Effect of a single dose of mifepristone on expression of pinopodes in endometrial surface of mice1
Introduction
Mifepristone (RU486), a progesterone antagonist steroid, has been largely used for both planned and emergency contraception[1], as well as for termination of pregnancy[2]. Although the effect of RU486 for contraception has been described previously, its mechanism has not been completely elucidated. Anovulation, inhibition of fertilization and transportation of embryo through the Fallopian tube, and embryo implantation dysfunction have all been postulated. Recent data sustain the hypothesis that mifepristone reduces perivascular decidual haemostasis and increases extracellular matrix-degrading protease activity[3].
It is now well established that the cross-talk between the implanting embryo and the endometrial epithelium is a versatile and dynamic process, which requires a series of rather complicated and synchronous morphological and biochemical changes. Large and smooth membrane protrusions with pinocytotic function, known as pinopodes, have been observed in the endometrial surface during the window of implantation[4,5]. The formation and appearance of pinopodes appears to advance or regress, depending on hormonal milieu and other physiological modifications throughout the menstrual cycle[6]. Furthermore, a positive correlation between the pinopodes number and blastocyst implantation has been reported[7]. Fully developed pinopodes have been considered as the characteristic morphologic markers to assess endometrial receptivity and to locate the implantation window[8,9].
The aim of this experimental study was to investigate the timing of pinopodes expression in the endometrial surface epithelium of mice following a single dose administration of RU486.
Materials and methods
Animals and reagents Seventy female (weight: 28–30 g) and 15 male (weight: 40–45 g) adult Kunming mice (age 8–12 weeks) were provided by Sanitary Epidemic-Prevention Station of Hubei Province, China (Certificated N
Animal treatment Female and male mice in a ratio of 2:1 were kept overnight in a cage for mating. The following morning, the display of a vaginal plug in female mice was designated as day 1 of pregnancy (Pd1). The pregnant mice were divided into five groups, as follows: a) non-treated group; b) treated group 1; c) treated group 2; d) treated group 3; and e) treated group 4. The non-treated group was considered the control. All mice in the treated groups received mifepristone subcutaneously (0.1 mL solution containing 0.1 mg mifepristone) between 07:00 and 08:00 AM on Pd1, Pd2, Pd3, and Pd4, respectively.
Scanning electron microscopy Two mice selected randomly from each group were killed by cervical dislocation between 21:30 and 22:00 PM on Pd4, and another two mice in the same group were killed between 09:30 and 10:00 AM on Pd5. All remaining mice were killed on Pd7 in order to observe the pregnancy and the average number of implanted embryos.
The murine uterine horns were excised and then cut open along the longitudinal axis. The endometrial tissue was rinsed in saline solution, fixed in 2.5% (w/v) glutaraldehyde solution in a sodium cacodylate buffer (0.15 mol/L, pH 7.3) and post-fixed in a 1% (w/v) osmium tetroxide solution in a sodium cacodylate buffer (0.15 mol/L, pH 7.3) containing sucrose (75 mmol/L). The specimens were dehydrated in a graded series of acetone, then dried in a critical-point drier with carbon dioxide, mounted on the specimen holder, coated with gold palladium, and observed by scanning electron microscopy (SEM) (S-520, Hitachi, Tokyo, Japan).
To avoid inter-observer bias, all specimens were analyzed by the same observer. Based on development stage and abundance, pinopodes were scored as developing, fully developed, or regressing, and then as few (<20%), moderate (20%–50%), or abundant (>50%), respectively. If different development stages were observed in the same specimen, only the commonest pattern was reported[6].
Statistical analysis Data were expressed as mean±SD. Unpaired t-test and χ2-test were used. Software SPSS 11.0 for Windows was used. P<0.05 was considered to be statistically significant.
Results
Pregnancy rate and average implanted embryos No mice in treated groups 1, 2, or 3 conceived. In contrast, one mouse in group 4 achieved a pregnancy. Number of pregnant mice and average number of implanted embryos was significantly lower in treated group 4 as compared with controls (1 vs 9, P<0.01; 6 vs 14.67±1.35, P<0.01, respectively).
