Recombinant bispecific antibodies for cancer therapy
Introduction
The concept of using bispecific molecules in therapy is based on the selective recruitment of an effector mechanism to a defined disease-related target structure. Thus, bispecific molecules serve as mediators (adaptors) between an effector and a target. A plethora of effector mechanisms can be envisaged for therapeutic applications and a large number have already been evaluated. These include the recruitment of effector molecules (e.g. toxins, drugs, prodrugs, cytokines, radionuclides), the retargeting of effector cells (e.g. cytotoxic T lymphocytes, NK cells, macrophages, granulocytes) and the retargeting of carrier systems (e.g. viral vectors for gene therapy)[1].
Antibodies are ideally suited as starting material for the construction of bispecific molecules as they normally bind specifically and with high affinity to antigens. Using different technologies, for instance hybridoma or phage display technology, antibodies can be generated against virtually any given antigen. Smaller portions of an antibody retaining antigen-binding activity can be produced by proteolytic cleavage or in recombinant form. Several methods have been developed for the generation of bispecific antibodies. Besides chemical cross-linking of two IgG molecules or two Fab or Fab’ fragments, bispecific antibodies can also be produced by fusion of two hybridomas. This hybrid-hybridoma technology results in cell lines (quadroma) that produce bispecific IgG molecules. However, the production and random association of two different heavy chains and two different light chains within one cell leads to the assembly of a substantial proportion of non-functional molecules. Thus, the hybrid-hybridoma technology and also chemical methods produce poorly defined products and require elaborate purification steps to obtain defined and clinically useful material. Nevertheless, several clinical studies have been performed to analyze the therapeutic potentials of bispecific antibodies. Most of these studies were focused on the retargeting of effector cells (cytotoxic T lymphocytes, NK cells) of the immune system to tumor cells. Mostly disappointing findings were obtained from these studies[1,2]. The main reasons were the induction of neutralizing antibodies against the murine bispecific antibodies and Fc-mediated side effects, including cytokine-release syndrome, thrombo-cytopenia and leukopenia, which limited the maximal applicable dose. Further studies indicate that the induction of inflammation within the tumor, for example, the co-application of inflammatory cytokines, is essential to achieve an effective treatment. It was concluded that besides strong and selective binding to a disease-related antigen clinically useful bispecific antibodies should fulfill several require-ments[2]: They should be non-immunogenic to avoid a neutralizing immune response. They should have a defined structure and should bind monovalently to the effector cells to induce activation of the effector cells only after binding to the target cells. They should not contain an Fc-region to avoid Fc-mediated side effects. They should have a size which allows penetration into the tumor tissue but should circulate sufficiently long to induce therapeutic effects.
Recombinant bispecific antibodies
Recombinant bispecific antibodies offer several advantages over conventional bispecific antibodies made by chemical cross-linking or fusion of two hybridoma clones and can be designed to meet the requirements described above. By using only the variable domains as building blocks, recombinant antibodies lack the Fc-region of an antibody, and thus do not induce Fc-mediated effects. Recombinant antibodies are constructed by genetic means allowing for the generation of human molecules in order to reduce or even avoid the induction of a neutralizing antibody response. Human antibody molecules used as starting blocks can be isolated, for instance, from human antibody libraries by means of phage display[3] or by using transgenic mice expressing human antibodies[4]. Recent studies have shown, however, that even fully human antibodies can be immunogenic leading to the generation of human anti-human antibodies (HAHA)[5]. By identification and removal of the responsible T cell epitopes (“deimmunization”) the immunogenicity risk associated with human antibodies can be further reduced[6]. These developments in the generation of recombinant antibody molecules have led to a revival in the use of bispecific antibodies for therapeutic applications[7].
A wide variety of different recombinant bispecific antibody formats have been developed over the past years[8]. Amongst them tandem single-chain Fv molecules and diabodies and various derivatives there of are the most widely used formats for the construction of recombinant bispecific antibodies (Figure 1). Routinely, construction of these molecules starts from two single-chain Fv (scFv) fragments that recognize different antigens[9].
