Role of amygdala in mediating sexual and emotional behavior via coupled nitric oxide release1
Introduction to the structure and function of the amygdala
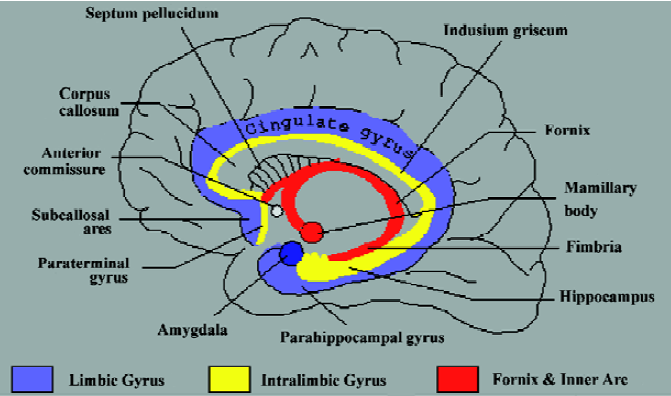
The region of the human brain commonly referred to as the amygdala comprises an area of approximately 3 cm3 [1,2]. At the dorsal base of the brain, the elevation of the para-hippocampus at the uncus is in part a result of the amygdala, which resides dorsal to it. Although neuro-anatomists often make reference to this portion as a single unitary structure, the amygdala is actually three distinct collections of nuclei. The largest portion of the amygdaloid complex is the basolateral nuclear group, consisting of the lateral nucleus, the irregular basal nucleus, and the accessory basal nucleus. The other major portion consists of the centro-medial group, which comprises the central nucleus and the medial nucleus. The centromedial group communicates via fibers of the stria terminalis to the bed nucleus of the stria terminalis (BST)[2] (Figure 1). Cell types in the BST are identical to those in the centromedial, causing the BST to be included in the classification of amygdalar tissue. The BST lies in the basal forebrain, which also contains the basal nucleus of Meynert, the nucleus accumbens, and the ventral portions of the putamen and globus pallidus. Anatomically, the smallest portion of the amygdaloid complex is the cortical nucleus; with primary input originating from the olfactory bulb and olfactory cortex, undoubtedly this plays a role in emotion-associated olfaction[2].
Nitric oxide correlates amygdalar function When we examine nitric oxide (NO) signaling, we notice two constitutive enzymatic components, the constitutive NO synthase (cNOS), including endothelial (eNOS) and neuronal (nNOS) isoforms. cNOS, as the name implies, is always expressed. When cNOS is stimulated, NO release occurs for a short period of time, but this level of NO can exert profound physiological actions for a long period of time[3]. NO not only is an immune, vascular, and neural autoregulatory signaling molecule, but also performs vital physiological activities via its constitutive expression[4,5].
Both the amygdala and the hippocampus contain numerous receptors for varying neurotransmitters. The central nucleus of the amygdala is most strongly modulated by dopamine, norepinephrine (NE), epinephrine, and serotonin[6,7]. The basal nuclei receive moderately high inputs of dopamine, NE, and serotonin[6,7], each of which has been demonstrated to exert their desired effect via NO[4]. Taken together, we surmise that NE initially promotes a slight vasoconstriction of the artery during the amygdalar compensatory response, which is defined as the limbic system's inherent mechanism to maintain homeostasis and lower stress levels. This mechanism is indicated by a slight enhancement of sympathetic activity on stimulation (ie, emotional), and is immediately followed by the release of NO from the peripheral nitroxi-dergic nerve, which mediates a concentration-dependent vasodilation[5]. In primates, the cerebral arterial diameter, under resting conditions, is maintained by tonic release of NO from the nerve (10%-20%), or from the nerve and endothelium (30%)[8]. This observation is supported by other data from our laboratory because of the fact that basal NO is cNOS-derived and keeps particular types of cells in a state of inhibition[5]. Endogenous superoxide dismutase in the cerebral artery appears to protect the relaxation induced by NO from perivascular nerves from the NO scavenger action of superoxide anions[9]. This NO then produces the longer-lived phenomenon of smooth muscle relaxation. In another report, it was found that NE vascular hyperresponsiveness in hypertension was dependent on an impairment of NO activity that was realized through NE-induced oxygen free radical production[10], providing an important contribution to the understanding of this regulatory process.
