Ascorbic acid improves impaired venous and arterial endothelium-dependent dilation in smokers1
Introduction
Chronic cigarette smoking is a major risk factor for cardiovascular disease and is associated with dose-dependent arterial endothelial dysfunction and hemodynamic changes[1–3]. The cause of endothelial dysfunction in smokers is not known. Carbon monoxide and nicotine have been implicat-ed[4–6], but the mechanism that seems to have the greatest effect is oxidant injury[7,8].
Although probably multifactorial, it has been hypothesized that the adverse effects of smoking may result from an accumulation of oxidative damage caused by the reactive oxygen species (ROS) on endothelial cells[9]. Low plasma concentrations of nitric oxide (NO)[10], which is a possible sign of endothelial dysfunction, along with low plasma concentrations of ascorbic acid (AA) have been reported in long-term habitual smokers[11]. AA has a broad spectrum of anti-oxidant activities because of its ability to react with numerous aqueous free radicals and ROS and it effectively protects lipids in human plasma against peroxidative damage[12]. It has been demonstrated that the administration of AA in arterial beds markedly improves endothelium-mediated vasodilation in chronic smokers[13], but its in vivo effect in veins has not been studied yet.
In contrast to the continuous production of NO in the arterial endothelium, a very low basal production of NO has been demonstrated in the venous endothelium[14,15]. In contrast, in the cultured endothelial cells of rats, higher endothelial isoform of the nitric oxide synthase (NOS) protein levels, nitric oxide synthase (NOS) activity, and intracellular L-arginine have been found in veins compared with that in arteries[16]. There is considerable evidence of heterogeneity between the arterial and the venous endothelium[17]. The goal of the present study was to compare the acute response of AA in both vascular beds in smokers.
Materials and methods
Study population The volunteers for this study were as follows: the control group comprised 26 healthy subjects without familial history of coronary artery disease or arterial hypertension (blood pressure<140/90 mmHg), who were also non-hypercholesterolemic, non-diabetic and non-smokers without a history of being regular passive smokers. The smoker group comprised 23 regular smokers (normally 20 cigarettes daily) without familial history of coronary artery disease or arterial hypertension, who were also non-hypercholesterolemic and non-diabetic.
None of the subjects was taking regular medications and all were clinically well. Subjects with cardiac or cerebral ischemic vascular disease, impaired renal function, or other major pathologies were excluded from the study. In accordance with current legislation, all patients were aware of the investigational nature of the study and gave their written informed consent before participating. The Institutional Committee for Ethics in Research approved this study.
Experimental procedure All studies were initiated at 8:00 AM after overnight fasting, with the subjects lying in the supine position in a quiet air-conditioned room (22–24 °C). The subjects were admitted in the outpatient clinic of the Campinas State University Hospital (HC-UNICAMP)on 2 different occasions for 4 h studies. During the first study, measurements were performed after infusion of saline solution at a rate of 75 mL/h, iv. The second study was performed with infusion of saline solution (75 mL/h, iv) plus AA at a rate of 25 mg/min, iv.
