Paradoxical effects of ginkgolide B on cardiomyocyte contractile function in normal and high-glucose environments
Introduction
Ginkgo biloba is a Chinese herb used for many centuries in traditional Chinese medicine[1]. Ginkgo biloba extract, an extract of the leaves of G biloba, has been used as a multi-component therapeutic agent in cardiovascular and neurological disorders, including ischemic heart disease, Alzheimer’s disease, dementia, cerebral and ocular blood flow occlusion, premenstrual problems and altitude sickness[1–4]. The primary active constituents of G biloba include 24% ginkgo-flavone glycosides and 6% unique diterpenes known as ginkgolides or bilobalides[4]. Due to its potent free radical scavenging and antioxidant properties[5–7], G biloba extract has displayed unusual promise in the treatment of cardiac, cerebrovascular and peripheral vascular disorders[4,8]. Although many pharmacological effects of G biloba extract have been attributed to ginkgolide constituents, including ginkgolide types A, B, M, and J, the precise mechanism of action of these terpenoids on cardiovascular and cerebrovascular function is still essentially unknown. It is thought that ginkgolide B (C20H24O10) elicits cardiac protective properties against ischemic heart diseases and arrhythmias[3,9,10] possibly related to its capacity as a specific platelet activating factor (PAF) receptor antagonist. This is consistent with the beneficial effect of ginkgolide B in PAF-induced ischemic damage[11]. Nevertheless, the impact of ginkgolide B on heart function or cardiac electromechanical function is still uncertain.
Previous reports have documented myocardial contractile effects from the G biloba extract EGb 761, ginkgolide A and ginkgolide B. Oral administration of EGb 761 or ginkgolide A significantly improves myocardial functional recovery after myocardial ischemia[12,13]. This is supported by in vitro observations that pre- and post-ischemic perfusion of rat heart with ginkgolide A and ginkgolide B but not EGb 761 enhanced all hemodynamic parameters[12]. It has been postulated that inhibition of the formation of free radicals such as hydroxyl radicals may play an essential role in EGb 761- or ginkgolide-elicited beneficial effect on myocardial function[14,15]. However, the direct impact of G biloba extract or ginkgolide on functioning of the essential cardiac unit, the cardiomyocyte, has not yet been established. More recently, G biloba extract has been proven beneficial for myocardial ultrastructure and biochemical function in diabetes mellitus[16,17]. However, the clinical indications of G biloba extract in diabetic patients with heart disease are not clear. Given the fact that heart disease is the leading cause of death in diabetes characterized by diastolic dysfunction and depressed cardiac contractility[18,19], the aim of the present study was to examine the effect of ginkgolide B on cardiomyocyte contractile function under normal and high extracellular glucose environments. We took advantage of a “diabetes-like” rat cardiomyocyte culture model developed in our laboratory by culturing ventricular myocytes in a high glucose milieu for 6–24 h[20]. Myocytes cultured in high-glucose medium exhibit a phenotype with prolonged diastole, which is reminiscent of that found in in vivo diabetes[18,20]. State-of-the-art cell biological and cell physiological techniques were used to evaluate cardiomyocyte mechanical function and expression of key cardiac Ca2+ regulatory proteins, including sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2a), phospholamban (PLB) and Na+-Ca2+ exchanger (NCX).