Amygdalar NO release and its relationship to sexual behavior
In addition to NO and the amygdala, new knowledge has emerged concerning the role of hypothalamic, limbic, and brainstem structures, neuropeptides, and brain monoamines in the control of partner preference, sexual desire, erection, copulation, ejaculation, orgasm, and sexual satiety ? the details of which are discussed below. At least one important sex difference exists between the male and female amygdala of many species. Owing to the interplay of the differing sex hormones, males and females will experience pleasure from differing experiences (eg, it has been shown that males are more visually stimulated than females[7,11]). In addition, modulating the concentration of testosterone may cause a male to partake in stereotypical “male behavior.” Likewise, modifying the concentration of estrogen may cause the female to partake in specified, sstereotypical “female behavior” [7,11]. The amygdala is intimately involved in sex and sexuality. It is important to note that the male amygdala is slightly bigger than that of the female. The medial part of the female amygdala plays an important role in pregnancy and appropriate coordination of the endocrine system. Stimulation of the amygdala will produce penile erection, sexual sensation, representations/memories of intercourse, and orgasm[7,12,13]. Furthermore, precortical region epilepsy has been shown to elicit spontaneous sexual arousal and orgasm, thus clearly demonstrating the role of the amygdala in sexual pleasure[12,13].
Stimulation of the corticomedial amygdala has been shown to induce ovulation in the female, and cutting the stria terminalis abolishes this effect. The introduction of tract lesions to the rat amygdala, including the medial nucleus, eliminates male libido, but not female libido[2,7,11,14]. In humans, temporal lobe epilepsy has been associated with sexual arousal in women to the point of orgasm; however, evidence of this in men is unsubstantiated[12,13].
Nitric oxide release has been demonstrated as the critical link between corticomedial stimulation and its relationship with the densely packed estrogen/androgen regions within the amygdala[15-19]. NO has been shown to be crucial for the occurrence of basal luteinizing hormone (LH) release in males[15], and for the LH surge in ovariectomized females treated with estradiol plus progesterone[16-18]. Furthermore, NO donors induce an LH surge in estradiol-treated ovariectomized females[16-20], and thus, have a progesterone-like effect. Concomitant findings show that estradiol stimulates nNOS expression in the preoptic area and exerts a helping influence on NO-producing neurons[17]. The released NO appears to be able to modulate the activity of gonadotrophic releasing hormone neurons (GnRH)[17]. These observations implicate neuronal NO in the regulation of GnRH cell activity in the preoptic area[20-23]. It is important to note that some studies suggest that at the median eminence (ME) level, the NO implicated in the modulation of GnRH release is endothelial in origin, rather than neuronal[23]. This is consistent with the fact that, unlike in the preoptic area where GnRH perikarya are surrounded by nNOS-containing cells, nNOS fibers and GnRH fibers in the ME are distributed separately in the internal and external zones, respectively[19]. Furthermore, in the ME, eNOS immunoreactivity is observed in endothelial cells of the pituitary portal blood vessels[20], located in immediate proximity to the GnRH terminals[21]. The endothelial origin of NO secreted from ME fragments is further substantiated by the results of prior reports that show that central administration of eNOS antisense is more efficacious than nNOS antisense administration in suppressing an estradiol-/progesterone-induced LH surge in ovariectomized females[21]. These findings are directly related to amygdalar function by way of neuronal projections extending from the amygdala precortical region to the ME (interestingly, this relationship can be made without regard to whether ME signaling occurs via neuronal or endothelial NO). Thus, we can hypothesize a more robust signaling system involving both NO from amygdalar origins, as well as hypothalamic hormonal relationships.
Emotional stressors mediated via amygdalar NO release
Morphine and related compounds mediating NO release within the amygdala The endocannabinoids, anandamide, and 2-arachidonyl glycerol, are naturally occurring, constitutively expressed, NO-stimulating signaling molecules[24]. Anandamide and morphine can also cause NO release from human immune cells, neural tissues, and human vascular endothelial cells[25]. Moreover, both anandamide and morphine can initiate invertebrate immune cell cNOS-derived NO[26]. Additionally, estrogen can stimulate cNOS-derived NO in human immune and vascular cells[27,28]. Anandamide, as part of the ubiquitous arachidonate and eicosanoid signaling cascade, serves to maintain and augment tonal NO in vascular tissues[24].