Dorsal hand vein technique The dorsal hand vein technique, previously modified by Aellig[18], as used in our laboratory has been described in detail[5]. Briefly, a 23 G butterfly needle was inserted into a suitable vein on the back of the hand, with the arm positioned at an upward angle of 30 o to allow the complete emptying of the veins. A tripod, holding a linear variable differential transformer (LVDT; Schaevitz Engineering, Pennsauken, New Jersey, USA) was mounted on the back of the hand with its central aperture, containing a movable metal core, at a distance of 10 mm downstream from the tip of the needle. The signal output of the LVDT, which is linearly proportional to the vertical movement of the core, gave a measurement of the diameter of the vein. Readings were taken under a congestive pressure of 40 mmHg by inflating a blood pressure cuff placed on the upper portion of the arm under study. Results were presented as normalized dose-response curves in which the diameter of the vein during saline infusion is defined as 100% dilation. The vein was pre-constricted to 20% of the baseline size by infusing increasing doses of phenylephrine (12–3166 ng/min). This dose rate of phenylephrine was defined as the ED80 dose and this degree of constriction was defined as 0% dilation for the purposes of subsequent calculations. The vasodilation effects expressed in this study were calculated as a percentage in the range between 0% and 100% dilation. Drugs were infused using a Harvard infusion pump (Harvard Apparatus, South Natick, MA, USA) at a flow rate of 0.3 mL/min. Blood pressure and heart rate were monitored in the opposite arm with a Dynamap Blood Pressure Monitor (Critikon, Tampa, FL, USA). After pre-constriction of the vein by phenylephrine, dose-response curves of bradykinin (1–278 ng/min) and sodium nitroprusside (0.0187 ng/min–3166 ng/min) were constructed with 5 ng/min infusion rates in both smoker and non-smoker volunteers. AA (25 mg/min) was co-infused during the second study of each volunteer. Infusions at each rate lasted for 5 min with the sphygmomanometer cuff inflated to 45 mmHg for the last 2 min of the infusion.
Flow-mediated dilation Brachial artery flow-mediated dilation was measured with a 7.0 MHz linear array transducer and an ATL HDI system (Advanced Technology Labora-tories, Seattle, WA, USA) according to the manufacturer’s instructions[19]. The brachial artery was scanned longitudinally 5–10 cm above the elbow and a holder probe was used to hold the transducer in the same position throughout the procedure. The focus zone was set to the depth of the near wall of the artery. Depth and gain settings were set to optimize images of the lumen-arterial wall interface. The images were magnified by a resolution box function and measurements were taken from the anterior to posterior “m” line at the R-wave peak of the electrocardiogram. The brachial artery diameter was measured by identifying a clear section of the vessel in B-mode.
After the baseline-resting scan, a pneumatic cuff, placed at the wrist, was inflated to 300 mmHg for 5 min. The second scan was performed 45–120 s after cuff deflation. Fifteen minutes were allowed for vessel recovery, after which a second baseline scan was performed. Glyceryl trinitrate (0.4 mg; Nitrostat, Parke-Davis, Morris Plainf, New Jersey, USA) was then administered and the 4th scan of the brachial artery was undertaken. When the brachial artery diameter and blood flow had returned to baseline, flow-mediated dilation was determined after AA infusion (25 mg/min, iv; 10 min). Blood flow and brachial artery diameter data for glyceryl trinitrate and vitamin C represent measurements during the last minute of each infusion[20]. Two independent observers unaware of the subjects’ clinical details, and the type and stage of the study measured the vessel diameter. Repeated measurements in individuals using this technique are consistent and reproducible.
Statistical analysis Descriptive data were expressed as mean±SD. The dose-response curves were fitted to a sigmoid model[21], and the maximum effect (Emax) and the dose that causes 50% of the Emax (ED50), were determined. Parametric tests (Student’s paired and unpaired t-tests) were used to compare the Emax and lgED50 values. The sample size was calculated for a power of 0.80. P<0.05 was considered to be statistically significant.
Results
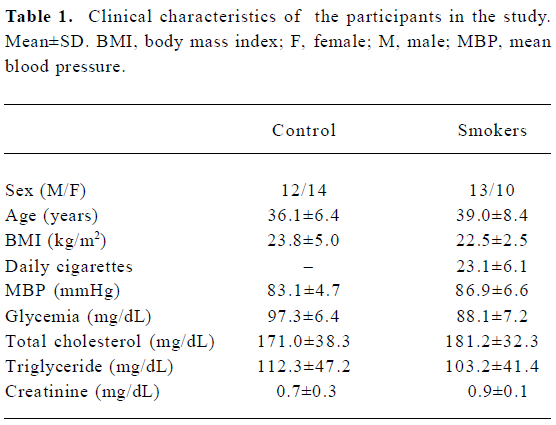
Clinical characteristics No statistical difference between smokers and non-smokers in terms of clinical characteristics was detected (Table 1). Chronic smokers had a history of 23.1±6.1 cigarettes a day. No side-effect was observed during the studies.
