ONO-1078 reduces NMDA-induced brain injury and vascular cell adhesion molecule-1 expression in rats1
Introduction
It is well known that N-methyl-D-aspartate (NMDA) receptors play a critical role in the initiation of cerebral ischemic injury[1]. The excitotoxicity mediated by NMDA receptors contributes to the damage that occurs in the early stages (min to h), and inflammation and programmed cell death in the late stages (h to weeks) of cerebral ischemia[1,2]. Among many factors involved in inflammation, adhesion molecules, including vascular cell adhesion molecule 1 (VCAM-1), can modulate ischemic injury in the brain[3,4]. However, cysteinyl leukotrienes (CysLTs, including LTC4, LTD4, and LTE4), 5-lipoxygenase (5-LOX) metabolites of arachidonic acid, are potent inflammatory mediators[5] that are also involved in pathogenic processes of cerebral ischemia. CysLT levels were elevated after global and focal cerebral ischemia and 5-lipoxygenase inhibitor attenuated CysLT production and ischemic brain injury[6–8]. Further-more, we have previously found that ONO-1078 {pranlukast, 4-oxo-8-[p-(4-phenylbutyloxy)benzoyl-amono] -2-(tetrazol-5-yl)-4H-1-benzopyran hemihydrate}, a CysLT1 receptor anta-gonist, protects rats and mice against focal cerebral ischemic injury[9,10].
Recently, we reported that global cerebral ischemia induces brain injury and time-dependently increases the expressions of NMDA receptor subunit proteins and VCAM-1 in different regions of the brain in rats[11]. We also found that ONO-1078 attenuates ischemic injury and inhibits the increased expressions of NMDA receptor subunit NR2A and VCAM-1[12]. However, whether ONO-1078 protects rats from chemically induced injury, and if NMDA receptor activation directly links to the increased VCAM-1 expression, remains unknown. Thus, in the present study, we induced cortical injury by NMDA microinjection to evaluate the protective effect of ONO-1078. Also, we observed VCAM-1 expression after NMDA receptor activation and the influence of ONO-1078. Edaravone (MCI-186, 3-methyl-1-phenyl-2- pyrazolin-5-one), a novel neuroprotective agent for ischemic stroke[13,14], was used as a control drug.
Materials and methods
Drugs and reagents NMDA was purchased from Sigma (St Louis, MO, USA); ONO-1078 was provided by Dr Masami TSUBOSHIMA (Ono Pharmaceutical Co, Osaka, Japan). This compound was dissolved in 100% ethanol (10 g/L), and freshly diluted with saline before use. Edaravone injection was obtained from Hangzhou Conba Pharmaceutical Co; polyclonal goat antibody against VCAM-1 from Santa Cruz, CA, USA; rabbit anti-goat IgG-HRP was obtained from Zhong-Shan Biotech Co, Beijing; enhanced chemiluminescence (ECL) reagent was obtained from Renaissance, New England Nuclear-Dupont. Other reagents were commercial products with analytic purity.
NMDA microinjection in neocortex Male Sprague-Dawley rats weighting 250–300 g were from the Experimental Animal Center of Zhejiang Academy of Medical Sciences (Grade II, Certificate N
After these procedures, the incisions were closed and rats were returned to their cages. ONO-1078 (0.03, 0.1, and 0.3 mg/kg), edaravone (10 mg/kg) or the same volumes of saline were ip injected 30 min before and after NMDA injection.
Evaluation of tissue injury Twenty-four hours after NMDA microinjection, the rats were anesthetized with chloral hydrate again and decapitated. Brains were removed immediately and sectioned coronally into 6 slices (2 mm-thick). Brain slices were stained in 0.5% 2,3,5-triphenyl tetrzolium chloride at 37 ºC for 30 min, followed by fixation with 10% formalin, overnight. All the brain slices were photographed using a CCD camera (MV-CP-230, Panasonic, Japan) on an anatomy microscope (XTL 2600, Shanghai, China). The volume and area of NMDA-injured cortex were measured by an image analyzer (AnalyPower1.0, Zhejiang University, Hangzhou, China).
Histopathological assessment Under chloral hydrate anesthesia, the rats were infused with 100 mL of heparinized saline followed by 300 mL of 10% formalin, before being decapitated. Brains were removed and fixed in 10% formalin for 7 d; paraffin sections (5 µm) were then cut and stained with hematoxylin and eosin. The densities of survival neurons in the cortex were counted using the image analyzer (AnalyPower1.0, Zhejiang University, Hangzhou, China).