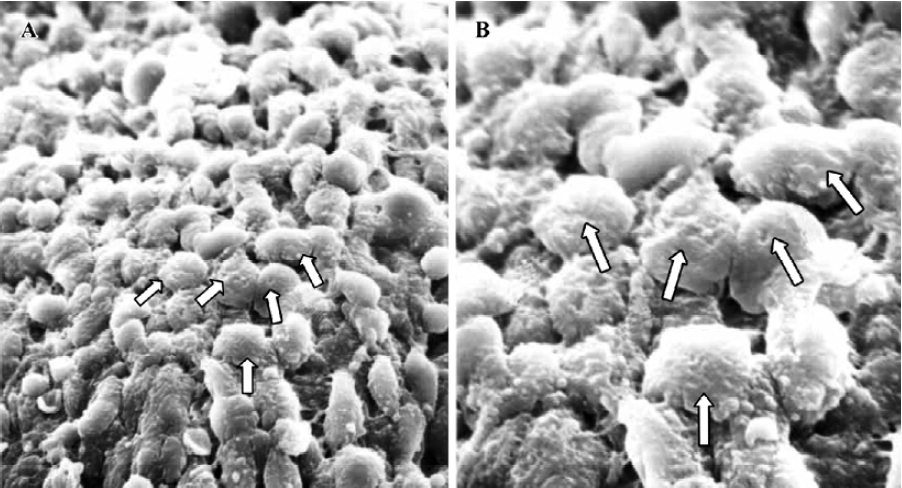
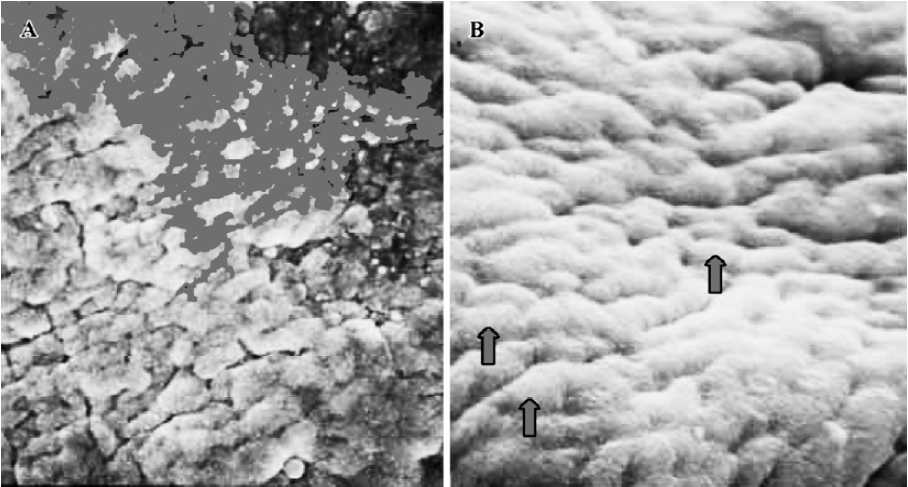
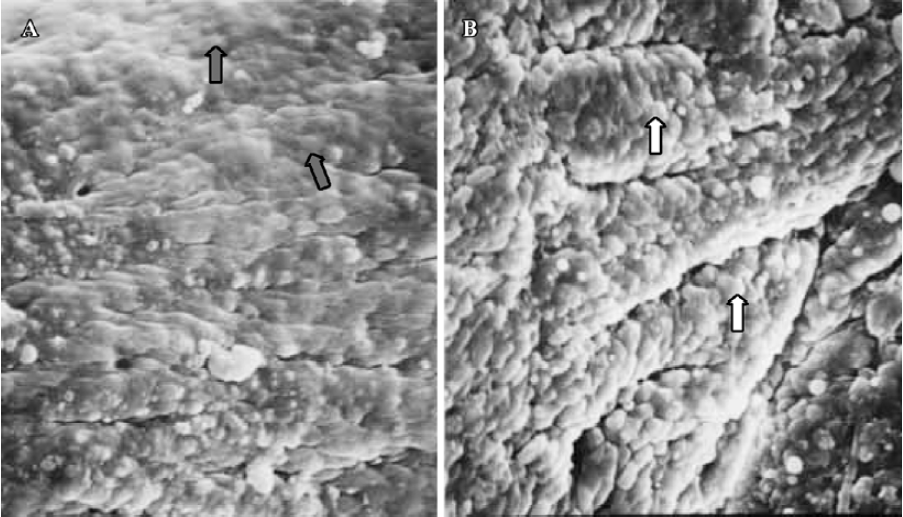
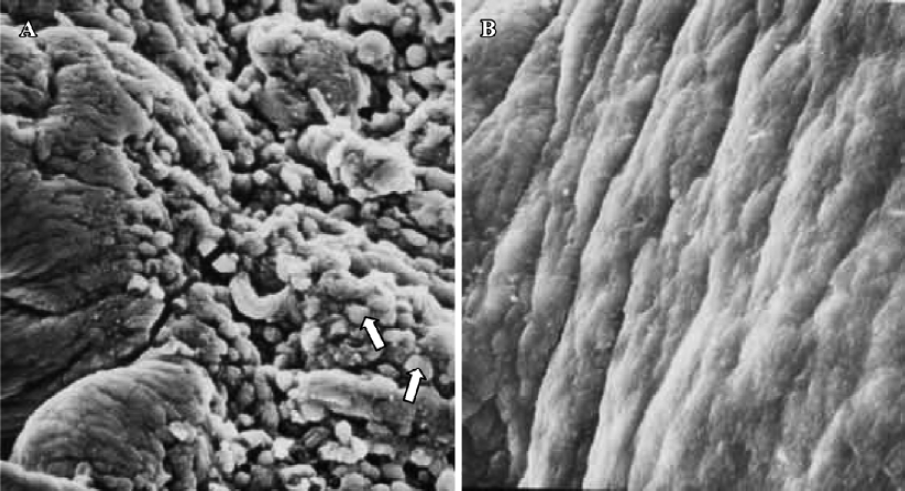
Pinopodes expression in endometrial surface In the control group, specimens collected between 21:30 and 22:00 PM on Pd4 showed abundant membrane projections widely distributed in the endometrial luminal surface. Clear, smooth, and slender projections were covered by short microvilli (developing pinopodes) (Figure 1). In specimens collected between 09:30 and 10:00 AM on Pd5, the endometrial surface was covered by membranous structures protruding and folding maximally (fully developed pinopodes). No microvilli were observed (Figure 2).
In treated group 1, the specimens collected between 21:30 and 22:00 PM on Pd4 showed smooth membrane projections. These structures, resembling developing pinopodes, were smaller, less abundant, and slightly lagged behind those present in controls (Figure 3). In specimens collected between 09:30 and 10:00 AM on Pd5, mainly fully developed pinopodes and developing pinopodes were present. Fully developed pinopodes were observed exclusively in epithelial cell depressions, while the neighboring surface was covered with short tips of microvilli (Figure 4).
