood vessel identification. Immunohistochemical detection of specific antigens was performed with α-SMA antibody, then the sections were dyed with 3,3'-diaminobenzidine (DAB) and redyed with brazilin. Results were observed with an optical microscope and photomicrographs were taken using a digital image analysis system.
α-SMA+ arteriole number Tissue samples obtained from three locations within the transitional zones of each animal (12 pigs) were identified. α-SMA+ arterioles in each tissue section were counted by an observer who was blind to which groups the samples came from (×400). The number of α-SMA+ arterioles in 5 high-power fields in each section were averaged, and expressed as α-SMA+ arterioles/mm2 (the area of 400 high-power fields is equal to 0.152 mm2). The averages of 5 fields for 3 samples from each animal were used for comparison.
Data analysis Continuous variable data are expressed as mean±SE. SPSS 11.5 software was used for all analysis. P<0.05 was considered significant. Differences in quantitative data were assessed by using the independent-sample t-test and the pair-sample t-test. Difference in categorical data was assessed by using the rank sum test.
Results
HGF expression HGF expression was examined by ELISA. Compared with the null-Ad5 group, higher expression of human HGF was observed in the myocardium of the Ad5-HGF group (109.3±7.8 vs 6.2±2.6, t=30.685, P<0.01).
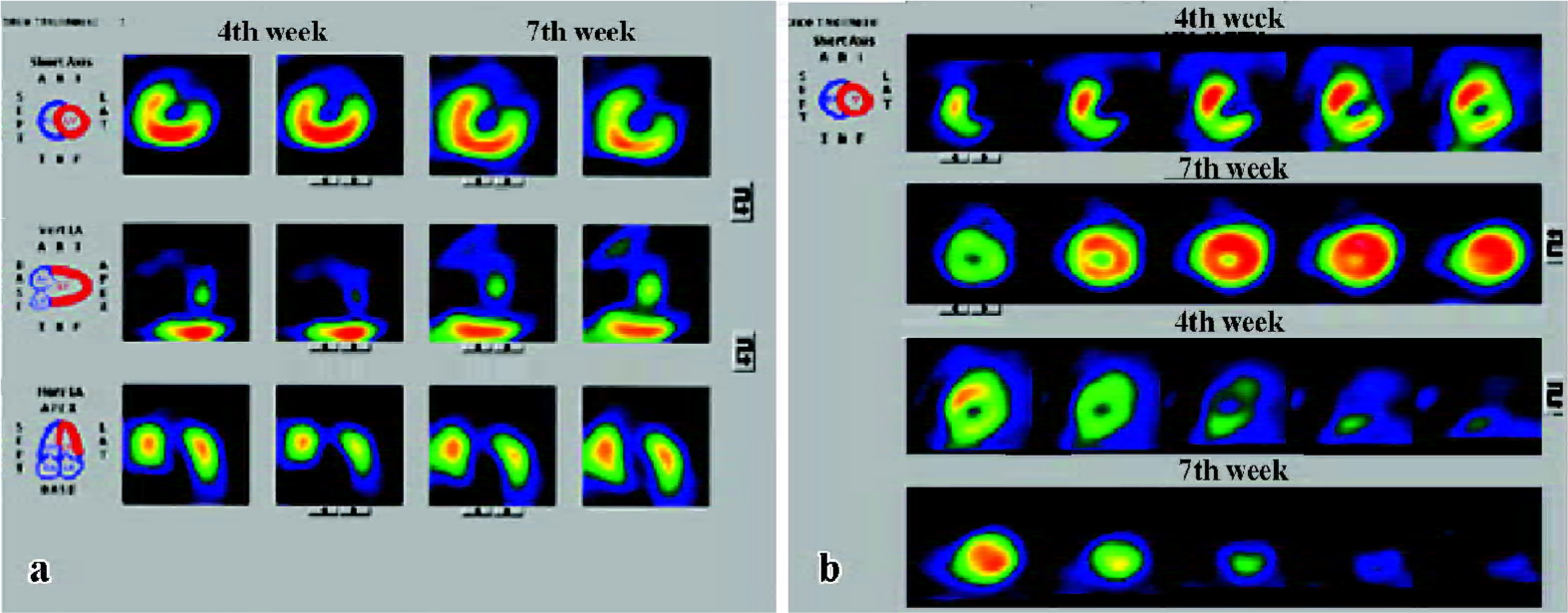
Heart function and cardiac perfusion Four weeks after LAD ligation, perfusion defects in the left ventricular apex and interventricular septum were detected in both groups, and there was no difference in the left ventricular ejection fraction (LVEF) between the two groups. After 7 weeks, cardiac perfusion was significantly improved in the Ad5-HGF group, but was unchanged or worse in the null-Ad5 group (Figure 2). From the 4th week to the 7th week after operation, left ventricular end diastolic volume (LVEDV), left ventricular end systolic volume (LVESV), and LVEF were ameliorated in the Ad5-HGF group, but LVEDV, LVESV and LVEF were worse in the null-Ad5 group. The improvement in LVEF was greater in the Ad5-HGF group than in the null-Ad5 group in the 7th week (Table 1).