Western blot analysis Western blot analysis was performed as described by Kang et al[16]. The injured and contralateral cortices were dissected and homogenized in buffer A (10 mmol/L Tris-HCl, pH 7.4, containing 320 mmol/L sucrose). The homogenate was centrifugated at 700×g for 10 min at 4 ºC, and the supernatant was further centrifuged at 37 000×g for 40 min at 4 ºC. The resultant pellet was resuspended in buffer B (10 mmol/L Tris-HCl, pH 7.4), and protein concentration was determined by the Lowry method.
The protein samples of 7.5 µg were separated by 7.5% SDS-PAGE and transferred to a nitrocellulose membrane in transfer buffer (Tris 25 mmol/L, glycine 192 mmol/L, 20% methanol, 0.05% SDS, pH 8.3). The nonspecific binding was blocked in 5% fat-free dry milk in TBST (Tris-HCl 20 mmol/L, NaCl 140 mmol/L, 0.1% Tween-20, pH 7.4) for 30 min at room temperature. The membrane was incubated with polyclonal goat antibody against VCAM-1 (1:500) overnight at 4 ºC. After washing with TBTS, the membrane was then incubated with HRP-conjugated rabbit anti-goat IgG (1:2000) for 2 h at room temperature followed by repeated washing with TBST. At the end, the membrane was incubated in enhanced chemiluminescence (ECL) solution for 1 min and then exposed to X-ray film. The protein bands on an X-ray film were scanned by a GS-800 Laser Densitometer (Bio-Rad, USA) and analyzed by Met Imaging Series 5.0 (Bio-Rad, USA). As a standard, a protein sample from normal rat brains was used and the relative expression of VCAM-1 was calculated as the ratio of tested/standard sample densities.
Statistical analysis Data were reported as mean±SD. Statistical evaluation was carried out by the independent-sample t-test or one-way ANOVA (SPSS 11.0 for Windows, SPSS Inc, USA), according to the experimental design. P<0.05 was considered statistically significant.
Results
NMDA-induced injury in vivo NMDA microinjection produced well-defined focal lesions (Figure 1) dose- and time-dependently in TTC-stained brain slices. The lesion volumes induced by NMDA 0.3 μmol (32.4±10.7, n=5) were significantly larger than those by 0.1 and 0.2 µmol (11.1±5.5 and 15.6±7.6, n=5, P<0.01, respectively) 24 h after NMDA injection. The lesion volumes at 24 h after NMDA (0.3 µmol) microinjection (35.4±7.0, n=4) were larger than those at 1, 3, or 6 h after injection (21.8±8.0, 25.1±8.8, or 30.7±9.2, respectively, n=4, P<0.05).

Histopathological changes in the lesion regions 24 h after NMDA (0.3 μmol) injection were characterized by neuronal damage with eosinophilic cytoplasm and serious pyknotic nucleus, and survival neuron decreased markedly in the injured cortices; however, no cell damage was observed in the contralateral cortices or in PBS-injected control cortices (Figure 2).
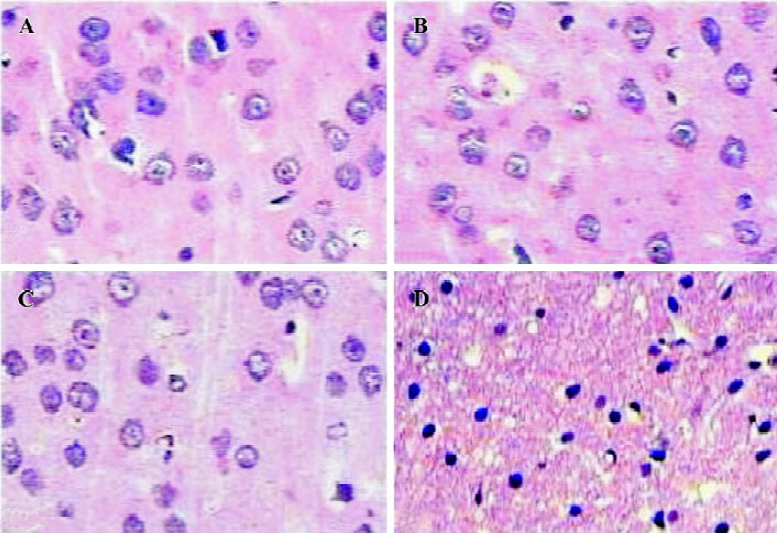
Effects of ONO-1078 and edaravone on NMDA-induced injury ONO-1078 at 0.1 and 0.3 mg/kg significantly reduced the lesion volume (P<0.05 or P<0.01, Table 1) and the lesion areas in individual 2 mm-thick consecutive slices (P<0.05 or P<0.01, Table 2), and the enlargement of injected cortices (indicated as I/C ratio) at 0.1 mg/kg (P<0.05, Table 1). The survival neuron densities in NMDA-injured regions showed a tendency to increase in the rats treated with ONO-1078, but no significant difference was found among these groups (P>0.05, Table 1). Edaravone (10 mg/kg) exhibited a similar effect to ONO-1078 (Tables 1, 2).