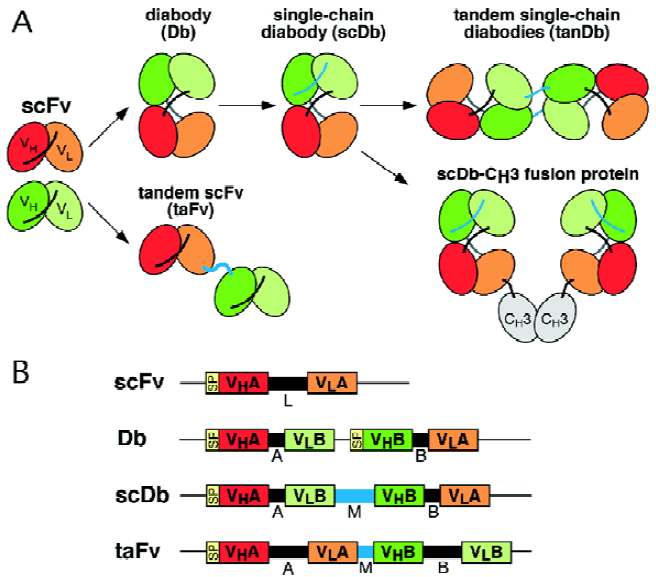
Tandem scFv molecules (taFv) represent a straightforward format simply connecting the two scFv molecules with an additional peptide linker (Figure 1). The two scFv fragments present in these tandem scFv molecules form separate folding entities. Thus various linkers can be used to connect the two scFv fragments and linkers with a length of up to 63 residues have been reported[10]. Although the parental scFv fragments can normally be expressed in soluble form in bacteria, it is, however, often observed that tandem scFv molecules form insoluble aggregates in bacteria. Hence, refolding protocols or the use of mammalian expression systems are routinely applied to produce soluble tandem scFv molecules. In a recent study, in vivo expression by transgenic rabbits and cattle of a tandem scFv directed against CD28 and a melanoma-assocated proteoglycan was reported[11]. In this construct the two scFv molecules were connected by a CH1 linker and serum concentrations of up to 100 mg/L of the bispecific antibody were found. Various strategies including variations of the domain order or using middle linkers with varying length or flexibility were employed to allow soluble expression in bacteria. A few studies have now reported expression of soluble tandem scFv molecules in bacteria[12–14] using either a very short Ala3 linker or long glycine/serine-rich linkers. In a recent study, we employed phage display of a tandem scFv repertoire containing randomized middle linkers with a length of 3 or 6 residues to enrich for those molecules which are produced in soluble and active form in bacteria. This approach resulted in the isolation of a preferred tandem scFv molecule with a 6 amino acid residue linker[15]. At present it is unclear if this linker sequence represents a general solution to the soluble expression of tandem scFv molecules. Nevertheless, this study demonstrate that phage display of tandem scFv molecules in combination with directed mutagenesis is a powerful tool to enrich for those molecules which can be expressed in bacteria in active form.
Bispecific diabodies (Db) utilize the diabody format for expression. Diabodies are produced from scFv fragments by reducing the length of the linker connecting the VH and VL domain to approximately 5 residues[16]. This reduction forces dimerization of two polypeptide chains by crossover pairing of the VH and VL domains (Figure 1). Bispecific diabodies are produced by expressing two polypeptide chains with either the structure VHA-VLB and VHB-VLA (VH-VL configuration) or VLA-VHB and VLB-VHA (VL-VH configuration) within the same cell. A large variety of different bispecific diabodies have been produced in the past and most of them could be expressed in soluble form in bacteria (Table 1). A recent comparative study demonstrate, however, that the orientation of the variable domains can influence expression and formation of active binding sites[17]. Nevertheless, the soluble expression in bacteria represents an important advantage over tandem scFv molecules. However, since two different polypeptide chains are expressed within a single cell inactive homodimers can be produced together with active heterodimers. This puts some obstacles towards the use as therapeutic molecules as it necessitates the implementation of additional purification steps in order to obtain homogenous preparations of bispecific diabodies.
