Orexin A promotes histamine, but not norepinephrine or serotonin, release in frontal cortex of mice1
Introduction
The neuropeptides orexin A and B (also called hypocretins 1 and 2) have recently been isolated from rat hypothalamic extracts, and have been reported to be involved in sleep-wake regulation[1–3]. Intracerebroventricular (icv) application of orexin A strongly enhances arousal in rats and mice[4–6]. Furthermore, c-fos expression in orexin neurons and prepro-orexin mRNA levels show a diurnal variation, with the strongest expression being observed during waking[7,8]. Mice lacking either the orexin gene (preproorexin knock-out mice) or orexin neurons (orexin/ataxin-3 transgenic mice) have phenotypes remarkably similar to the human sleep disorder narcolepsy[9–11], a disabling neurological disorder characterized by symptoms including excessive daytime sleepiness, sleep attacks, sleep fragmentation, cataplexy, and sleep-onset periods of rapid eye movement[12]. Lesions of the lateral hypothalamus by orexin-2-saporin produce narcoleptic-like sleep behavior in rats[13]. Consistent with these findings, recent reports suggest that human narcolepsy is accompanied by a loss of orexin neuropeptide production and specific destruction of orexin neurons[14–17]. These results suggest that the orexinergic system is involved in sleep-wake regulation and mainly contributes to arousal.
Orexin neurons are located specifically in the lateral hypothalamic area and project to almost all parts of the brain except the cerebellum[2,10,18]. Particularly dense projections of these neurons are observed in monoaminergic nuclei, such as the noradrenergic locus ceruleus (LC), serotonergic raphe nuclei (DRN), and histaminergic tuberomammillary nucleus (TMN). These monoaminergic nuclei expressing orexin receptors (OX1R and/or OX2R)[19] play important roles in the promotion of wakefulness. Electro-physiological studies have revealed that orexins had mainly excitatory effects on all monoaminergic neurons in vitro[20–24], suggesting that the arousal effect of orexins is mediated by these monoaminergic systems. However, which type(s) of these monoaminergic systems is involved in orexin-induced arousal remains to be elucidated.
In the present study, we investigated the effects of orexin A on the release of the monoaminergic neurotransmitters histamine, norepinephrine (NE), and serotonin (5-HT) in the frontal cortex (FrCx) of mice by using in vivo microdialysis to further clarify the mechanism underlying the arousal effects of orexin A.
Materials and methods
Animals Male C57BL/6 mice (Shizuoka Laboratory Animal Center, Shizuoka, Japan) weighing 24–28 g (11–13 weeks old) were housed at a constant temperature (24±0.5 ºC) and relative humidity (60%±2%), an automatically controlled 12:12 h light/dark cycle (light on at 8 AM), and ad libitum access to food and water. All animal experiments used in this study were approved by the Animal Care Committee of Osaka Bioscience Institute.
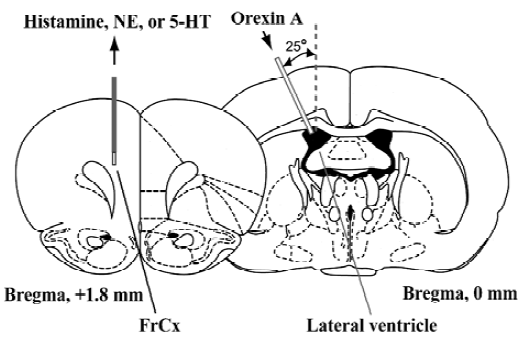
Microdialysis procedure The microdialysis was performed as previously described[5]. As shown in Figure 1, under urethane anesthesia (1.8 g/kg, ip), a micro-dialysis probe (CUP7, membrane length of 2-mm; Carnegie Medicin, Stockholm, Sweden) was inserted in the FrCx of mice at a position 1.8 mm anterior and 0.8 mm lateral to the bregma and 2.3 mm depth from the dura. One stainless steel cannula (outer diameter, 0.2 mm) was stereotaxically placed at the site of 2.0 mm lateral to the bregma and inserted to a depth of 2.2 mm from the surface of the cortex at an angle of 25° from the midsagittal plane according to the atlas of Franklin and Paxinos[25]. The microdialysis probe was perfused with Ringer’s solution (NaCl 147 mmol/L, KCl 4.0 mmol/L, and CaCl2 2.3 mmol/L; pH 7.3) at a flow rate of 2 µL/min to stabilize the release of histamine, NE, and 5-HT. Two hours after insertion of the microdialysis probe, dialysates were continuously collected from the FrCx at 20-min interval (40 µL each) for 1 h as the basal value before the orexin A injection, and until 4 h after administration of the peptide.