Full table

Full table
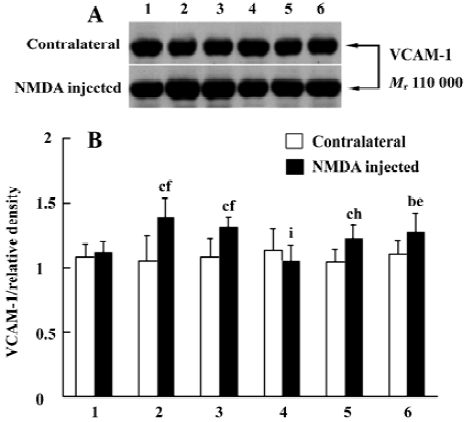
Effects of ONO-1078 and edaravone on VCAM-1 expression in NMDA-injected cortex To assess the relation between the protection of ONO-1078 and the expression of inflammatory adhesion molecule VCAM-1, we detected the local expression of VCAM-1 in contralateral and injured cortices 24 h after NMDA injection. VCAM-1 level did not remarkable change among the groups in the contralateral cortices without damage. In the injured cortices, however, VCAM-1 expression was up-regulated. ONO-1078 (0.1 and 0.3 mg/kg) significantly inhibited the increased VCAM-1 expression (P<0.05 or P<0.01), however, edaravone did not show a significant effect (P>0.05, Figure 3).
Discussion
The findings in the present study show that NMDA microinjection produces serious neocortical damage and a CysLT1 receptor antagonist ONO-1078 possessed protective effect on NMDA-induced brain injury. This effect of ONO-1078 on chemically-induced brain injury is consistent with its protective effect on brain ischemic injuries discussed in our previous studies[12]. Furthermore, the results of the present study indicates that NMDA microinjection up-regulates the local expression of VCAM-1, and ONO-1078 inhibits the enhanced VCAM-1 expression in the injured cortices.
The excessive release of excitory amino acids and activation of NMDA receptor are important events in cerebral ischemia and other brain injuries. NMDA receptor activation has been reported to be related to CysLT production in focal cerebral ischemia because NMDA receptor antagonist MK 801 reduces both CysLT production and ischemic injury[17]. Also, LTC4 synthesis inhibitor azelastine protects cultured hippocampal slices from hypoxic or NMDA-induced injury in vitro[18]. Therefore, the protective effect of ONO-1078 in this study might be via antagonizing the actions of resultant CysLTs after NMDA receptor activation.
However, the increased VCAM-1 expression by NMDA injection suggests a possibility that excitotoxicity might initiate inflammation in the brain. It has been reported that NMDA receptor activation can up-regulate the expression of inflammatory cytokines, such as TNF-α and MCP-1[19,20], and induce neuronal signal transduction-associated adhesion molecules, such as PSA-NCAM and NCAM-180[21,22]. Our results further confirmed that NMDA increases the expression of inflammatory adhesion molecule VCAM-1. Evidence has shown the involvement of VCAM-1 in cerebral ischemic injury and inflammation. Serum level of soluble VCAM-1 increased in patients with ischemic stroke, at its peak 5 d after ischemia[4]. VCAM-1 expression also increased in astrocytes and endothelial cells from the infarcted brain areas in patients who died of acute ischaemic stroke[23], and in cultured human cerebromicrovascular endothelial cells after being subjected to ischemia-like insults or inflammatory cytokinins (IL-1 and TNF-α)[24]. VCAM-1 promotes intracerebral inflammation by affecting leukocyte rolling, adhesion[25] or earlier steps of interaction between inflammatory cells (such as T lymphocytes) and microvascular endothelial cells[26]. Furthermore, we found that ONO-1078 inhibits NMDA-increased VCAM-1 expression. In other reported studies, CysLTs only slightly augmented VCAM-1 expression in human umbilical vein endothelial cells[27]; CysLT1 receptor antagonist ONO-1078[16,28] and montelukast[29] suppressed VCAM-1 expression in the lung after allergen challenge. Based on these findings, we hypothesized that NMDA receptor activation induces CysLT production, CysLTs activate their receptors followed by VCAM-1 expression. This might partially explain our previous results that ONO-1078 reduces VCAM-1 expression in rat global ischemia[12].