Full table
Collateral artery growth Four weeks after LAD ligation, no collateral artery growth was detected in either group. After 7 weeks, collateral artery growth was obviously greater in the Ad5-HGF group than in the null-Ad5 group (average rank sum 9.17 vs 3.83, n=6, u=–2.687, P<0.01; Figure 3).
Immunohistochemistry In the Ad5-HGF group, the number of α-SMA+ arterioles that were surrounded by a layer of smooth muscle cells in every mm2 was significantly higher than in the null-Ad5 group (56.14±4.2 vs 16.45±3.5, t=17.731, P<0.01; Figure 4).
Discussion
HGF, a heparin-binding glycoprotein, is a disulfide-linked heterodimeric molecule that was initially identified, purified and cloned as a potent mitogen for hepatocytes. The angiogenic properties of HGF may depend on upregulation of an essential transcription factor for angiogenesis, ets-1. HGF may upregulate the transcription activity and mRNA expression of ets-1. Moreover, ets-1 may activate HGF, MMP-1, c-Met and vascular endothelial-derived growth factor (VEGF) expression in vascular smooth muscle cells and endothelial cells, which in turn upregulate the degradation pathway of the extracellular matrix and stimulate the migration of vascular smooth muscle cells and endothelial cells, to accomplish angiogenesis[7]. In the present study, we demonstrated that HGF could not only promote the formation of functional arterioles that are coated with smooth muscle cells (diameter<200 µm), but also promote collateral artery growth (diameter >200 µm).
Vasculogenesis refers to the formation of blood vessels from endothelial progenitor cells, a process that was initially described as occurring during embryonic development and, more recently, also in adult animals. Angiogenesis involves the sprouting of new capillaries from preexisting vessels, whereas arteriogenesis refers to the remodeling of newly formed or preexisting vascular channels into larger and well-muscularized arterioles and collateral vessels. The generation of new vascular channels by vasculogenesis and angiogenesis must accomplish arteriogenesis, involving coating with smooth muscle cells, then the formation of mature blood vessels. Recruitment of smooth muscle cells provides these vessels with essential viscoelastic and vasomotor properties and enables accommodation of the changing needs for tissue perfusion. This stage has a major role in collateral growth[8,9]. In prior studies regarding neovascularization, endothelial cell-related antigen (VIII factor; vWF) has been most commonly used to evaluate the degree of neovasculari-zation, but this method cannot distinguish between perfusion vessels and nonperfusion vessels. Therefore, an increase in the number of vessels as indicated by this method might not necessarily indicate an improvement in perfusion function. VEGF, a well-known angiogenic growth factor, has been shown to produce no effect on vascular smooth muscle cells (VSMC), resulting in a delay in the maturation of blood vessels during angiogenesis when VEGF is used as an inducer. Therefore, we only evaluated the number of mature vessels that were surrounded by α-SMA in the present study. Importantly, we discovered that HGF could also improve the growth of collateral arteries with a diameter >200 µm. After HGF transfer, coronary angiography showed that the growth of collateral blood vessels was obviously greater in the Ad5-HGF group. The function of new blood vessels was evaluated using gated cardiac perfusion imaging, and we found that cardiac perfusion was significantly improved in the Ad5-HGF group.