Both the hippocampus and the amygdala (particularly the lateral nucleus) contain high concentrations of receptors for the endocannabinoids[29,30]. In fact, reports have found endogenous morphine within the structure of the hippocampus[29,30]. In addition, this morphine activates pleasure pathways via NO and has been shown to do so in the rat brain hippocampus and amygdala[31-34]. Studies from our laboratory confirm the mediated release of NO via real-time amperometric measurement from the rat brain hippocampus[34] and amygdala[31]. This information can further be used to understand some of the pleasurable aspects of sexual activity that, indeed, are often found to have morphine-like properties and, perhaps, are mediated via these endocannabinoid and morphine laden amygdalar pathways[31,35]. Further credence to these findings stems from lesional data. Humans with amygdala lesions show a decrease in emotional tension and related sexual dysfunction[6,7]. It has been postulated that endocannabinoids and endogenous morphine may act on the lateral nucleus to prevent the linkage of sexual significance to sensory stimuli prior to conscious processing, thus interfering with the perception of sexually and emotionally charged stimuli[36].
Estrogen mediates NO release within the amygdala Estro-gen, through NO release, provides an additional pathway by which the system can downregulate immunocyte and vascular function in women[37]. This may be because of both the immune and vascular trauma associated with cyclic reproductive activities, such as endometrial buildup, when a high degree of vascular and immune activities occur. Given the extent of proliferative growth capacity during peak estrogen levels in this cycle, NO may function to enhance down regulation of the immune system to allow for these changes. Therefore, enhanced cNOS activity would be a beneficial effect within the concept and time framework of amygdalar compensation (as defined earlier) and the subsequent sense of calm it induces. Thus, these signal molecules, especially endocannabinoid and opiate alkaloids, have the potential to make you "feel" good and relax[38] by releasing NO, which may once again be part of the sexual resolution (post coitus) phase of the sexual cycle.
Emotionally charged events mediating NO release within the amygdala Within this context of varying stimuli evoking NO release, emotional stresses such as fear and anxiety can induce cardiovascular alterations, such as cardiac dysrhyth-mias. These are some of the same events that occur when one is exposed to sexually charged stimulus, or engaged in a sexual act[39-42]. These cardiovascular events are initiated at the level of the cingulated, amygdalar, and hypothalamic processes, as well as their projection into the higher level cerebral cortex, further altering the heart rate under stressful or sexually aroused conditions[43]. Neurons in the insular cortex, the central nucleus of the amygdala, and the lateral hypothalamus, owing to their role in the integration of emotional and ambient sensory input, may be involved in the emotional link to the cardiovascular phenomenon[44]. These include changes in cardiac autonomic tone with a shift from the cardioprotective effects of parasympathetic predominance to massive cardiac sympathetic activation[45]. This autonomic component, carried out with parasympathetic and sympathetic preganglionic cells via subcortical nuclei from which descending central autonomic pathways arise, may therefore be a major pathway in how emotional state may affect cardiovascular function. The importance of an elicited emotional response (and therefore limbic activation) was further demonstrated in ischemic heart disease when patients with frequent and severe ventricular ectopic rhythms were subjected to psychological stress[46]. The frequency and severity of ventricular ectopic beats increased dramatically during emotional activation of sympathetic mechanisms, but not during reflexively induced increased sympathetic tone. Perhaps we can even relate this mechanism to sexual orgasm, a process dominated by increased sympathetic tone.
The hard-wiring of emotional and sexual sensations coupled to cardiovascular neural processes probably involves many subcortical descending projections from the forebrain, midbrain, and, specifically, the amygdala[47-50]. Cardiovascular changes were observed in experiments where the motor cortex surface was stimulated, eliciting tachycardia accompanied by and independent of changes in arterial blood pressure[51]. The “sigmoid” cortex [52] and frontal lobe[53-55], and, in particular, the medial agranular region[56], subcallosal gyrus[57], septal area[58], temporal lobe[59], and cingulate gyrus[60-62] appear to be involved. The insular cortex in cardiac regulation is important because of its high connectivity with the limbic system, suggesting that the insula is involved in cardiac rate and rhythm regulation under emotional stress[53,54]. This form of regulation is mediated via a parasympathetic response, and is probably active in the resolution phase following orgasm[2,6,12,13].