As a control drug used in this study, edaravone protected NMDA-induced injury but did not reduce VCAM-1 expression compared with ONO-1078. Edaravone is a scavenger reacting with hydroxyl radical (OH) [30] and might act on the signal pathways other than those of ONO-1078. In cultured human dermal microvascular endothelial cells, antioxidants show different actions on TNF-α-induced expressions of VCAM-1, ICAM-1 and E-selectin: pyrrolidine dithiocarbamate had inhibiting effects, but N-acetylcysteine did not[31]. In our previous study of rat global ischemia, edaravone also did not significantly inhibit VCAM-1 expression[12]. Its neuroprotective effect might be mediated by other actions, such as inhibition of mitochondrial permeability transition pore, upregulation of Bcl-2[32], normalization of irradiation-reduced endothelial nitric oxide synthase expression[33], and protection against hypoxia/ischemia-induced endoplasmic reticulum dysfunction[34].
In the present study, we found that ONO-1078 reduced NMDA-induced lesion size, but did not increase survival neurons in the lesion, suggesting incomplete protection. We have not yet explained the details of NMDA receptor activation in CysLTs production, VCAM-1 expression and brain injury, however, the results of the present study indicate that CysLT1 receptor antagonist ONO-1078 inhibits NMDA- induced brain injury and pro-inflammatory VCAM-1 expression, supplying further evidence for its neuroprotec-tive effect on ischemic brain injury.
Acknowledgment
We thank Dr Masami TSUBOSHIMA of Ono Pharmaceutical Co Ltd, Osaka, Japan, for providing us with ONO-1078.
References
- Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature 1999;399:A7-14.
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci 2003;26:248-54.
- Zaremba J, Losy J. Adhesion molecules of immunoglobulin gene superfamily in stroke. Folia Morphol (Warsz) 2002;61:1-6.
- Bitsch A, Klene W, Murtada L, Prange H, Rieckmann P. A longitudinal prospective study of soluble adhesion molecules in acute stroke. Stroke 1998;29:2129-35.
- Stewart LR, White AR, Jobling MF, Needham BE, Maher F, Thyer J, et al. Involvement of the 5-lipoxygenase pathway in the neurotoxicity of the prion peptide PrP106–126. J Neurosci Res 2001;65:565-72.
- Rao AM, Hatcher JF, Kindy MS, Dempsey RJ. Arachidonic acid and leukotriene C4: role in transient cerebral ischemia of gerbils. Neurochem Res 1999;24:1225-32.
- Shishido Y, Furushiro M, Hashimoto S, Yokokura T. Effect of nordihydroguaiaretic acid on behavioral impairment and neuronal cell death after forebrain ischemia. Pharmacol Biochem Behav 2001;69:469-74.
- Baskaya MK, Hu Y, Donaldson D, Maley M, Rao AM, Prasad MR, et al. Protective effect of the 5-lipoxygenase inhibitor AA-861 on cerebral edema after transient ischemia. J Neurosurg 1996;85:112-6.
- Zhang WP, Wei EQ, Mei RH, Zhu CY, Zhao MH. Neuroprotective effect of ONO-1078, a leukotriene receptor antagonist, on focal cerebral ischemia in rats. Acta Pharmacol Sin 2002;23:871-7.
- Zeng LH, Zhang WP, Wang RD, Wang LP, Wei EQ. Protective effect of ONO-1078, a leukotriene antagonist, on focal cerebral ischemia in mice. Acta Pharm Sin 2001;36:148-50.
- Zhang LH, Wei EQ. Time course of brain damage and expression of related molecules in rats with transient global cerebral ischemia. J Zhejiang Univ Med Sci 2002;31:77-80.
- Zhang LH, Wei EQ. Neuroprotective effect of ONO-1078, a leukotriene receptor antagonist, on transient global cerebral ischemia in rats. Acta Pharmacol Sin 2003;24:1241-7.
- Toyoda K, Fujii K, Kamouchi M, Nakane H, Arihiro S, Okada Y, et al. Free radical scavenger, edaravone, in stroke with internal carotid artery occlusion. Neurol Sci 2004;221:11-7.
- Yasuoka N, Nakajima W, Ishida A, Takada G. Neuroprotection of edaravone on hypoxic-ischemic brain injury in neonatal rats. Dev Brain Res 2004;151:129-39.
- Iadecola C, Niwa K, Nogawa S, Zhao X, Nagayama M, Araki E, et al. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci USA 2001;98:1294-9.