Materials and methods
Adult rat ventricular myocyte isolation and high-glucose culture All animal experimental procedures were approved by our institutional animal care and use committee. Ventricular myocytes were isolated from adult male Sprague-Dawley rats (approximately 250 g), under sterile conditions by perfusing collagenase (176 U/mL) and hyaluronidase (0.1 mg/mL) through coronaries, and were further digested by trypsin (0.02 mg/mL) after the tissue was minced. The viability of freshly isolated rat cardiomyocytes was approximately 70%, which was not significantly affected by short-term culture with either high-glucose or ginkgolide B at the concentrations described in this study. Isolated myocytes were maintained for 6 h in a defined medium consisting of Medium 199 with Earle’s salts containing 25 mmol/L N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES) and NaHCO3 supplemented with albumin (2 mg/mL), L-carnitine (2 mmol/L), creatine (5 mmol/L), taurine (5 mmol/L), insulin (0.1 µmol/L), penicillin (100 U/mL), streptomycin (100 µg/mL) and gentamicin (100 µg/mL). This medium also contained either normal glucose (NG; 5.5 mmol/L) or high-glucose (HG; 25.5 mmol/L) concentrations. The high glucose concentration is comparable to serum glucose levels in severely diabetic rats[18]. Subsets of each medium were also supplemented with ginkgolide B (0.5–2.0 µg/mL, obtained from the National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China). The myocytes in either the NG or HG medium were incubated at 37 oC with 100% humidity and 5% CO2 [21].
Cell shortening/relengthening The mechanical properties of ventricular myocytes were assessed using an IonOptix MyoCam system (IonOptix Corporation, Milton, MA, USA)[21]. In brief, cells were placed in a Warner chamber mounted on the stage of an inverted microscope (Olympus, IX-70) and superfused (approximately 1 mL/min at 30 oC) with a buffer containing (in mmol/L): 131 NaCl, 4 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, 10 HEPES at pH 7.4. Cells were field stimulated with suprathreshold voltage and at a frequency of 0.5 Hz, 3 ms duration, using a pair of platinum wires placed on opposite sides of the chamber and connected to an electrical stimulator (FHC Inc, Brunswick, ME, USA). The myocyte being studied was displayed on the computer monitor using an IonOptix MyoCam camera. A SoftEdge software (IonOptix) was used to capture changes in cell length during shortening and relengthening. Cell shortening and relengthening were assessed using the following indices: peak shortening (PS), the amplitude myocytes shortened upon electrical stimulation, indicative of peak ventricular contractility; time-to-PS (TPS), the duration of myocyte shortening, indicative of systolic duration; time-to-90% relengthening (TR90), the duration to reach 90% relengthen-ing, indicative of diastolic duration (90% rather 100% relengthening was used to avoid noisy signal at baseline level); and maximal velocities of shortening/relengthening, maximal slope (derivative) of shortening and relengthening phases, indicative of maximal velocities of ventricular pressure increase/decrease.
Western blot analysis of SERCA2a, NCX and PLB Expression of SERCA2a, NCX, and PLB in cardiomyocytes was assessed by Western blotting. In brief, NG or HG cultured cardiomyocytes were sonicated in lysis buffer containing (in mmol/L): Tris 10, NaCl 150, ethylenediamine tetraacetic acid (EDTA) 5, 1% Triton X-100 and protease inhibitor cocktail followed by centrifugation at 6000×g for 15 min at 4 °C. The supernatant was transferred to a clean microtube and protein was quantified spectrophotometrically using the Bradford method. Protein samples (10–50 µg/lane) were separated by polyacrylamide gel electrophoresis (PAGE) using 7% (SERCA2a and NCX) or 15% (PLB) sodium dodecylsul-fate (SDS)-polyacrylamide gels. Proteins were transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA, USA) and stained with Ponceau S red to assess equal protein loading and transfer. Membranes were incubated for 1 h in a blocking solution containing 5% milk in Tris-buffered saline-Tween (TBST), then membranes were washed briefly in TBST and incubated overnight at 4 °C with mouse monoclonal anti-SERCA2a (1:1000, Affinity Bio-Reagents, Golden, CO, USA), mouse monoclonal anti-PLB (1:5000, provided by Dr Steven CALA from Wayne State University, Detroit, MI, USA) and rabbit polyclonal anti-NCX antibody (1:1000; Swant, Bellinzona, Switzerland). After washing blots to remove excessive primary antibody binding, blots were incubated for 1 h with horseradish peroxidase (HRP)-conjugated secondary antibody (1:5000). The membrane was then exposed to 2 mL of a mixture of luminol plus hydrogen peroxide under alkaline conditions (Super-Signal West Dura Extended Duration Substrate; Pierce, Rockford, IL, USA) for 3 min, and the resulting chemiluminescent reaction was detected by Kodak X-OMAT AR Film (Eastman Kodak, Rochester, NY, USA). Immunoreactive bands were visualized by imaging densitometry (GS-800, Imaging Densitometer; Bio-Rad)[21].