The amygdala, with respect to autonomic-emotional integration[63,64], is composed of numerous subnuclei, which play a major role in the elaboration of autonomic responses[65]. There are profuse inputs to this region from the insular and orbitofrontal cortices, the parabrachial nucleus, and the nucleus tractus solitarius[66]. Amygdalo-tegmental projections are viewed as a critical link in cerebral cortical control of autonomic function[8,67]. This level of input allows for cerebral control of sexual behavior, such as showing sexual restraint and the ability to pass on sexual gratification. Indeed, a great deal of research center on sex-offenders? inability for, or lack of, the above-mentioned amygdalo-tegmental projections[68,69].
Mechanisms of amygdala-induced emotional compensation
As noted above, once individuals are exposed to sexually explicit or emotionally charged information, they experience peripheral vasodilation: warming of the skin, an increase in heart rate, and an ensuing sense of agitation[5,70]. This experience is remarkably similar to the physiological state that exists throughout the sexual cycle, from initial arousal through to resolution. It is the function of the amygdala to aid in the relief of these altered states, through the amygdalar primary compensatory response as defined above[2,6,7,53]. In examining a potential mechanism for this relief, besides the overriding central nervous system output via the autonomic nervous system, peripheral neuro-vascular processes would appear to be important. We surmise that NO is of fundamental importance in this response because of the increase in peripheral temperature (ie, vasodilation[5]). For a complete review of possible related mechanisms as well as the related mechanisms outlined above, see the studies by Toda et al[8], Lembo et al[10], Okamura et al[66], and Toda[67].
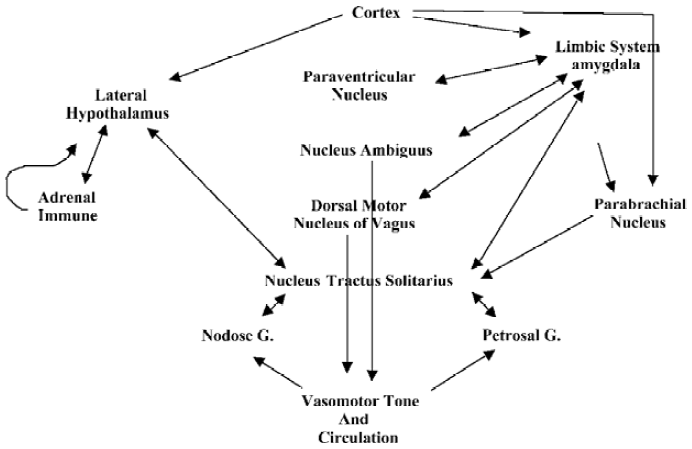
We also surmise, based on current studies, that endothelial-derived NO, released through normal pulsations as a result of vascular dynamics responding to heart beat[38], as well as acetylcholine-stimulated endothelial NO release, may contribute to the effect of NO in inducing smooth muscle relaxation[5,70]. Furthermore, vascular pulsations may be of sufficient strength to also stimulate nNOS-derived NO release, limiting any basal NE actions[5,70]. Interestingly, nitrosative stress, mediated by involvement of the reactive nitrogen oxide species, N2O3, does inhibit dopamine hydroxylase, which, in turn, inhibits NE synthesis and contributes to the regulation of neurotransmission and vasodilation[5,70]. This system may provide an autoregulatory mechanism involved in the neuronal control of peripheral vasomotor responses and may, once again, aid in the resolution phase of sexual intercourse (Figure 2).
Conclusion
Our conclusion is two-fold. We demonstrate that amygdalar regulation of the male and female sexual cycle is medicated by estrogen-/androgen-related signaling molecules, both of which exert their respective influences on ovulation and sexual behavior via coupled NO release. Furthermore, we propose that amygdalar-induced homeostatic control mechanisms acting in response to emotionally charged stimuli, including sexually stimulating sensations, appear to be mediated by a system of regulation involving NO as a neurotransmitter and as a locally acting hormone. Hence, these two principal roles of the amygdala exert their respective behaviors via NO.
In final summary, we have demonstrated numerous mechanisms and neurochemical pathways with regard to both emotion and sexual behavior (ovulation, arousal, etc), and we have shown a link between each of these complex pathways systems, as well as the use of NO as a major biochemical messenger. Moreover, throughout each of the aforementioned pathways, we have attempted to offer a possible relationship to sex, either as a mediator of direct sexual activity, or as a mediator of an individual aspect of the sexual cycle.
Acknowledgment
We thank John R HESSELINK, Professor of Radiology and Neurosciences at University of California San Diego Medical Center, San Diego, CA, for permission to use his figure of the limbic system (Figure 1).