An important component of postinfarction cardiac failure is ventricular remodeling with chamber dilatation and wall thinning[10]. In the present study we found that the Ad5-HGF group had preserved chamber dilation compared with the null-Ad5 group. Three weeks after HGF transfer, LVESV and LVEDV were lower in the Ad5-HGF group than in the null-Ad5 group. LVEF was significantly increased in the Ad5-HGF group, which signified an improvement in systolic function. Moreover, Vasant et al[1] reported that an adeno-HGF group had a significantly greater border zone wall thickness compared with an adeno-null group of rats. However, the improvement in heart function was possibly not only due to the potential angiogenic benefits, but also the anti-apoptotic, anti-fibrotic benefits of HGF[4].
Our study also demonstrated that Ad5-HGF injection via non-infarct-related vessels was an alternative method for gene transfer. Clinically, some patients’ infarction-related vessels cannot be opened, so transfer cannot occur via an infarction-related vessel, thus a new and more convenient method should be sought. Importantly, in our experiment, 3 weeks following intracoronary virus administration, human HGF protein was still expressed at high levels in the myocardium, and produced a marked effect. Furthermore, similar to the results of White et al[11] and owing to adenovirus immunogenicity, we found that human HGF protein was almost not expressed in myocardium at the 4th week after transfer (data not shown). Therefore, we believe that this method of gene transfer via non-infarct-related vessels is feasible. Small amounts of Ad5-HGF entered the peripheral circulation because of intracoronary transfer, but were only able to produce transient expression (3 weeks). Excluding patients with special needs (eg those with tumors), this treatment will potentially have little effect on patients[4].
In conclusion, HGF can effectively induce collateral artery growth and improve heart function when administered via non-infarction-related vessels in a postinfarction model.
References
- Jayasankar V, Woo YJ, Pirolli TJ, Bish LT, Berry MF, Burdick J, et al. Induction of angiogenesis and inhibition of apoptosis by hepatocyte growth factor effectively treats postischemic heart failure. J Card Surg 2005;20:93-101.
- Scheubel RJ, Bartling B, Simm A, Silber RE, Drogaris K, Darmer D, et al. Apoptotic pathway activation from mitochondria and death receptors without caspase-3 cleavage in failing human myocardium. J Am Coll Cardiol 2002;39:481-8.
- Salam AM. Selective aldosterone blockade with eplerenone in patients with congestive heart failure. Expert Opin Invest Drugs 2003;12:1423-7.
- Jin H, Wyss JM, Yang R, Schwall R. The therapeutic potential of hepatocyte growth factor for myocardial infarction and heart failure. Curr Pharm Des 2004;10:2525-33.
- Matsumoto K, Nakamura T. Hepatocyte growth factor: Renotropic role and potential therapeutics for renal diseases. Kidney Int 2001;59:2023-38.
- Rentrop KP, Cohen M, Blanke H, Phillips RA. Change in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol 1985;5:587-92.
- Tomita N, Morishita R, Taniyama Y, Koike H, Aoki M, Shimizu H, et al. Angiogenic property of hepatocyte growth factor is dependent on upregulation of essential transcription factor for angiogenesis, ets-1. Circulation 2003;107:1411-7.
- Fam NP, Verma S, Kutryk M, Stewart DJ. Clinician guide to angiogenesis. Circulation 2003;108:2613-8.
- Schwartz Y, Kornowski R. Progenitor and embryonic stem cell transplantation for myocardial angiogenesis and functional restoration. Eur Heart J 2003;24:404-11.
- White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation 1987;76:44-51.
- Wu DL, Zhang YR, Lao MF, Qiu ZH, Ha XQ, Wu ZZ. Gene therapy of adenovirus-mediated human hepatocyte growth factor on the rat model of myocardial ischemia. Chin Sci Bull 2002;22:1726-9.