Full table
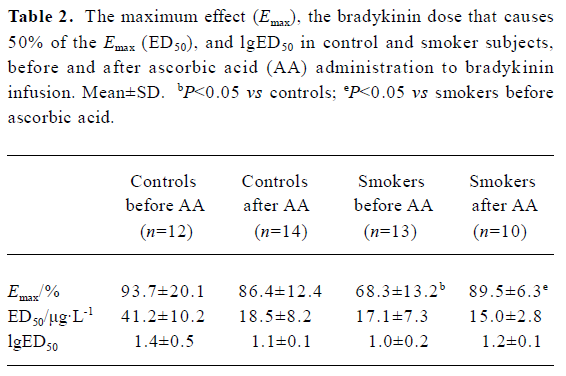
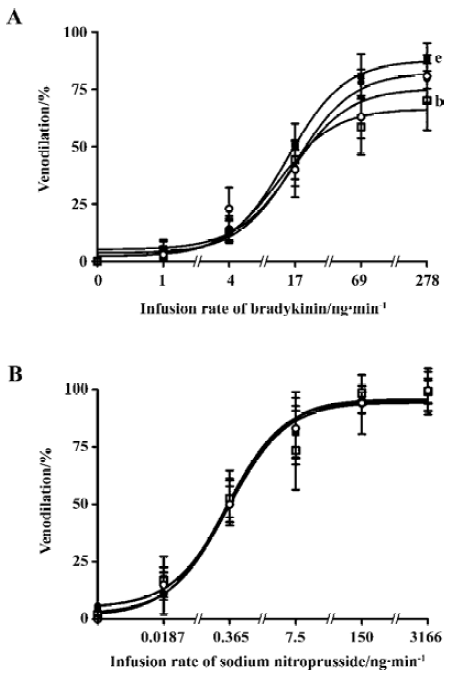
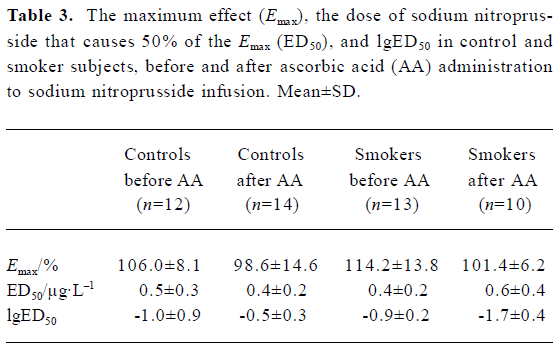
Dorsal hand vein Smokers had an impaired endothelium-dependent venodilation with bradykinin compared with non-smokers (68.3%±13.2% and 93.7%±20.1%, respectively; P<0.05) (Table 2; Figure 1). AA administration significantly increased the venodilation to bradykinin in smokers (68.3%±13.2% and 89.5%±6.3% before and after AA infusion, respectively; P<0.05), restoring it to levels similar to those observed in non-smokers (93.7%±20.1% and 86.4%±12.4% before and after AA infusion, respectively; P>0.05) (Table 2). The endothelium-independent venodilation response did not show significant differences (Table 3; Figure 1).
Full table
Full table
Flow-mediated dilation The arterial response measured by flow-mediated dilation showed an impaired endothelium-dependent vasodilation in smokers compared with non-smokers (8.8%±2.7% vs 15.2%±2.3%, respectively; P<0.05). AA administration significantly increased the endothelium-dependent vasodilation in response to reactive hyperemia in smokers (8.8%±2.7% vs 18.7%±6.5% before and after AA infusion, respectively; P<0.05), restoring it to similar levels to those observed in non-smokers (15.2%±2.3% vs 14.0%± 4.4% before and after AA, respectively). The endothelium- independent vasodilation response did not show significant difference (Table 4).