References
- Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer's loop of the optic radiation. AJNR Am J Neuroradiol 2004;25:677-91.
- Thomas A, Woolsey JH, Mokhtar HG. The brain atlas. 2nd ed. New York: Wiley-Liss; 2002.
- Frank M. MAL, a proteolipid in glycosphingo lipid enriched domains: functional implications in myelin and beyond. Prog Neurobiol 2000;60:531-44.
- Cadet P, Zhu W, Mantione K, Rymer M, Dardik I, Reisman S, et al. Cyclic exercise induces anti-inflammatory signal molecule increases in the plasma of Parkinson's patients. Int J Mol Med 2003;12:485-92.
- Stefano GB, Fricchione GL, Slingsby BT, Benson H. The placebo effect and relaxation response: neural processes and their coupling to constitutive nitric oxide. Brain Res Brain Res Rev 2001;35:1-19.
- Joseph JT, Cardozo DL. Functional neuroanatomy: an interactive text and manual. New York: Wiley-Liss; 2004.
- Smith CM. Elements of molecular neurobiology. 3rd ed. New York: Wiley-Liss; 2002.
- Toda N, Tanaka T, Ayajiki K, Okamura T. Cerebral vasodilatation induced by stimulation of the pterygopalatine ganglion and greater petrosal nerve in anesthetized monkeys. Neuroscience 2000;96:393-8.
- Tanaka T, Okamura T, Handa J, Toda N. Neurogenic vasodilation mediated by nitric oxide in porcine cerebral arteries. J Cardiovasc Pharmacol 1999;33:56-64.
- Lembo G, Vecchione C, Izzo R, Fratta L, Marino G, Pilato G, et al. Noradrenergic vascular hyper-responsiveness in human hypertension is dependent on oxygen free radical impairment of nitric oxide activity. Circulation 2000;102:552-7.
- McClure EB, Monk CS, Nelson EE, Zarahn E, Leibenluft E, Bilder RM, et al. A developmental examination of gender differences in brain engagement during evaluation of threat. Biol Psychiatry 2004;55:1047-55.
- Janszky J, Szucs A, Halasz P, Borbely C, Hollo A, Barsi P, et al. Orgasmic aura originates from the right hemisphere. Neurology 2002;58:302-4.
- Tanuri FD, Thomaz RB, Tanuri JA. Temporal lobe epilepsy with aura of pleasure. Arq Neuropsiquiatr 2000;58:178-80.
- Rasia-Filho AA, Fabian C, Rigoti KM, Achaval M. Influence of sex, estrous cycle and motherhood on dendritic spine density in the rat medial amygdala revealed by the Golgi method. Neuroscience 2004;126:839-47.
- Rettori V, Belova N, Dees WL, Nyberg CL, Gimeno M, McCann SM. Role of nitric oxide in the control of luteinizing hormone-releasing hormone release and . Proc Natl Acad Sci USA 1993; 90: 10 130-4.
- Bonavera JJ, Sahu A, Kalra PS, Kalra SP. Evidence that nitric oxide may mediate the ovarian steroid-induced luteinizing hormone surge: involvement of excitatory amino acids. Endocrinology 1993;133:2481-7.
- Bonavera JJ, Sahu A, Kalra PS, Kalra SP. Evidence in support of nitric oxide (NO) involvement in the cyclic release of prolactin and LH surges. Brain Res 1994;660:175-9.
- Bonavera JJ, Kalra PS, Kalra SP. -Arginine/nitric oxide amplifies the magnitude and duration of the leuteinizing hormone surge induced by estrogen: involvement by neuropeptide Y. Endocrinology 1996; 137: 1956-62.
- Pu S, Kalra PS, Kalra SP. Ovarian steroid-independent diurnal rhythm in cyclic GMP/nitric oxide efflux in the medial preoptic area: possible role in preovulatory and ovarian steroid-induced LH surge. J Neuroendocrinol 1998;10:617-25.
- Prevot V, Rialas C, Croix D, Salzet M, Dupouy JP, Puolain P, et al. Morphine and anandamide coupling to nitric oxide stimulated GnRH and CRF release from rat median eminence: neurovascular regulation. Brain Res 1998;790:236-44.
- Herbison AE, Simonian SX, Norris PJ, Emson PC. Relationship of neuronal nitric oxide synthase immunoreactivity to GnRH neurons in the ovariectomized and intact female rat. J Neuroendo-crinol 1996;8:73-82.