Determination of histamine, NE, and 5-HT levels by HPLC The histamine levels in the dialysates were measured by using a fluorometric HPLC system[26], and NE and 5-HT levels were determined by using HPLC with electrochemical detection[27]. Since the absolute basal release of histamine, NE, and 5-HT varied between subjects, the mean of the first 3 fractions before administration of orexin A was defined as the mean basal release, and subsequent fractions were expressed as a percentage of the mean basal release.
Drugs Orexin A (Peptide Institute, Osaka, Japan) was diluted in saline to the concentrations needed and was injected into the lateral ventricle of mice from the cannula at doses of 200, 50, or 12.5 pmol per mouse in 2 µL of saline at a speed of 2 µL/min. Fluoxetine (Sigma-Aldrich, St Louis, MO, USA), a 5-HT reuptake inhibitor which has been reported to also increase NE release through activation of postsynaptic 5-HT1A receptors by increased 5-HT[28,29], was dissolved in saline and injected ip (20 mg/kg).
Statistical analysis Data were expressed as mean±SD. Differences between groups were analyzed by analysis of variance (ANOVA) followed by the post-hoc Newman-Keuls test. The significant level of difference was set at P<0.05.
Results
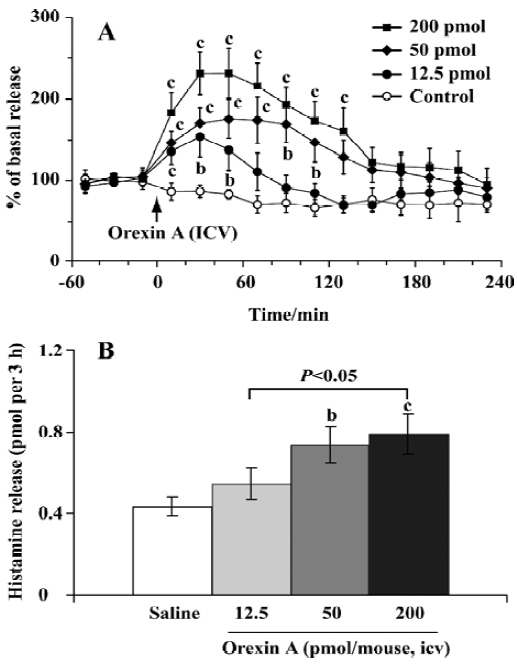
Effect of orexin A on histamine release The mean basal release of histamine was 0.06±0.01 pmol per 20 min. Compared with the control, orexin A at doses of 12.5 and 50 pmol produced a rapid and significant elevation of histamine release, with the maximal magnitude being 150% and 175% over the mean basal release, respectively; and these higher levels maintained for approximately 1 and 2 h (Figure 2A), respectively. At the highest dose (200 pmol) tested, orexin A markedly promoted histamine release, and the release reached its maximal level of 230% over the mean basal level. The significant increase lasted 140 min.
For comparison of the differences between different dosage groups, we calculated the total amount of histamine released over a 3-h period after the administration of orexin A. The total amounts of histamine released were 0.55±0.08, 0.74±0.09, and 0.79±0.11 pmol per 3 h in the groups treated with orexin A at doses of 12.5, 50, and 200 pmol, respectively. In the latter 2 groups the release was significantly higher than that of the control (0.43±0.05 pmol per 3 h, P<0.05) (Figure 2B). These results indicated that orexin A induced histamine release in a dose-dependent manner.
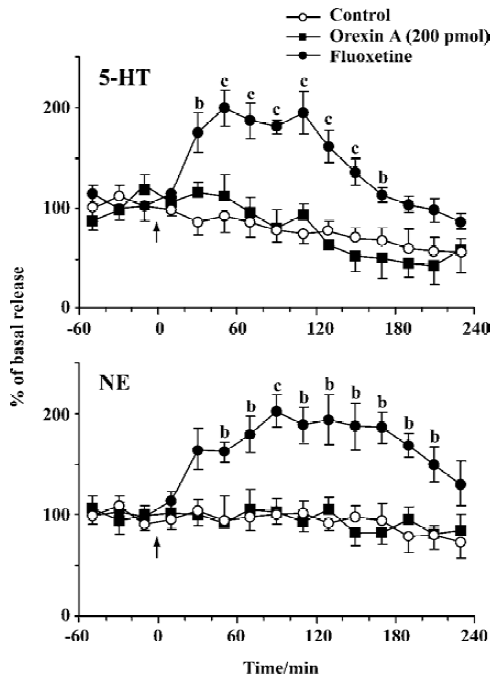
Effects of orexin A on NE and 5-HT release The mean basal release of NE and 5-HT was 1.75±0.21 and 16.9±2.33 pg per 20 min, respectively. No difference was observed in NE or 5-HT release between the group treated with orexin A (200 pmol) and the control. As the positive control, fluoxetine, significantly elevated the extracellular levels of NE and 5-HT, with the maximal magnitude being approximately 200% over the mean basal release at approximately 1.5 h and 1 h after administration (20 mg/kg, ip), respectively. Compared with the control, the increase in NE and 5-HT lasted about 180 and 160 min, respectively (Figure 3).