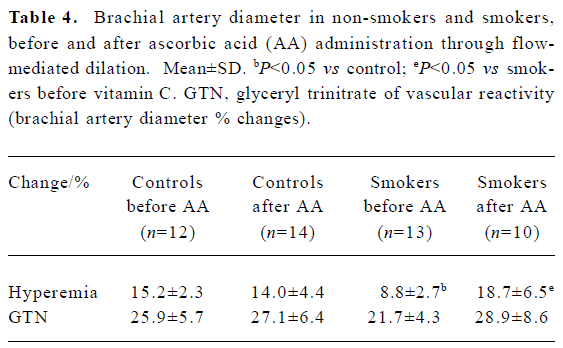
Full table
Hemodynamic findings Infusion of AA in smokers and non-smokers did not cause significant vascular changes, as evidenced using Portapress (TNO BMI, Amsterdam, Netherlands) device (Tables 5, 6), excepting a mild increase in the heart rate.
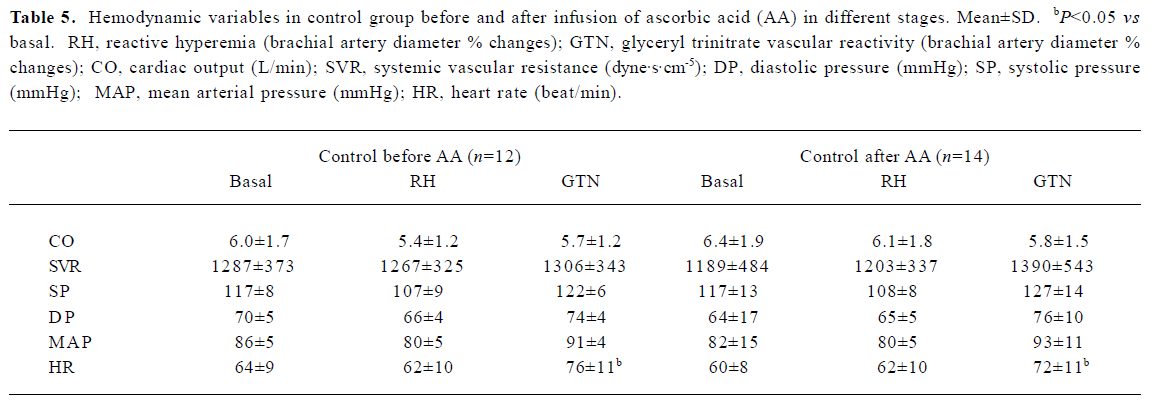
Full table
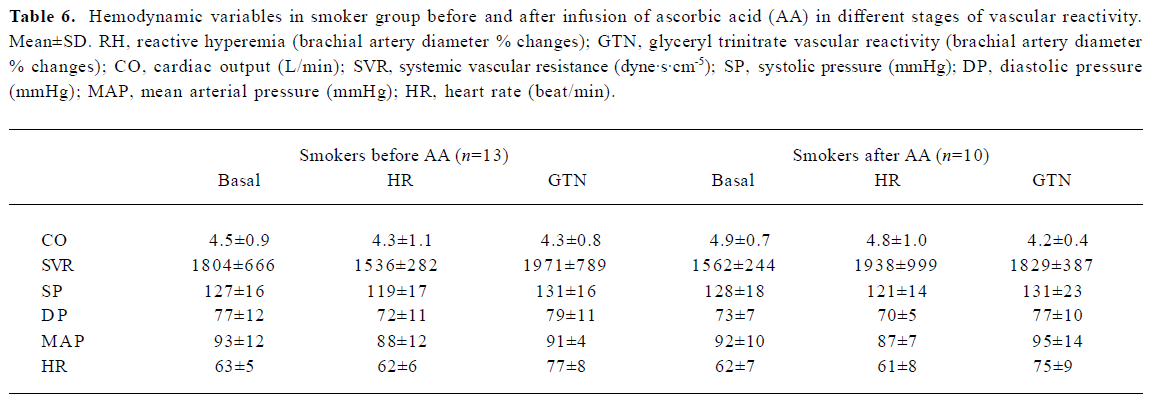
Full table
Discussion
The present study demonstrates that impaired endothelium-dependent vasodilation in chronic smokers could be markedly improved in both arterial and venous endothelium beds by acute administration of the anti-oxidant AA. In contrast to the continuous production of NO by the arterial endo-thelium, the basal production of NO from the venous endothelium is very low[22]. However, its production from the venous endothelium can be increased in response to bradykinin or other molecules[23]. Recently, Wagner et al found higher eNOS protein levels, NOS activity, and intra-cellular L-arginine levels exhibited in cultured venular endothelial cells from rat mesenteric circulation compared with cultured endothelial cells from their paired arterioles[16]. Bohlen reported that, although the mean NO concentration was not significantly different between vessel types because of the high variability in absolute values, the venous wall NO concentration was higher than the arterial wall NO concentration in approximately 80% in terms of small mesenteric artery vein pairs studied in vivo[24]. Other studies have suggested that the venous endothelium does not have a larger capacity for NO synthesis than the arterial endothelium and in some cases may generate less NO. For example, shear stress and acetylcholine stimulate less NO release from the jugular vein than from the nearby carotid artery[25] and shear stress elicits a smaller NO-dependent dilation in coronary venules than in their respective arterioles[26].