Full table
One approach to force the generation of bispecific diabodies is the production of knob-into-hole diabodies[18]. This was demonstrated for a bispecific diabody directed against HER2 and CD3. A large knob was introduced in the VH domain by exchanging Val37 with Phe and Leu45 with Trp and a complementary hole was produced in the VL domain by mutating Phe98 to Met and Tyr87 to Ala, either in the anti-HER2 or the anti-CD3 variable domains. By using this approach the production of bispecific diabodies could be increased from 72% by the parental diabody to over 90% by the knob-into-hole diabody. Importantly, production yields did only slightly decrease by these mutations. However, a reduction in antigen-binding activity was observed for several analyzed constructs. Thus, this rather elaborate approach requires the analysis of various constructs in order to identify those mutations which produce heterodimeric molecule with unaltered binding activity.
Single-chain diabodies (scDb) represent an alternative strategy to improve the formation of bispecific diabody-like molecules[19,20]. Bispecific single-chain diabodies are produced by connecting the two diabody-forming polypeptide chains with an additional middle linker with a length of approximately 15 amino acid residues (Figure 1). Consequently, all molecules with a molecular weight corresponding to monomeric single-chain diabodies (50-60 kDa) are bispecific. Several studies have demonstrated that bispecific single-chain diabodies are expressed in bacteria in soluble and active form (Table 1) with the majority of purified molecules present as monomers[19–22]. Thus, single-chain diabodies combine the advantages of tandem scFvs (all monomers are bispecific) and diabodies (soluble expression in bacteria).
Stability improvements
Stability of the recombinant bispecific antibodies under storage conditions as well as after in vivo application is a critical parameter with strong impact for clinical application. The antibody has to be sufficiently stable to allow the molecules to induce a therapeutic benefit before being degrad-ed[23]. Unfortunately, several studies showed that tandem scFv molecules as well as diabodies were inactivated under physiological conditions, with varying half-lives depending on the antibody construct tested[15,20].
One approach to improve the stability of antibody molecules is the generation of disulfide-stabilized molecules introducing cysteine bridges between the VH-VL interfaces to inhibit dissociation of the VH and VL domains. That this results in an increased thermal stability has been shown for a bivalent anti-CEA diabody[24]. A subsequent study with a disulfide-stabilized bispecific diabody demonstrated that this approach also resulted in an increase in the formation of heterodimers similar to the knob-into-hole approach[18]. However, a marked reduction in production yield was reported for this disulfide-stabilized bispecific diabody in E coli. Principally, this approach to improve stability by introducing disulfide-bonds between the VH and VL domains is applicable for any recombinant bispecific format including tandem scFv molecules.
Several comparative studies have revealed that single-chain diabodies are more stable than diabodies and tandem scFv molecules[14,15,19,20]. This is probably a result of the physical linkage of the four variable domains, similar to the improved stability of single-chain Fv fragments compared to Fv fragments[25]. A recent study demonstrated that the length and composition of the three linkers present in a single-chain diabody molecule and its dimeric tandem form had a strong influence on stability and functional activity[26]. In this study tandem diabodies composed of 10 residue long flanking linkers and a long middle linker (27 residues) showed highest stability and activity. These results emphasize the fact that modest variations in the composition of recombinant bispecific antibodies have strong impacts on their biological properties.
Improvement of pharmacokinetics
One of the drawbacks of small recombinant bispecific antibodies for therapeutic applications is the short circulation time in the body. Diabodies, single-chain diabodies and tandem-scFv molecules have a molecular weight of 50-60 kDa. This causes rapid clearance from circulation by extravasation and renal elimination with an initial half-life (t1/2α) below 30 min[20]. This is much shorter than the half-life of whole antibody molecules, which can be in the range of several weeks, due to its larger size and Fn receptor mediated recycling.