- Kang H, Wei EQ, Yang XH, Zhang WP, Shen JZ. VCAM-1 expression, eosinophil infiltration, and pharmacological modulation in rat allergic airway inflammation. Acta Pharmacol Sin 2002;23:157-61.
- Ciceri P, Rabuffetti M, Monopoli A, Nicosia S. Production of leukotrienes in a model of focal cerebral ischaemia in the rat. Br J Pharmacol 2001;133:1323-9.
- Wallis RA, Panizzon KL. Protection from hypoxic and N- methyl-D-aspartate injury with azelastine, a leukotriene inhibitor. Eur J Pharmacol 1993;238:165-71.
- Block F, Loos M, Frohn C, Schwarz M. Association between inflammation and nigral neuronal damage following striatal excitotoxic lesion. Brain Res 2004;998:29-35.
- Katayama T, Minami M, Nakamura M, Ito M, Katsuki H, Akaike A, et al. Excitotoxic injury induces production of monocyte chemoat-tractant protein-1 in rat cortico-striatal slice cultures. Neurosci Lett 2002;328:277-80.
- Bouzioukh F, Tell F, Jean A, Rougon G. NMDA receptor and nitric oxide synthase activation regulate polysialylated neural cell adhesion molecule expression in adult brainstem synapses. J Neurosci 2001;21:4721-30.
- Hoffman KB, Murray BA, Lynch G, Munirathinam S, Bahr BA. Delayed and isoform-specific effect of NMDA exposure on neural cell adhesion molecules in hippocampus. Neurosci Res 2001;39:167-73.
- Blann A, Kumar P, Krupinski J, McCollum C, Beevers DG, Lip GY. Soluble intercelluar adhesion molecule-1, E-selectin, vascular cell adhesion molecule-1 and von Willebrand factor in stroke. Blood Coagul Fibrinolysis 1999;10:277-84.
- Stanimirovic DB, Wong J, Shapiro A, Durkin JP. Increase in surface expression of ICAM-1, VCAM-1 and E-selectin in human cerebromicrovascular endothelial cells subjected to ischemia-like insults. Acta Neurochir Suppl (Wien) 1997;70:12-6.
- James WG, Bullard DC, Hickey MJ. Critical role of the alpha 4 integrin/VCAM-1 pathway in cerebral leukocyte trafficking in lupus-prone MRL/fas(lpr) mice. J Immunol 2003;170:520-7.
- Laschinger M, Engelhardt B. Interaction of alpha4-integrin with VCAM-1 is involved in adhesion of encephalitogenic T cell blasts to brain endothelium but not in their transendothelial migration in vitro. J Neuroimmunol 2000;102:32-43.
- Saito H, Shimizu H, Mita H, Maeda Y, Akiyama K. Histamine augments VCAM-1 expression on IL-4- and TNF-alpha-stimulated human umbilical vein endothelial cells. Int Arch Allergy Immunol 1996;111:126-32.
- Yang XH, Wei EQ. Expression of vascular cell adhesion molecule-1 in lung slices from antigen sensitized rats and pharmacological modulation. J Zhejiang Univ Med Sci 2003;32:319-22.
- Wu AY, Chik SC, Chen AW, Li Z, Tsang KW, Li W. Anti-inflammatory effects of high-dose montelukast in an animal model of acute asthma. Clin Exp Allergy 2003;33:359-66.
- Abe S, Kirima K, Tsuchiya K, Okamoto M, Hasegawa T, Houchi H, et al. The reaction rate of edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one (MCI-186)) with hydroxyl radical. Chem Pharm Bull (Tokyo) 2004;52:186-91.
- Jiang MZ, Tsukahara H, Ohshima Y, Todoroki Y, Hiraoka M, Maeda M, et al. Effects of antioxidants and nitric oxide on TNF-alpha-induced adhesion molecule expression and NF-kappaB activation in human dermal microvascular endothelial cells. Life Sci 2004;75:1159-70.
- Rajesh KG, Sasaguri S, Suzuki R, Maeda H. Antioxidant MCI-186 inhibits mitochondrial permeability transition pore and upregulates Bcl-2 expression. Am J Physiol Heart Circ Physiol 2003;285:H2171-8.
- Zhang XH, Matsuda N, Jesmin S, Sakuraya F, Gando S, Kemmotsu O, et al. Normalization by edaravone, a free radical scavenger, of irradiation-reduced endothelial nitric oxide synthase expression. Eur J Pharmacol 2003;476:131-7.
- Qi X, Okuma Y, Hosoi T, Nomura Y. Edaravone protects against hypoxia/ischemia-induced endoplasmic reticulum dysfunction. J Pharmacol Exp Ther 2004;311:388-93.