Many studies have demonstrated the superior long-term patency of arterial grafts compared with the saphenous vein, based on endothelial function[27]. A higher basal release of NO and endothelium-derived hyperpolarizing factor in arterial grafts[15], intact endothelial function and absence of intimal thickening in arterial grafts when compared with saphenous grafts[28], and the maintenance of the physiological NO and prostacyclin metabolisms, both soon after surgery and in long-term follow up, in arterial rather than venous grafts[29] suggest a higher bioavailability of NO in arterial beds. All these findings highlight the growing consensus that there is a marked regional and segmental heterogeneity in vascular endothelial function, with NO release and/or its vascular effects varying between and within specific vascular beds[17].
Previously, we demonstrated an impaired vasodilation in smokers using the dorsal hand vein technique[30] and flow-mediated dilation[31]. Now, our data show that acute AA administration reverses this abnormal response caused by cigarette smoking in both vascular beds. Clinical observations have shown oxygen-derived free radicals as mediators of smoking-induced endothelial dysfunction. Ascorbate improves or reverses endothelial dysfunction suggesting that there is a link between this and NO production or its metabolism. Depleted levels of AA, have been demonstrated in chronic smokers[11,32]. Ascorbate has been shown to reverse NO-dependent endothelial dysfunction present in the coronary arteries of patients with atherosclerosis[33], hyperhomocysteinemia[34], hypercholesterolemia[35], hypertension[36], and diabetes[37]. There are many mechanisms for the improvement of endothelial function by ascorbate[38]: (i) prevention of endothelial dysfunction induced by oxidized cholesterol low-density lipoproteins; (ii) ascorbate-induced release of NO from S-nitrosothiols in plasma; (iii) reduction of nitrite to NO; (iv) scavenging of superoxide by ascorbate; (v) thiol-dependent redox regulation of eNOS interaction with ascorbate; (vi) regulation of eNOS through tetra-hydrobio-pterin; (vii) ascorbate as a cofactor for eNOS; and (viii) effects on stimulation of guanylyl cyclase by NO. Many studies have shown these improvements in the arterial beds of regular smokers using flow-mediated dilation, impedance plethysmography, and intra-arterial infusions, but not in venous vascular beds.