Statistical analyses For each experimental series, data are presented as mean±SEM. Statistical significance (P<0.05) for each variable was estimated by analysis of variance (ANOVA) or t-test, where appropriate.
Results
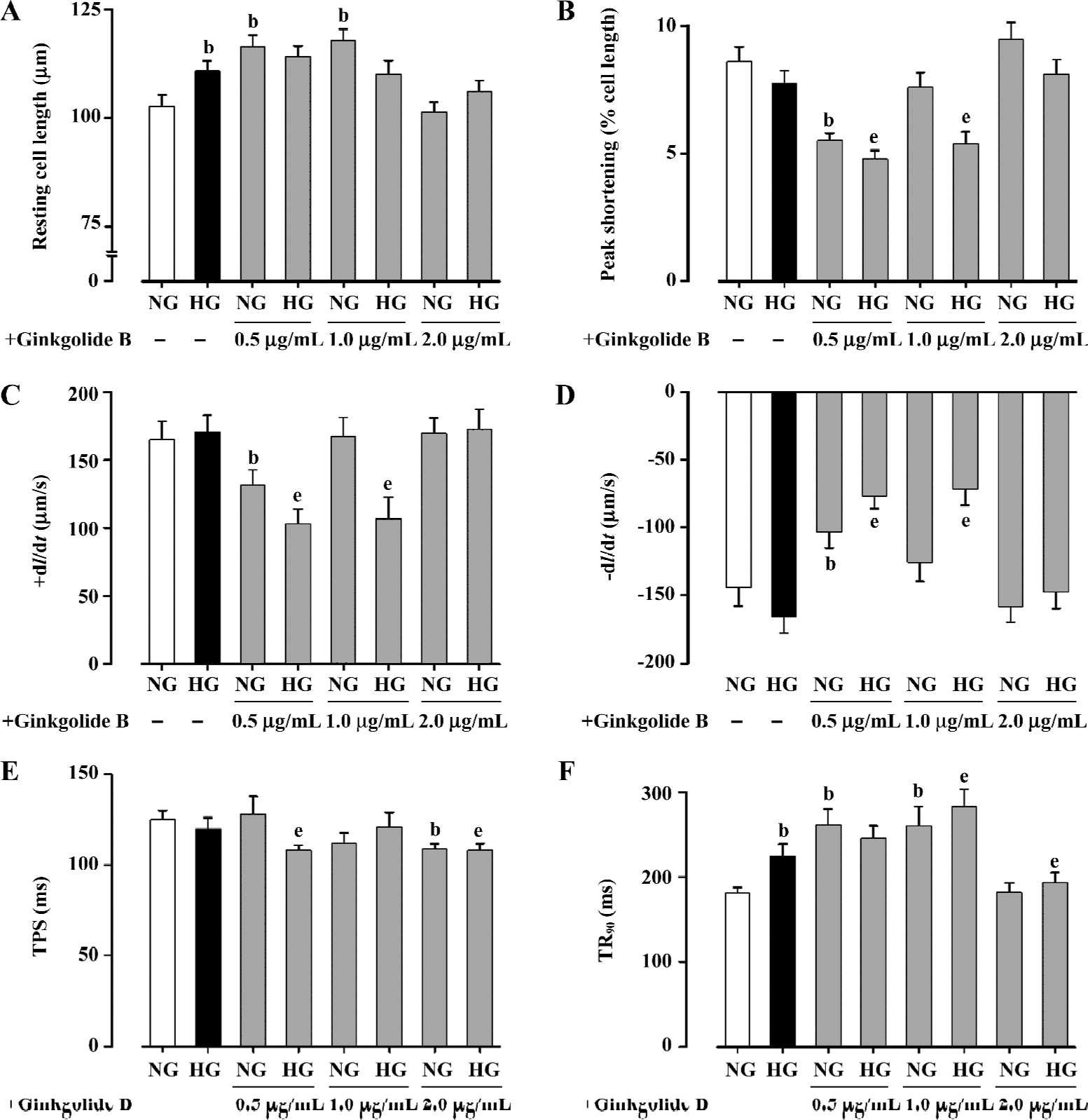
Effect of ginkgolide B on myocyte shortening in normal and high glucose-cultured myocytes Short-term HG culture significantly elongated the resting cell length of cardio-myocytes. Lower concentrations (0.5 and 1.0 µg/mL) of ginkgolide B significantly lengthened resting cell length in NG myocytes, whereas high concentrations of ginkgolide B (2.0 µg/mL) failed to affect the resting cell length in NG cells. The resting cell length in HG myocytes was not affected by ginkgolide B at the concentration range tested (Figure 1A). The peak shortening (PS normalized to resting cell length) amplitude was not affected by the HG milieu. Short-term incubation of ginkgolide B significantly reduced PS in NG myocytes at 0.5 µg/mL and decreased PS in HG myocytes at 0.5 and 1.0 µg/mL. The high concentration of ginkgolide B (2.0 µg/mL) did not affect PS in either myocyte group (Figure 1B). Myocytes cultured in high glucose medium exhibited similar maximal velocity of cell shortening and relengthening (±dl/dt) to those maintained in NG medium. Consistent with its effect on PS, ginkgolide B significantly reduced ±dl/dt in NG myocytes at 0.5 µg/mL and decreased ±dl/dt in HG myocytes at 0.5 and 1.0 µg/mL. Higher concentrations of ginkgolide B (2.0 µg/mL) failed to affect ±dl/dt in either myocyte group (Figure 1C, 1D). HG culture did not affect duration of shortening (TPS). Ginkgolide B significantly shortened TPS in both NG (2.0 µg/mL) and HG (0.5 and 2.0 µg/mL) cells (Figure 1E). HG significantly prolonged the duration of relengthening (TR90) compared with NG cells. Although ginkgolide B effectively nullified HG-induced prolongation of TR90 at the highest concentration (2.0 µg/mL), it paradoxically prolonged TR90 in both NG and HG myocytes at lower concentrations (with the exception of the HG group at 0.5 µg/mL; Figure 1F). These data suggest that ginkgolide B exerts paradoxical effects on cardiomyocyte contractile indices under both NG and HG culture conditions.