Several approaches have been undertaken to improve the pharmacokinetics of recombinant antibodies. One approach is to increase the size of these molecules. For example, this was achieved by fusion of a bispecific single-chain diabody to the IgG CH3 or Fc region via an IgG hinge[27]. A similar approach was also applied for bispecific diabodies fusing one of the two diabody chains to a CH3 domain (di-diabody)[28]. These fusions result in the formation of dimeric molecules with molecular weights of 150-180 kDa containing four functional antigen-binding sites, two for each antigen. The advantage of using single-chain diabodies for this approach is that a single polypeptide chain is expressed resulting in the assembly of defined molecules with identical size and binding activity. In contrast, the usage of two different polypeptide chains for the expression of di-diabody may cause formation of a mixture of non-functional diabody molecules produced by homodimeric assembly of two identical polypeptide chains and functional diabody-CH3 fusion proteins. Interestingly, Lu and co-workers[28] could not detect such non-functional diabody molecules in their preparations indicating that heterodimeric assembly of the two polypeptide chains is favored, at least for the described construct. Heterodimer-forming CH3 domains containing knob-into-hole structures were also used to generate bispecific antibodies by fusion of two different scFv fragments directed against HER2 and CD16 to these domains[29]. Although not tested in vivo this bispecific minibody was stable in mouse and human serum at 37 °C for several days and biologically active.
Dimeric single-chain diabody molecules with a molecular weight of 100-115 kDa can also be generated by varying the length of the linkers connecting the variable domain. Reducing the middle linker of a single-chain diabody to less than 13 amino acid residues results in the formation of dimeric single-chain diabody (tandem diabodies, tanDb) most likely with a linear arrangement of the two polypeptide chains (Figure 1)[20,21]. Similarly, reduction of the flanking linkers to 0-1 amino acid residue results also in dimeric molecules, presumably in a tetrabody-like arrangement[21]. For tandem diabodies it has been shown that this dimerization results in a four- to eight fold increase in circulation time compared to diabodies and an improved therapeutic efficacy[20,30].
The covalent attachment of polyethylene glycol (PEG) chains may represent another possibility to improve the pharmacokinetics of recombinant bispecific antibodies. Several PEGylated protein therapeutics which exhibit increased half-lives and improved therapeutic efficacy compared to their non-PEGylated forms have been clinically approved[31]. Although as yet not applied for recombinant bispecific antibodies, it was shown for single-chain Fv fragments that PEGylation could prolong serum half-life up to 200-fold[32]. In this study single reactive cysteine residues were introduced into the scFv molecules at defined positions, e.g. at the C-terminus or in the linker region. This additional cysteine residue allowed for a site-specific coupling of a single PE.G. chain without impairing binding activity of the scFv fragment.
A rather new approach is the combination of recombinant bispecific antibodies with gene-therapeutic protocols aiming at a direct in vivo expression of the antibody molecules[33]. This should result in high and constant levels of antibody molecules over an extended period of time. Furthermore, this approach obviates the need for extensive purification and characterization of the therapeutic proteins. However, one has to keep in mind that for therapeutic applications it is essential that expression is tightly controlled in order to stall expression in the event of severe side effects. In 1999 we postulated that this approach should be applicable for the in vivo production of recombinant bispecific antibody molecules[19]. In this study we show that bispecific single-chain diabodies are secreted in an active form from mammalian producer cells in vitro and are able to selectively recruit a prodrug-converting enzyme to tumor cells. Subsequently, we developed an adenoviral system containing the gene for an anti-CD3 x anti-CEA bispecific single-chain diabody. Injection of these recombinant adenoviruses into mice resulted in high-level expression of the antibody molecule over a period of several weeks[34]. The therapeutic effects of in vivo produced proteins were recently demonstrated with an anti-CEA x anti-CD3 bispecific diabody in combination with a B7-anti-CEA scFv fusion protein[35]. In vivo expression was achieved by implanting near the tumor site 293 producer cells stably transfected with the DNA encoding these proteins. After injection of human T cells, anti-tumor effects were observed in a colon carcinoma tumor model demonstrating the feasibility of this approach. Further studies are, however, needed to establish the most effective and safe protocol, including an evaluation of in vivo and ex vivo gene transfer, a comparison of local versus systemic production, identification of suitable transcriptional control elements and safe gene transfer vehicles.