Discussion
In the present study we found that orexin A activated a histaminergic system in mice. An increasing body of evidence indicates the interaction between the orexinergic and histaminergic systems. For example, with respect to neuro-anatomy, orexin cells densely innervate the histaminergic TMN[18,30], a nucleus enriched in orexin 2 receptors[19]. Most human narcolepsy is caused by a loss of orexin neurons[16] and a consequent reduction in orexin levels[15,31]. Gliosis accompanies this loss of orexin neurons and is most intense in the posterior hypothalamus where the histaminergic TMN is located[16,17], suggesting that the orexinergic terminals are lost in this region and that the consequent loss of orexinergic innervation of histaminergic cells is an important component of the pathology of narcolepsy. Based on neurochemical studies, Nishino et al[31] reported that the histamine content was markedly decreased in the cortex of orexin-2 receptor-mutated narcoleptic Dobermans and that the decrease was due to a lack of excitatory input of orexin neurons caused by a loss of function of orexin-2 receptors in histaminergic TMN cell groups. Furthermore, Lin et al[32] found that orexin A and B contents were significantly lower in histamine H1 receptor knockout mice. These results indicate that there exist functional connections between histaminergic and orexinergic systems.
The FrCx is a brain region that has higher EEG frequency during waking[33], and which receives projections of monoaminergic neurons such as the noradrenergic, serotonergic, and histaminergic neurons that originate from the LC, DRN, and TMN, respectively. Microdialysis studies have revealed that extracellular levels of histamine and 5-HT in the FrCx showed typical changes across the sleep-wake cycle, with their highest levels during the waking period[34–37]. Thus, orexin-activated release of these neurotransmitters in the FrCx reflects their contributions to the arousal effect of orexin. In the present study, we found that orexin A significantly promoted histamine release in the FrCx in a dose-dependent manner, but not release of NE or 5-HT, although orexin A excites noradrenergic and serotonergic neurons in vitro[20–22]. We previously reported that a prostaglandin E2 receptor subtype EP4 agonist enhanced histaminergic neuron activity with an increase in histamine release in the FrCx to produce arousal[38]. Together with our previous observations that orexin induced wakefulness in wild-type mice but not all in histamine H1 receptor knockout mice[5], these findings suggest that the arousal effect of orexin is largely mediated by histaminergic systems and activation of H1 receptors. In contrast to the histaminergic activity linked to the maintenance of wakefulness, John et al[39] found that noradrenergic and serotonergic neurons were more tightly coupled to the maintenance of muscle tone during wakefulness and its loss during rapid eye movement sleep and cataplexy.
Taken these findings together, we conclude that the arousal effect of orexin A is mainly mediated by the activation of histaminergic systems.
Acknowledgment
We sincerely thank Dr Yoshihiro URADE and Dr Larry FYRE for their critical readings of the manuscript and valuable comments.
Footnote
Project supported in part by a grant-in-aid for scientific research from the Japan Society for the Promotion of Science (ZLH) and Osaka City.
References
- Gautvik KM, de Lecea L, Gautvik VT, Danielson PE, Tranque P, Dopazo A, et al. Overview of the most prevalent hypothalamus- specific mRNAs, as identified by directional tag PCR subtraction. Proc Natl Acad Sci USA 1996;93:8733-8.
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998;92:573-85.
- Hayaishi O, Huang ZL. Role of orexin and prostaglandin E2 in activating histaminergic neurotransmission. Drug News Perspect 2004;17:1-5.
- Piper DC, Upton N, Smith MI, Hunter AJ. The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. Eur J Neurosci 2000;12:726-30.
- Huang ZL, Qu WM, Li WD, Mochizuki T, Eguchi N, Watanabe T, et al. Arousal effect of orexin A depends on activation of the histaminergic system. Proc Natl Acad Sci USA 2001;98:9965-70.
- Mieda M, Willie JT, Hara J, Sinton CM, Sakurai T, Yanagisawa M. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc Natl Acad Sci USA 2004;101:4649-54.
- Taheri S, Sunter D, Dakin C, Moyes S, Seal L, Gardiner J, et al. Diurnal variation in orexin A immunoreactivity and prepro-orexin mRNA in the rat central nervous system. Neurosci Lett 2000;279:109-12.
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci 2001;21:1656-62.
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 2001;30:345-54.
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 1999;98:437-51.
- Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. J Neurosci 2004;24:6291-300.