Our findings demonstrate that endothelial dysfunction in smokers can be reversed by AA in venous and arterial vascular beds. Arteries and veins have different biological activities in terms of the endothelium, probably by marked regional and segmental heterogeneity in vascular endothelial function, but we have demonstrated similar findings in this specific vascular bed. The precise mechanism for this needs further investigation. Because veins are not susceptible to atherosclerosis, we have demonstrated that the dorsal hand vein technique can be used as a tool to assess vascular responsiveness in smokers.
These results show that there is a similar impaired responsiveness in arterial and venous endothelial-dependent vascular reactivity in smokers, and that after acute infusion of AA there is an improved responsiveness in both vascular beds.
Acknowledgments
This study, Heitor MORENO Jr, Maria Cláudia IRIGOYEN and Fernanda CONSOLIN-COLOMBO were supported by CNPq (São Paulo, Brasil). Márcio Gonçalves de SOUSA, Marcelo RUBIRA, Sílvia Elaine FERREIRA-MELO, Rodrigo PLENTZ and Deise BARBIERI thank FAPESP (São Paulo, Brasil) and CNPq (Brasília, Brasil).
References
- Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 1993;88:2149-55.
- Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog Cardiovasc Dis 2003;46:91-111.
- Tanus-Santos JE, Toledo JC, Cittadino M, Sabha M, Rocha JC, Moreno H Jr. Cardiovascular effects of transdermal nicotine in mildly hypertensive smokers. Am J Hypertens 2001;14:610-4.
- Samet JM. The 1990 report of the surgeon general: the health benefits of smoking cessation. Am Rev Respir Dis 1990;142:993-4.
- Sabha M, Tanus-Santos JE, Toledo JC, Cittadino M, Rocha JC, Moreno H Jr. Transdermal nicotine mimics the smoking-induced endothelial dysfunction. Clin Pharmacol Ther 2000;68:167-74.
- Neunteufl T, Heher S, Kostner K, Mitulovic G, Lehr S, Khoschsorur G, et al. Contribution of nicotine to acute endothelial dysfunction in long-term smokers. J Am Coll Cardiol 2002;39:251-6.
- Kalra J, Chaudhary AK, Prasad K. Increased production of oxygen free radicals in cigarette smokers. Int J Exp Pathol 1991;72:1-7.
- Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med 1995;332:1198-203.
- Reilly M, Delanty N, Lawson JA, FitzGerald GA. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation 1996;94:19-25.
- Node K, Kitakaze M, Yoshikawa H, Kosaka H, Hori M. Reversible reduction in plasma concentration of nitric oxide induced by cigarette smoking in young adults. Am J Cardiol 1997;79:1538-41.
- Tsuchiya M, Asada A, Kasahara E, Sato EF, Shindo M, Inoue M. Smoking a single cigarette rapidly reduces combined concentrations of nitrate and nitrite and concentrations of antioxidants in plasma. Circulation 2002;105:1155-7.
- Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA 1989;86:6377-81.
- Heitzer T, Just H, Munzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation 1996;94:6-9.
- Luscher TF, Diederich D, Siebenmann R, Lehmann K, Stulz P, von Segesser L, et al. Difference between endothelium-dependent relaxation in arterial and in venous coronary bypass grafts. N Engl J Med 1988;319:462-7.
- Liu ZG, Ge ZD, He GW. Difference in endothelium-derived hyperpolarizing factor-mediated hyperpolarization and nitric oxide release between human internal mammary artery and saphenous vein. Circulation 2000;102 Suppl 3:III296-301.
- Wagner L, Hoey JG, Erdely A, Boegehold MA, Baylis C. The nitric oxide pathway is amplified in venular vs arteriolar cultured rat mesenteric endothelial cells. Microvasc Res 2001;62:401-9.
- Boegehold MA. Heterogeneity of endothelial function within the circulation. Curr Opin Nephrol Hypertens 1998;7:71-8.
- Aellig WH. A new technique for recording compliance of human hand veins. 1981. Br J Clin Pharmacol 2004;58:S768-74.
- Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;39:257-65.