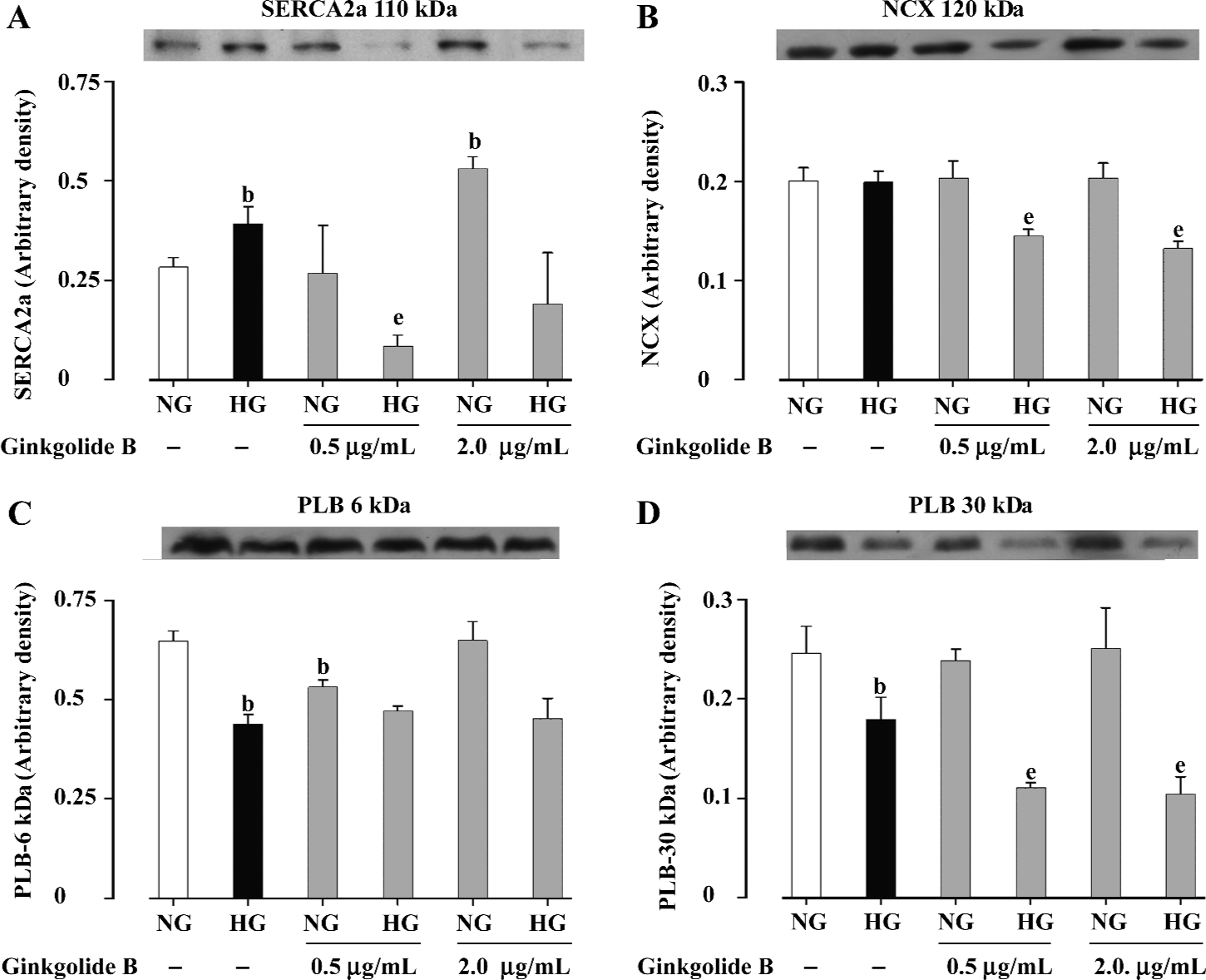
Effect of ginkgolide B on SERCA2a, PLB, and NCX expression To delineate the possible mechanisms responsible for ginkgolide B-induced cardiomyocyte contractile response, expression of key intracellular Ca2+-cycling proteins SERCA2a, PLB, and NCX were evaluated in cardiomyocytes maintained in NG and HG medium without or with low (0.5 µg/mL) or high (2.0 µg/mL) concentrations of ginkgolide B. These proteins are closely associated with myocardial mechanical properties[22]. Our results indicated that cardiac expression of SERCA2a was significantly elevated in HG-cultured myocytes. Low concentrations of ginkgolide B reduced SERCA2a expression in HG myocytes without affecting that in NG cells. In contrast, high concentrations of ginkgolide B upregulated SERCA2a expression in NG cells without affecting that in HG myocytes (Figure 2A). Expression of NCX was comparable between NG and HG-cultured cells. Ginkgolide B did not affect NCX expression in NG cells, whereas it significantly reduced NCX expression in HG cells, at both concentrations tested (Figure 2B). In addition, the HG milieu significantly downregulated PLB expression (both the 6 kDa monomer and the 30 kDa pentamer). Although ginkgolide B treatment did not affect the pattern of PLB expression (6 or 30 kDa) between NG and HG cells, 0.5 µg/mL ginkgolide B significantly reduced monomer expression in NG cells and pentamer expression in HG cells. Ginkgolide B 2.0 µg/mL significantly reduced pentamer expression in HG cells (Figure 2C, 2D). These data indicate that ginkgolide B may regulate cardiomyocyte contractile function through alteration of the expression of cardiac Ca2+ regulatory proteins SERCA2a, NCX and PLB.