- Yamada K, Emson P, Hokfelt T. Immunohistochemical mapping of nitric oxide synthase in the rat hypothalamus and coloca-lization with neuropeptides. J Chem Neuroanat 1996;10:295-316.
- Prevot V, Dutoit S, Croix D, Tramu G, Beauvillain JC. Semi-quantitative ultrastructural analysis of the localization and neuropeptide content of gonadotropin releasing hormone nerve terminals in the median eminence throughout the estrous cycle of the rat. Neuroscience 1998;84:177-91.
- Stefano GB, Salzet M, Magazine HI, Bilfinger TV. Antagonist of LPS and IFN-γ induction of iNOS in human saphenous vein endothelium by morphine and anandamide by nitric oxide inhibition of adenylate cyclase. J Cardiovasc Pharmacol 1998;31:813-20.
- Stefano GB, Salzet M, Bilfinger TV. Long-term exposure of human blood vessels to HIV gp120, morphine and anandamide increases endothelial adhesion of monocytes: uncoupling of nitric oxide. J Cardiovasc Pharmacol 1998;31:862-8.
- Stefano GB, Salzet B, Salzet M. Identification and characterization of the leech CNS cannabinoid receptor: coupling to nitric oxide release. Brain Res 1997;753:219-24.
- Stefano GB, Cadet P, Breton C, Goumon Y, Prevot V, Dessaint JP, et al. Estradiol-stimulated nitric oxide release in human granulocytes is dependent on intracellular calcium transients: evidence for a cell surface estrogen receptor. Blood 2000;95:3951-8.
- Stefano GB, Goumon Y, Casares F, Cadet P, Fricchione GL, Rialas C, et al. Endogenous morphine. Trends Neurosci 2000;9:436-42.
- Bianchi E, Guarna M, Tagliamonte A. Immunocytochemical localization of endogenous codeine and morphine. Adv Neuro-immunol 1994;4:83-92.
- Spector S, Munjal I, Schmidt DE. Endogenous morphine and codeine. Possible role as endogenous anticonvulsants. Brain Res 2001;915:155-60.
- Zhu W, Ma Y, Bell A, Esch T, Guarna M, Bilfinger TV, et al. Presence of morphine in rat amygdala: evidence for the 3 opiate receptor subtype via nitric oxide release in limbic structures. Med Sci Monit 2004;10:BR433-9.
- Steffens M, Engler C, Zentner J, Feuerstein TJ. Cannabinoid CB1 receptor-mediated modulation of evoked dopamine release and of adenylyl cyclase activity in the human neocortex. Br J Pharmacol 2004;141:1193-203.
- Castellano C, Rossi-Arnaud C, Cestari V, Costanzi M. Cannabinoids and memory: animal studies. Curr Drug Targets CNS Neurol Disord 2003;2:389-402.
- de la Torre JC, Pappas BA, Prevot V, Emmerling MR, Mantione K, Fortin T, et al. Hippocampal nitric oxide upregulation precedes memory loss and A beta I-40 accumulation after chronic brain hypoperfusion in rats. Neurol Res 2003;25:635-41.
- Esch T, Guarna M, Bianchi E, Zhu W, Stefano GB. Commonalities in the central nervous system抯 involvement with complementary medical therapies: limbic morphinergic processes. Med Sci Monit 2004;10:MS6-17.
- Azad SC, Zieglgansberger W. What do we know about the state of chronic pain? Schmerz 2003;17:441-4.
- Stefano GB. Endocannabinoid immune and vascular signaling. Acta Pharmacol Sin 2000;21:1071-81.
- Stefano GB, Prevot V, Cadet P, Dardik I. Vascular pulsations stimulating nitric oxide release during cyclic exercise may benefit health: a molecular approach. Int J Mol Med 2001;7:119-29.
- Lown B, DeSilva RA. Roles of psychologic stress and autonomic nervous system changes in provocation of ventricular premature complexes. Am J Cardiol 1978;41:979-85.
- Lown B, Verrier RL. Neural activity and ventricular fibrillation. N Engl J Med 1976;294:1165-70.
- Wellens HJ, Vermeulen A, Durrer D. Ventricular fibrillation occurring on arousal from sleep by auditory stimuli. Circulation 1972;46:661-5.