- Aldrich MS. Narcolepsy. N Engl J Med 1990;323:389-94.
- Gerashchenko D, Kohls MD, Greco M, Waleh NS, Salin-Pascual R, Kilduff TS, et al. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci 2001;21:7273-83.
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 2000;355:39-40.
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000;6:991-7.
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron 2000;27:469-74.
- Thannickal TC, Siegel JM, Nienhuis R, Moore RY. Pattern of hypocretin (orexin) soma and axon loss, and gliosis, in human narcolepsy. Brain Pathol 2003;13:340-51.
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 1998;18:9996-10015.
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 2001;435:6-25.
- Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline). J Neurosci 2002;22:8850-9.
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA 1999;96:10911-6.
- Liu RJ, van den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci 2002;22:9453-64.
- Yamanaka A, Tsujino N, Funahashi H, Honda K, Guan JL, Wang QP, et al. Orexins activate histaminergic neurons via the orexin 2 receptor. Biochem Biophys Res Commun 2002;290:1237-45.
- Nakamura T, Uramura K, Nambu T, Yada T, Goto K, Yanagisawa M, et al. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res 2000;873:181-7.
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic Coordinates. San Diego (CA): Academic Press; 1997.
- Yamatodani A, Fukuda H, Wada H, Iwaeda T, Watanabe T. High-performance liquid chromatographic determination of plasma and brain histamine without previous purification of biological samples: cation-exchange chromatography coupled with post-column derivatization fluorometry. J Chromatogr 1985;344:115-23.
- Ago Y, Sakaue M, Baba A, Matsuda T. Selective reduction by isolation rearing of 5-HT1A receptor-mediated dopamine release in vivo in the frontal cortex of mice. J Neurochem 2002;83:353-9.
- Suzuki M, Matsuda T, Asano S, Somboonthum P, Takuma K, Baba A. Increase of noradrenaline release in the hypothalamus of freely moving rat by postsynaptic 5-hydroxytryptamine1A receptor activation. Br J Pharmacol 1995;115:703-11.
- Gobert A, Rivet JM, Cistarelli L, Melon C, Millan MJ. Buspirone modulates basal and fluoxetine-stimulated dialysate levels of dopamine, noradrenaline and serotonin in the frontal cortex of freely moving rats: activation of serotonin1A receptors and blockade of alpha2-adrenergic receptors underlie its actions. Neuroscience 1999;93:1251-62.
- Eriksson KS, Sergeeva O, Brown RE, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci 2001;21:9273-9.
- Nishino S, Fujiki N, Ripley B, Sakurai E, Kato M, Watanabe T, et al. Decreased brain histamine content in hypocretin/orexin receptor-2 mutated narcoleptic dogs. Neurosci Lett 2001;313:125-8.
- Lin L, Wisor J, Shiba T, Taheri S, Yanai K, Wurts S, et al. Measurement of hypocretin/orexin content in the mouse brain using an enzyme immunoassay: the effect of circadian time, age and genetic background. Peptides 2002;23:2203-11.
- Buchsbaum MS, Mendelson WB, Duncan WC, Coppola R, Kelsoe J, Gillin JC. Topographic cortical mapping of EEG sleep stages during daytime naps in normal subjects. Sleep 1982;5:248-55.
- Chu M, Huang ZL, Qu WM, Eguchi N, Yao MH, Urade Y. Extracellular histamine level in the frontal cortex is positively correlated with the amount of wakefulness in rats. Neurosci Res 2004;49:417-20.
- Cespuglio R, Sarda N, Gharib A, Chastrette N, Houdouin F, Rampin C, et al. Voltammetric detection of the release of 5-hydroxyindole compounds throughout the sleep-waking cycle of the rat. Exp Brain Res 1990;80:121-8.
- Portas CM, Bjorvatn B, Fagerland S, Gronli J, Mundal V, Sorensen E, et al. On-line detection of extracellular levels of serotonin in dorsal raphe nucleus and frontal cortex over the sleep/wake cycle in the freely moving rat. Neuroscience 1998;83:807-14.
- de Saint Hilaire Z, Orosco M, Rouch C, Python A, Nicolaidis S. Neuromodulation of the prefrontal cortex during sleep: a microdialysis study in rats. Neuroreport 2000;11:1619-24.
- Huang ZL, Sato Y, Mochizuki T, Okada T, Qu WM, Yamatodani A, et al. Prostaglandin E2 activates the histaminergic system via the EP4 receptor to induce wakefulness in rats. J Neurosci 2003;23:5975-83.
- John J, Wu MF, Boehmer LN, Siegel JM. Cataplexy-active neurons in the hypothalamus: implications for the role of histamine in sleep and waking behavior. Neuron 2004;42:619-34.