Therapeutic applications
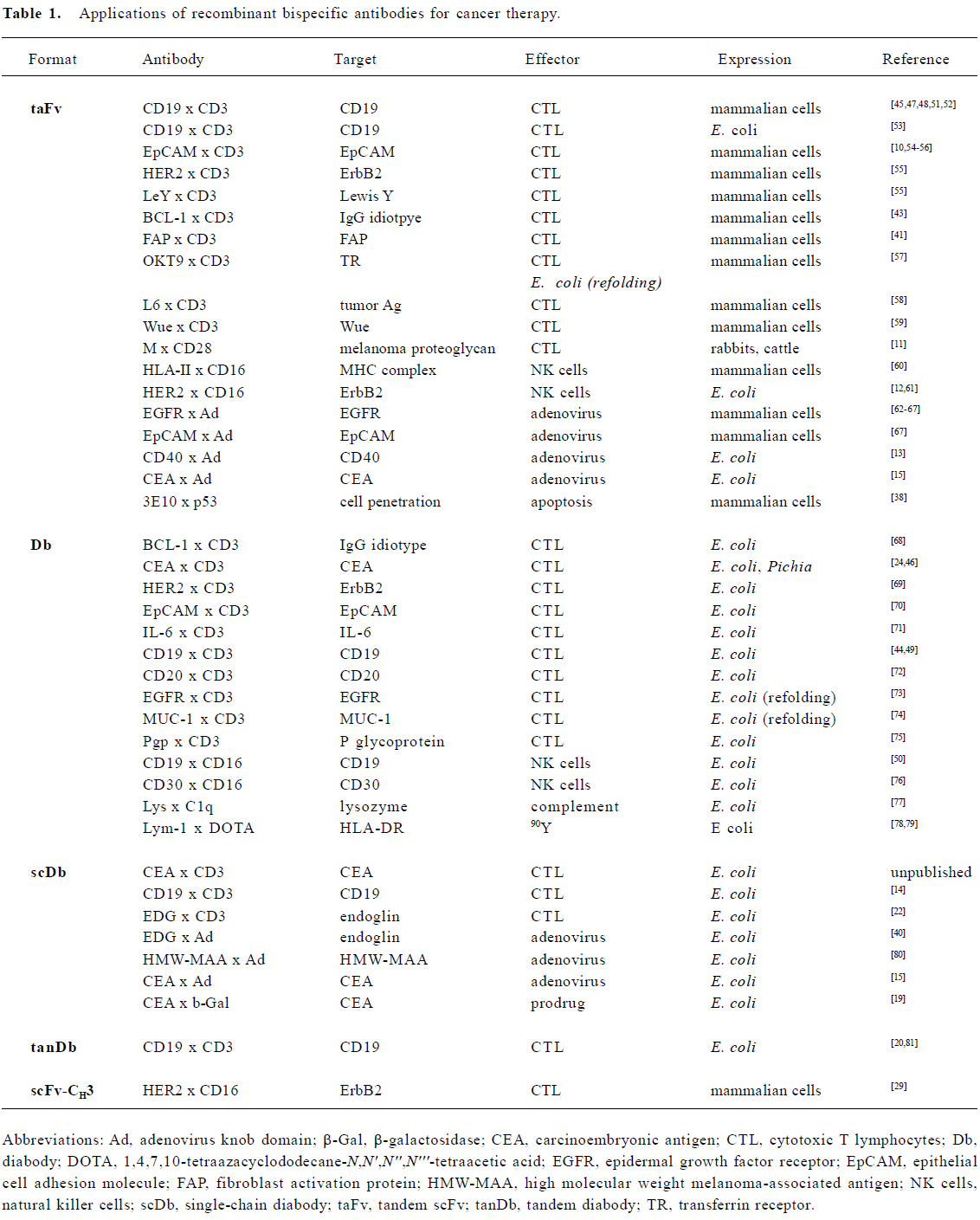
Recombinant bispecific antibodies have already been developed for a variety of different applications with potential use in cancer therapy. These applications include the retargeting of effector molecules (prodrug-converting enzymes, radio-isotopes, complement components), effector cells (CTLs, NK cells) and adenoviral vectors (Table 1), covering various therapeutic strategies, e.g. radiotherapy, chemotherapy, immunotherapy and gene therapy. In addition, recent work explored their use as intracellular bispecific antibodies (intrabodies)[36]. In one study an intracellularly expressed diabody was used to induce a functional knockout of two cell surface receptors[37]. In another study a bispecific tandem scFv with cell-penetrating abilities was applied to restore p53 wild-type function by intracellular binding to p53[38].
Most applications of recombinant bispecific antibodies for cancer therapy focus on the retargeting of effector cells of the immune system to tumor cells (Table 1). Extensive work has been done on the retargeting of cytotoxic T lymphocytes (CTLs) through binding to the T cell co-receptor molecule CD3. In addition, natural killer cells (NK) were retargeted with recombinant bispecific antibodies directed against Fcγ receptor III (CD16). A large variety of different target antigens have been evaluated. Most of them represent tumor-associated antigens (TAA) over-expressed by tumor cells, including CD19, CD20, epithelial cell adhesion molecule (EpCAM), epidermal growth factor receptor (EGFR), HER2, MUC-1, and carcinoembryonic antigen (CEA) (Table 1). In addition, recent focus has switched to tumor vasculature as a target structure. Targeting tumor vasculature of solid tumors has several advantages compared to direct targeting of tumor cells[39]. Tumor endothelial cells are directly accessible for circulating agents, ie extravasation of effector molecules and cells is not necessary. All solid tumors depend on neovascularization to grow beyond a few millimeters in