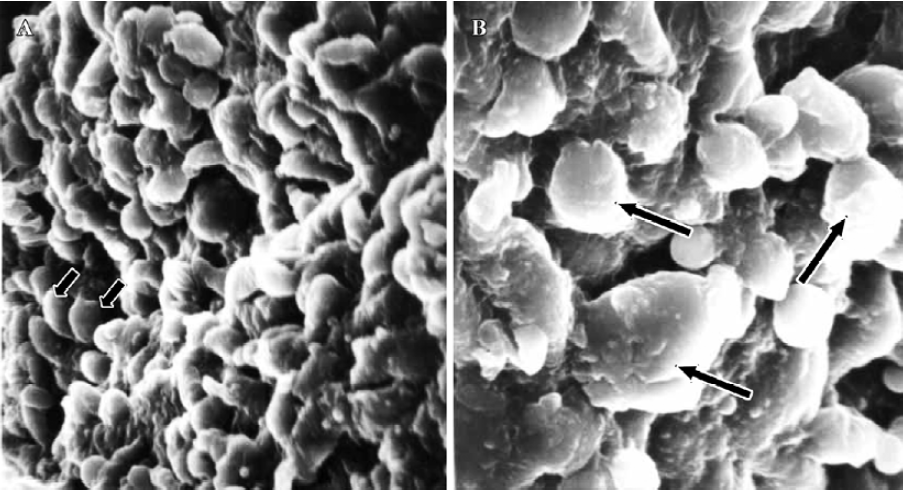
In treated group 2, the specimens taken between 21:30 and 22:00 PM on Pd4 showed endometrial surface covered by scanty membrane projections. The endometrial luminal surface was relatively smooth with few and slender membranous projections (developing pinopodes) (Figure 5). These structures appeared slightly more pronounced in specimens collected between 09:30 and 10:00 AM on Pd5, but no fully developed pinopode was observed (Figure 6).
In treated group 3, no membrane projections were present in the endometrial surface of specimens collected between 21:30 and 22:00 PM on Pd4. The epithelial cells lining the luminal surface were rather smooth and covered by small tips of microvilli (Figure 7). The specimens taken between 09:30 and 10:00 AM on Pd5 showed smooth endometrial surface covered by normal epithelial cells and short microvilli in one case, while in the other there were slender membranous projections (developing pinopodes) which were much smaller than those expressed in controls between 21:30 and 22:00 PM on Pd4 and between 09:30 and 10:00 AM on Pd5 (Figure 8).
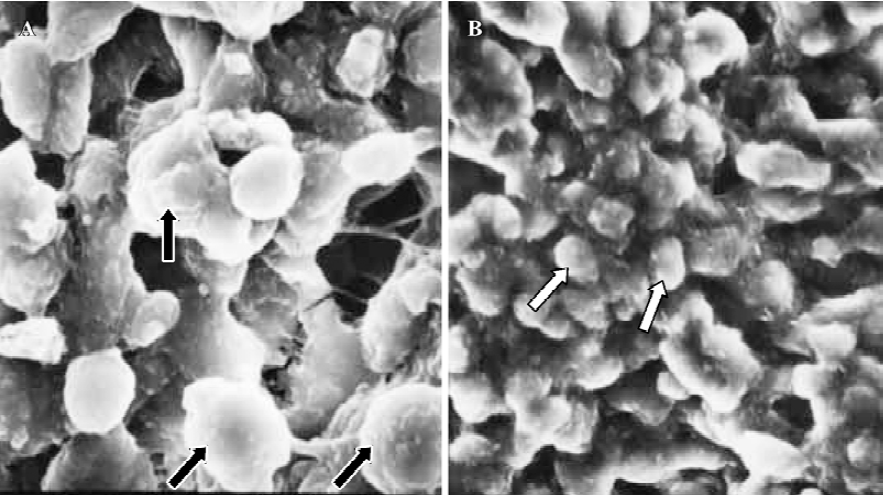
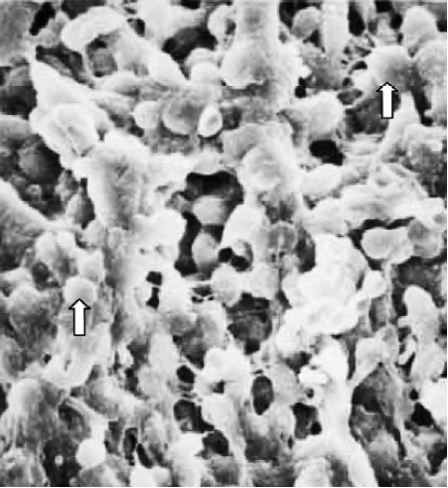
Finally, in treated group 4, no synchronously developed structure was expressed in all specimens collected between 21:30 and 22:00 h on Pd4. Few pinopodes in different development stages were present. The majority of endometrial surface expressed no membrane projections and was covered by epithelial cells with short and thick microvilli. The boundaries of epithelial cells were not clear (Figure 9). Specimens collected between 09:30 and 10:00 AM on Pd5 had no pinopodes, but abundant microvilli and clear boundaries on the epithelial cells (Figure 10).