Discussion
The primary findings of our study were that high concentrations of ginkgolide B (2.0 µg/mL) restored HG-induced prolongation of relengthening duration (TR90). However, low to medium concentrations (0.5−1.0 µg/mL) of ginkgolide B depressed peak shortening (PS), maximal velocity of shortening/relengthening (±dl/dt) and shortened shortening duration (TPS) in NG and HG cells. Given that it can nullify HG-induced prolongation in relengthening duration, ginkgolide B (low to medium levels) paradoxically prolonged TR90 in NG or HG cells. Our further analysis using Western blotting revealed that HG upregulated SERCA2a and downregulated PLB expression without affecting that of NCX. Ginkgolide B at low and high concentrations disrupted the NG-HG response pattern in SERCA2a and NCX without affecting that of PLB. These data collectively suggest that ginkgolide B may elicit paradoxical cardiomyocyte contractile effects in NG and HG-maintained myocytes through disparate regulation of cardiac Ca2+ regulatory proteins, including SERCA2a, NCX, and PLB.
Ginkgo biloba extract (such as EGb 761) is known to have cardiac protective actions against ischemia-reperfusion injury involving inhibition of free radical formation[14]. This is supported by the observation that G biloba extract protects against H2O2-induced cardiomyocyte damage with respect to lactate dehydrogenase (LDH) leakage, lipid peroxidation and ultrastructural alteration[15]. Further study suggested that G biloba extract might protect against ischemia or free radical (1.1-diphenyl-2-picryl hydrazyl)-induced cardiotoxicity (eg suppression of left ventricular pressure (LVP) and maximal velocity of pressure development (LV dp/dtmax) via free radical inhibition, scavenging or tocopheryl radical recycling to maintain membrane vitamin E[13,23]. A growing body of evidence suggests that G biloba extract participates in the regulation of cardiac function, although there is no evidence of any direct effect of G biloba extract on cardiomyocyte contractile function. Our current study revealed for the first time that ginkgolide B might exert a direct effect on cardiomyocyte contractile function in both NG and HG environments. The fact that a high concentration of ginkgolide B reconciles HG-elicited diastolic dysfunction is in line with a previous report that ginkgolide B is capable of inhibiting a rise in left ventricular end-diastolic pressure[24]. Although it is still premature to propose a precise mechanism of action for ginkgolide B or G biloba extract-elicited in isolated cardiomyocytes, several mechanisms may be postulated. Ginkgo biloba extract and ginkgolide have been demonstrated to prolong and shorten the action potential duration, respectively[25]. This disparate effect seems to be consistent with our current observation that ginkgolide B prolongs and shortens the duration of shortening (TR90) at low and high concentrations, respectively, suggesting a likely biphasic effect of ginkgolide on action potential configuration. Our data also revealed that ginkgolide B inhibited cardiomyocyte contractile function (PS and ±dl/dt) at low to medium doses. This is consistent with the notion that G biloba extract markedly inhibits Ca2+ current and delayed rectifier K+ current in cardiomyocytes[25]. In fact, inhibition of delayed rectifier K+current by G biloba extract is in line with prolongation of the action potential duration[25]. Ginkgolide B has also been shown to reduce the cardiomyo-cyte intracellular Ca2+ levels and may antagonize the Ca2+ overload triggered by myocardial ischemia[24,26]. Another mechanism that may be considered responsible for ginkgolide B-induced cardiomyocyte contractile