- Schiffer F, Hartley LH, Schulman CL, Abelmann WH. Evidence for emotionally-induced coronary arterial spasm in patients with angina pectoris. Br Heart J 1980;44:62-6.
- Holstege G. Some anatomical observations on the projections from the hypothalamus to brainstem and spinal cord: an HRP and autoradiographic tracing study in the cat. J Comp Neurol 1987;260:98-126.
- Holstege G, Meiners L, Tan K. Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Exp Brain Res 1985;58:379-91.
- Hopkins DA. Amygdalotegmental projections in the rat, cat and rhesus monkey. Neurosci Lett 1975;1:263-70.
- Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res 1978;32:529-47.
- Kuypers HGJM, Maisky VA. Retrograde axonal transport of horseradish peroxidase from spinal cord to brainstem cell groups in the cat. Neurosci Lett 1975;1:9-14.
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 1980;194:555-70.
- de la Torre JC, Stefano GB. Evidence that Alzheime's disease is a microvascular disorder: the role of constitutive nitric oxide. Brain Res Rev 2000;34:119-36.
- Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HHO, Das SK, Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest 1997; 100: 1538-46.
- MacLean PD. Discussion. Physiol Rev 1960;40:113-4.
- Smith WK. The functional significance of the rostral cingular cortex as revealed by its responses to electrical excitation. J Neurophysiol 1945;8:241-54.
- Ueda H. Arrhythmias produced by cerebral stimulation. Jpn Circ J 1962;26:225-30.
- Fimiani C, Liberty T, Aquirre AJ, Amin I, Ali N, Stefano GB. Opiate, cannabinoid, and eicosanoid signaling converges on common intracellular pathways: nitric oxide coupling. Prostaglandins Other Lipid Mediat 1999;57:23-34.
- Russchen FT. Amygdalopetal projections in the cat. I. Cortical afferent connections. A study with retrograde and anterograde tracing techniques. J Comp Neurol 1982;206:159-79.
- Calaresu FR, Ciriello J. Projections to the hypothalamus from buffer nerves and nucleus tractus solitarius in the cat. Am J Physiol 1980;239:R130-6.
- Bonvallet M, Bobo EG. Changes in phrenic activity and heart rate elicited by localized stimulation of amygdala and adjacent structures. Electroencephalogr Clin Neurophysiol 1972;32:1-16.
- Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol 1991;311:1-16.
- Kapp BS, Schwaber JS, Driscoll PA. Frontal cortex projections to the amygdaloid central nucleus in the rabbit. Neuroscience 1985;15:327-46.
- Beattie J, Brow GR, Long CNH. Physiological and anatomical evidence for the existence of nerve tracts connecting the hypothalamus with spinal sympathetic centres. Proc R Soc Lond B Biol Sci 1930;106:253-75.
- Magoun HW, Ranson SW, Heatherington A. Descending connections from the hypothalamus. Arch Neurol Psychiatry 1938;39:1127-49.
- Saper CB, Swanson LW, Cowan WM. An autoradiographic study of the efferent connections of the lateral hypothalamic area in the rat. J Comp Neurol 1979;183:689-706.
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol 1991;308:249-76.
- Hosoya Y, Matsushita M. Brainstem projections from the lateral hypothalamic area in the rat, as studied with autoradiography. Neurosci Lett 1981;24:111-6.
- ter Horst GJ, Luiten PG, Kuipers F. Descending pathways from hypothalamus to dorsal motor vagus and ambiguus nuclei in the rat. J Auton Nerv Syst 1984;11:59-75.
- Okamura T, Ayajiki K, Uchiyama M, Uehara M, Toda N. Neurogenic vasodilatation of canine isolated small labial arteries. J Pharmacol Exp Ther 1999;288:1031-6.
- Toda N. Mediation by nitric oxide of neurally-induced human cerebral artery relaxation. Experientia 1993;49:51-3.
- Dressing H, Obergriesser T, Tost H, Kaumeier S, Ruf M, Braus DF. Homosexual pedophilia and functional networks: an fMRI case report and literature review. Fortschr Neurol Psychiatr 2001;69:539-44.
- Howard RC. The neurophysiology of sexual desire, with particular reference to paedophilia. Ann Acad Med Singapore 1995;24:724-7.
- Salamon E, Kim M, Beaulieu J, Stefano GB. Sound therapy induced relaxation: down regulating stress processes and pathologies. Med Sci Monit 2003;9:RA96-101.