Discussion
The use of mifepristone for emergency contraception has been widely accepted[1,3], however, a large number of prospec-tive, randomized, double-blind studies are still in progress. Several clinical trials have shown that a single dose of mifepri-stone (10 mg) is effective for emergency contraception when given within 120 h from unprotected coitus, causing mild or no side-effects[10,11]. However the biological mechanism of RU486 remains a matter of much debate.
Wang et al[12] investigated the effects of a single dose of mifepristone (10 mg) on the endometrial expressions of HOXA-11, progesterone receptors (PR), and leukaemia inhibitory factor (LIF). The found that following oral administration of mifepristone on d 2 post-ovulation (LH+2) the development of endometrium was quite delayed. In the glandular epithelium, the expressions of HOXA-11 and PR increased significantly and that of LIF decreased. Conversely, in the stromal epithelium, the expressions of these markers remained unchange.
More recently, it has been proved that mifepristone could inhibit the establishment of uterine receptivity in some animals[13,14]. Marions et al[15] reported that following mifepristone administration during early luteal phase in fertile women, down-regulation of PR was inhibited and no significant modifications of the remaining markers of endometrial receptivity, such as pinopodes, integrin dimmers α4 and β3, cyclo-oxygenase-1 and -2 were found. These authors[15] have also observed that the endometrial changes happened irrespective of embryo; however, its influence on implantation could not be ignored. Furthermore, they observed the influence of RU486 on endometrium only after RU486 was given on LH+2 whereas RU486 is effective for emergency contraception when given within 120 h from unprotected coitus.
A large consensus of opinion sustains that fully developed pinopodes represent specific morphological markers of endometrial receptivity[6,8,9]. The appearance and disappearance of fully developed pinopodes coincide with the nidation window both in humans and animals. Synchronous expressions of fully developed pinopodes and other markers of uterine receptivity such as integrins (αvβ3)[16], heparin-binding epidermal growth factor (HB-EGF)[17], LIF and LIF-receptor[18] have been noted. Therefore, it is plausible that morphological and biochemical changes during the menstrual cycle may be reliable in evaluating endometrial function and receptivity.
In this study, mifepristone as a single dose was administered straight away after the murine successful coitus was confirmed. When RU486 was given on Pd4, only one mouse conceived and the number of implanted embryos was significantly lower compared to controls (P<0.01). No mice conceived when RU486 was given on Pd1, Pd2, and Pd3. These data show that a single dose of RU486 is effective when given on Pd1, Pd2, Pd3, and Pd4.
When RU486 was administered on Pd1, developing and fully developed pinopodes were observed in the endometrial surface, but its expression was reduced compared to that of controls. It is therefore likely that mifepristone administration on Pd1 inhibits zygote development or embryo transportation through the Fallopian tube rather than having an effect on the endometrial surface. When mifepristone was given on Pd2, the uterine receptivity was significantly impaired, hence elucidating the effects of this antiprogestogenic steroid on the endometrium and its rationale for contraception. Likewise, administration of RU486 on Pd3 and Pd4 appeared to impair development and maturation of pinopodes, so affecting endometrial receptivity and embryo implantation.
The effect of RU486 on the endometrium on Pd3 is stronger than that on Pd2 and Pd4. Conversely, the effect is relatively weak when this compound is given on Pd1. To our knowledge, this is the first report in the literature assessing pinopode expression as morphological marker of implantation window in mice following administration of mifepristone. Therefore, comparison with other data is not possible.
In conclusion, these findings suggest that mifepristone might inhibit endometrial receptivity and, to some extent, prevent embryo implantation as a result of morphological modifications of the luminal epithelial cells. Since the establishment of endometrial receptivity includes morphological immunological changes, further studies exploring the effects of RU486 on the expressions of cytokines, adhesion molecules and other immunological factors during the implantation window would provide new insights and improve our knowledge of implantation and early pregnancy.