function is manipulation of cardiac Ca2+ regulatory proteins including SERCA2a, phospholamban and Na+-Ca2+ exchanger, as shown in our current study. The enhanced SERCA2a expression and reduced phospholamban levels under the 6-h short-term HG milieu may reflect a compensatory mechanism for diabetes-induced depression of SERCA2a protein and elevation of phospholamban[27]. The ginkgolide B-elicited inhibitory effect on SERCA2a and Na+-Ca2+ exchanger protein is somewhat consistent with its depressant effect on myocyte contractile function (PS and ±dl/dt). The fact that ginkgolide B displays a predominant effect on SERCA2a and Na+-Ca2+ exchanger in HG-cultured cardiomyocytes implies that glycemic condition may be a critical determining factor for ginkgolide B-induced cardiac effect. Recent evidence has indicated the beneficial role of G biloba extract in the ultrastructural and biochemical properties of diabetic cardiomyocytes[16], suggesting the therapeutic potential of G biloba extract or ginkgolide in diabetic heart diseases. Nevertheless, our current observations have revealed that the effect of ginkgolide B on “diabetic-like” cardiomyocyte function is rather complicated, and may even be detrimental. Considering the complexity of the cardiomyocyte contractile effect of ginkgolide B in normal and HG environments, future research is warranted to clarify the impact of ginkgolide B and ginkgolide A (due to the apparent role of ginkgolide A in myocardial contractile regulation[12,14]) on intracellular Ca2+ homeostasis under normal and diabetic conditions. It may be speculated that ginkgolide B possesses therapeutic potential for cardiomyocyte dysfunction in diabetes, although caution must be urged with respect to its clinical value in the treatment of heart disease.
Acknowledgements
This work was supported in part by the American Heart Association. JK was supported in part by the University of Wyoming Experimental Program to Stimulate Competitive Research (EPSCoR).
References
- Kleijnen J, Knipschild P. Ginkgo biloba. Lancet 1992;340:1136-9.
- Cheng D, Chen W. Effects of ginkgolide B on isobaric hypoxic pulmonary hypertension in rats. Chin Med J (Engl) 1996;109:881-4.
- Koltai M, Tosaki A, Hosford D, Braquet P. Ginkgolide B protects isolated hearts against arrhythmias induced by ischemia but not reperfusion. Eur J Pharmacol 1989;164:293-302.
- McKenna DJ, Jones K, Hughes K. Efficacy, safety, and use of Ginkgo biloba in clinical and preclinical applications. Altern Ther Health Med 2001;7:70-90.
- Maitra I, Marcocci L, Droy-Lefaix MT, Packer L. Peroxyl radical scavenging activity of Ginkgo biloba extract EGb 761. Biochem Pharmacol 1995;49:1649-55.
- Marcocci L, Packer L, Droy-Lefaix MT, Sekaki A, Gardes-Albert M. Antioxidant action of Ginkgo biloba extract EGb 761. Methods Enzymol 1994;234:462-75.
- Chen JW, Chen YH, Lin FY, Chen YL, Lin SJ. Ginkgo biloba extract inhibits tumor necrosis factor-alpha-induced reactive oxygen species generation, transcription factor activation, and cell adhesion molecule expression in human aortic endothelial cells. Arterioscler Thromb Vasc Biol 2003;23:1559-66.
- Wang ZG, Ren J. Current status and future direction of Chinese herbal medicine. Trends Pharmacol Sci 2002;23:347-8.
- Koltai M, Lepran I, Szekeres L, Viossat I, Chabrier E, Braquet P. Effect of BN 52021, a specific PAF-acether antagonist, on cardiac anaphylaxis in Langendorff hearts isolated from passively sensitized guinea-pigs. Eur J Pharmacol 1986;130:133-6.
- Wainwright CL, Parratt JR, Bigaud M. The effects of PAF antagonists on ischaemia and reperfusion arrhythmias and ischaemia-induced platelet aggregation. Biomed Biochim Acta 1988;47:S224-7.