- Landmesser U, Merten R, Spiekermann S, Buttner K, Drexler H, Hornig B. Vascular extracellular superoxide dismutase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation 2000;101:2264-70.
- Mackay D. A generally useful modification of ALLFIT that facilitates the fitting of null equations to dose-response curves. Trends Pharmacol Sci 1988;9:121-2.
- Vallance P, Collier J, Moncada S. Nitric oxide synthesised from L-arginine mediates endothelium dependent dilation in human veins in vivo. Cardiovasc Res 1989;23:1053-7.
- Bedarida GV, Kim D, Blaschke TF, Hoffman BB. Characterization of an inhibitor of nitric oxide synthase in human-hand veins. Horm Metab Res 1994;26:109-12.
- Bohlen HG. Mechanism of increased vessel wall nitric oxide concentrations during intestinal absorption. Am J Physiol 1998;275:H542-50.
- Fukaya Y, Ohhashi T. Acetylcholine- and flow-induced production and release of nitric oxide in arterial and venous endothelial cells. Am J Physiol 1996;270:H99-106.
- Kuo L, Arko F, Chilian WM, Davis MJ. Coronary venular responses to flow and pressure. Circ Res 1993;72:607-15.
- Mangoush O, Nakamura K, Al-Ruzzeh S, Athanasiou T, Chester A, Amrani M. Effect of ascorbic acid on endothelium-dependent vasodilation of human arterial conduits for coronary artery bypass grafting. Eur J Cardiothorac Surg 2003;24:541-6.
- Komori K, Inoguchi H, Kume M, Shoji T, Furuyama T. Differences in endothelial function and morphologic modulation between canine autogenous venous and arterial grafts: endothelium and intimal thickening. Surgery 2002;131 Suppl:S249-55.
- Yang ZH, von Segesser L, Bauer E, Stulz P, Turina M, Luscher TF. Different activation of the endothelial L-arginine and cyclooxy-genase pathway in the human internal mammary artery and saphenous vein. Circ Res 1991;68:52-60.
- Moreno H Jr, Chalon S, Urae A, Tangphao O, Abiose AK, Hoffman BB, et al. Endothelial dysfunction in human hand veins is rapidly reversible after smoking cessation. Am J Physiol 1998;275:H1040-45.
- Yugar-Toledo JC, Tanus-Santos JE, Sabha M, Sousa MG, Cittadino M, Tacito LH, et al. Uncontrolled hypertension, uncompensated type II diabetes, and smoking have different patterns of vascular dysfunction. Chest 2004;125:823-30.
- Schectman G, Byrd JC, Gruchow HW. The influence of smoking on vitamin C status in adults. Am J Public Health 1989;79:158-62.
- Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 2001;104:2673-8.
- Chambers JC, McGregor A, Jean-Marie J, Obeid OA, Kooner JS. Demonstration of rapid onset vascular endothelial dysfunction after hyperhomocysteinemia: an effect reversible with vitamin C therapy. Circulation 1999;99:1156-60.
- Perticone F, Ceravolo R, Maio R, Cloro C, Candigliota M, Scozzafava A, et al. Effects of atorvastatin and vitamin C on endothelial function of hypercholesterolemic patients. Atherosclerosis 2000;152:511-8.
- Sherman DL, Keaney JF Jr, Biegelsen ES, Duffy SJ, Coffman JD, Vita JA. Pharmacological concentrations of ascorbic acid are required for the beneficial effect on endothelial vasomotor function in hypertension. Hypertension 2000;35:936-41.
- Hirai N, Kawano H, Hirashima O, Motoyama T, Moriyama Y, Sakamoto T, et al. Insulin resistance and endothelial dysfunction in smokers: effects of vitamin C. Am J Physiol Heart Circ Physiol 2000;279:H1172-8.
- May JM. How does ascorbic acid prevent endothelial dysfunction? Free Radic Biol Med 2000;28:1421-9.