diameter. Thus, this approach is broadly applicable. Endothelial cells are genetically stable and do not become resistant to therapy. A few studies have demonstrated that recombinant bispecific antibodies can be employed to retarget effector cells (CTLs) or adenoviral vectors to endothelial cells in vitro. These studies used endoglin (CD105) or fibroblast activation protein (FAP) as vascular targeting structures[22,40,41]. Other suitable antigens are described, e.g. vascular endothelial growth factor (VEGF) receptor, αv-integrins and the ED-B domain of a tumor-associated fibronectin splice variant[42], which allows these studies to be extended to other targets but also to other effector mechanisms.
Several animal studies have been performed with recombinant bispecific antibodies and curative effects have been demonstrated[10,30,35,43-45]. Most studies with anti-CD3 bispecific antibody molecules have shown that T cells need a second stimulus to efficiently lyse target cells. This second stimulus can be provided by pre-activation of isolated T lymphocytes, e g with IL-2 and anti-CD28 antibodies. Several studies developed approaches to provide this co-stimulus in vivo by co-administering anti-CD28 antibodies or B7-scFv fusion proteins[30,46]. Interestingly, one anti-CD3 antibody used for the construction of bispecific tandem scFv molecules was shown to induce a co-stimulation independent T cell response[47,48].
Other studies evaluated various combinations of recombinant bispecific antibodies with other compounds to further improve anti-tumor responses. These approaches include the co-application of anti-CD19 x anti-CD3 diabodies, anti-CD28 antibodies and anti-CD19 x anti-CD16 bispecific diabodies targeting and activating two different effector cell types and the co-application of an anti-CD19 x anti-CD16 bispecific diabody together with thalidomide as chemotherapeutic drug[49,50]. Interestingly, these studies described synergistic effects between the different compounds indicating that further dramatic improvements of the systems are possible.