- Kecskemeti V, Balogh I. Cardiac ultrastructural effects of the platelet-activating factor and its antagonist BN 52021. Exp Toxicol Pathol 1995;47:463-70.
- Chen X, Chen WZ. Recent pharmacological progress of Ginkgo biloba extract for cardiovascular and neuronal diseases. Chin J Integrated Tradit West Med 1996;2:300-4.
- Chen X, Liu LY, Lee TJF. Cardiovascular protective effects and NO-mediated cerebro-relaxant effects of extract of Ginkgo biloba leaves. Natl Med J China 1998;78:692-6.
- Pietri S, Maurelli E, Drieu K, Culcasi M. Cardioprotective and anti-oxidant effects of the terpenoid constituents of Ginkgo biloba extract (EGb 761). J Mol Cell Cardiol 1997;29:733-42.
- Niu YH, Yang XY, Bao WS. Protective effects of Ginkgo biloba extract on cultured rat cardiomyocytes damaged by H2O2. Acta Pharmacol Sin 1999;20:635-8.
- Fitzl G, Martin R, Dettmer D, Hermsdorf V, Drews H, Welt K. Protective effects of Gingko biloba extract EGb 761 on myocardium of experimentally diabetic rats. I: ultrastructural and biochemical investigation on cardiomyocytes. Exp Toxicol Pathol 1999;51:189-98.
- Welt K, Weiss J, Martin R, Dettmer D, Hermsdorf T, Asayama K, et al. Ultrastructural, immunohistochemical and biochemical investigations of the rat liver exposed to experimental diabetes and acute hypoxia with and without application of Ginkgo extract. Exp Toxicol Pathol 2004;55:331-45.
- Ren J, Davidoff AJ. Diabetes rapidly induces contractile dysfunctions in isolated ventricular myocytes. Am J Physiol 1997;272:H148-58.
- Ren J, Ceylan-Isik AF. Diabetic cardiomyopathy: do women differ from men? Endocrine 2004;25:73-83.
- Ren J, Dominguez LJ, Sowers JR, Davidoff AJ. Metformin but not glyburide prevents high glucose-induced abnormalities in relaxation and intracellular Ca2+ transients in adult rat ventricular myocytes. Diabetes 1999;48:2059-65.
- Ren J, Duan J, Hintz KK, Ren BH. High glucose induces cardiac insulin-like growth factor I resistance in ventricular myocytes: role of Akt and ERK activation. Cardiovasc Res 2003;57:738-48.
- Bers DM. Cardiac excitation-contraction coupling. Nature 2002;415:198-205.
- Kusmic C, Basta G, Lazzerini G, Vesentini N, Barsacchi R. The effect of Ginkgo biloba in isolated ischemic/reperfused rat heart: a link between vitamin E preservation and prostaglandin biosynthesis. J Cardiovasc Pharmacol 2004;44:356-62.
- Qi XY, Zhang ZX, Xu YQ. Effects of Ginkgolide B on action potential and calcium, potassium current in guinea pig ventricular myocytes. Acta Pharmacol Sin 2004;25:203-7.
- Satoh H. Effects of Ginkgo biloba extract and bilobalide, a main constituent, on the ionic currents in guinea pig ventricular cardiomyocytes. Arzneimittelforschung 2003;53:407-13.
- Zhang ZX, Qi XY, Xu YQ. Effect of ginkgolide B on L-type calcium current and cytosolic [Ca2+]i in guinea pig ischemic ventricular myocytes. Acta Physiol Sin 2003;55:24-8.
- Duan J, Zhang HY, Adkins SD, Ren BH, Norby FL, Zhang X, et al. Impaired cardiac function and IGF-I response in myocytes from calmodulin-diabetic mice: role of Akt and RhoA. Am J Physiol Endocrinol Metab 2003;284:E366-76.