Footnote
Project supported by the National Natural Science Foundation of China (N
References
- Cameron ST, Critchley HO, Thong KJ, Buckley CH, Williams AR. Effects of daily low dose mifepristone on endometrial maturation and proliferation. Hum Reprod 1996;11:2518-26.
- Basu R, Gundlach T, Tasker M. Mifepristone and misoprostol for medical termination of pregnancy: the effectiveness of a flexible regimen. J Fam Plann Reprod Health Care 2003;29:139-41.
- Papp C, Schatz F, Krikun G, Hausknecht V, Lockwood CJ. Biological mechanisms underlying the clinical effects of mifepristone (RU486) on the endometrium. Early Pregnancy 2000;4:230-9.
- Psychoyos A, Mandon P. Study of the surface of the uterine epithelium by scanning electron microscopy: observation in the rat at the 4th and 5th day of pregnancy. Crit Rev Acad Sci Paris 1971;272:2723-9.
- Enders AC, Nelson DM. Pinocytotic activity of the uterus of the rat. Am J Anat 1973;138:277-99.
- Nardo LG, Sabatini L, Rai R, Nardo F. Pinopode expression during human implantation. Eur J Obstet Gynecol Reprod Biol 2002;10:104-8.
- Nikas G, Makrigiannakis A, Hovatta O, Jones HW Jr. Surface morphology of the human endometrium: basic and clinical aspects. Ann N Y Acad Sci 2000;900:316-24.
- Psychoyos A, Nikas G. Uterine pinopodes as markers of uterine receptivity. Ass Reprod Rev 1994;4:26-32.
- Bentin-Ley U, Sjogren A, Nilsson L, Hamberger L, Larsen JF. Presence of uterine pinopodes at the embryo-endometrial interface during human implantation in vitro. Hum Reprod 1999;14:515-20.
- Von Hertzen H, Piaggio G, Ding J, Chen J, Song S, Bartfai G. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomized trial. Lancet 2002;360:1803-10.
- Xiao BL, Von Hertzen H, Zhao H, Piaggio G. A randomized double-blind comparison of two single doses of mifepristone for emergency contraception. Hum Reprod 2002;17:3084-9.
- Wang L, Wang H, Wu J, Luo HZ, Zhu ZM, Wang JD. The effect of low dose RU486 on the expression of HOXA11 in human endometrium during midluteal phase. J Reprod Med 2000;9:165-70. Chinese.
- Gao F, Xu FH, Zhou XC, Han XB, Liu YX. Mifepristone regulates expression of apoptosis related genes fas and fasL in mouse endometrium. Acta Pharmacol Sin 2001;22:524-9.
- Liu CQ, Wang ZX, Yuan Y. Effect of mifepristone on uterine receptivity in guinea pigs. Acta Pharmacol Sin 2002;23:177-82.
- Marions L, Hultenby K, Lindell I, Sun X, Stabi B, Gemzell Danielsson K. Emergency contraception with mifepristone and levonorgestrel:mechanism of action. Obstet Gynecol 2002;100:65-71.
- Nardo LG, Nikas G, Makrigiannakis A, Sinatra F, Nardo F. Synchronous expression of pinopodes and alpha v beta 3 and alpha 4 beta 1 integrins in the endometrial surface epithelium of normally menstruating women during the implantation window. J Reprod Med 2003;48:355-61.
- Stavreus-Evers A, Aghajanova L, Brismar H, Eriksson H, Landgren BM, Hovatta O. Co-expression of heparin-binding epidermal growth factor-like growth factor and pinopodes in human endometrium at the time of implantation. Mol Hum Reprod 2002;8:765-9.
- Aghajanova L, Stavreus-Evers A, Nikas Y, Hovatta O, Landgren BM. Co-expression of pinopodes and leukemia inhibitory factor, as well as its receptor, in human endometrium. Fertil Steril 2003;79 Suppl 1:808-14.