Currently there is one bispecific tandem scFv molecule (MT103) directed against CD19 and CD3 in a clinical phase I safety trial for the treatment of Non-Hodgkin’s Lymphoma (NHL). This antibody was shown to be very potent in destroying CD19-expressing tumor cells in vitro and in vivo in a T cell costimulation-independent way[48]. A final report of the outcome is currently not available.
Conclusions
The past decade has led to enormous improvements in the generation and application of recombinant bispecific antibodies. These molecules combine the potentials of bispecific molecules for therapeutic applications with the advantages provided by antibody engineering technologies. Although further improvements are necessary, recent studies have shown that recombinant bispecific antibodies can find their way into the clinic.
References
- van Spriel AB, van Ojik HH, van de Winkel JGJ. Immunotherapeutic perspectives for bispecific antibodies. Immunol Today 2000;21:391-6.
- Segal DM, Weiner GJ, Weiner LM. Bispecific antibodies in cancer therapy. Curr Opin Immunol 1999;11:558-62.
- Hoogenboom HR, Chames P. Natural and designer binding sites made by phage display technology. Immunol Today 2000;21:371-8.
- Brüggemann M, Taussig MJ. Production of human antibody repertoires in transgenic mice. Curr Opin Biotechnol 1997;8:455-8.
- Ritter G, Cohen LS, Williams C, Richards EC, Old LJ, Welt S. Serological analysis of human anti-human antibody responses in colon cancer patients treated with repeated doses of humanized monoclonal antibody A33. Cancer Res 2001;61:685-9.
- Hellendoorn K, Jones T, Watkins J, Baker M, Hamilton A, Carr F. Limiting the risk of immunogenicity by identification and removal of T-cell epitopes (DeImmunisationTM). Cancer Cell Int 2004;4:S20.
- Kufer P, Lutterbüse R, Baeuerle PA. A revival of bispecific antibodies. Trends Biotechnol 2004;22:238-44.
- Kriangkum J, Xu B, Nagata LP, Fulton RE, Suresh MR. Bispecific and bifunctional single chain recombinant antibodies. Biomol Eng 2001;18:31-40.
- Kontermann RE, Völkel T, Korn T. Production of recombinant bispecific antibodies. In: Antibody Engineering Protocols, 2 edition-Methods in Molecular Biology Vol 51. Lo BKC (Ed). Totowa: Humana Press; 2003. p 227–42.
- Ren-Heidenreich L, Davol PA, Douttab NM, Elfenbein GJ, Lum LG. Redirected T-cell cytotoxicity to epithelial cell adhesion molecule-overepressing adenocarcinomas by a novel recombinant antibody, E3Bi, in vitro and in an animal model. Cancer 2004;100:1095-103.
- Grosse-Hovest L, Müller S, Minoia R, Wolf E, Zakhartchenko V, Wenigerkind H, et al. Cloned transgenic farm animals produce a bispecific antibody for T cell-mediated tumor cell killing. Proc Natl Acad Sci USA 2004;101:6858-63.
- McCall AM, Adams GP, Amoroso AR, Nielsen UB, Zhang L, Horak E, et al. Isolation and characterization of an anti-CD16 single-chain Fv fragment and construction of an anti-HER2/neu/anti-CD16 bispecific scFv that triggers CD16-dependent tumor cytolysis. Mol Immunol 1999;36:433-45.
- Brandaõ JG, Scheper RJ, Lougheed SM, Curiel DT, Tillman BW, Gerritsen WR, et al. CD40-targeted adenoviral gene transfer to dendritic cells through the use of a novel bispecific single-chain Fv antibody enhances cytotoxic T cell activation. Vaccine 2003;21:2268-72.
- Kipriyanov SM, Moldenhauer G, Braunagel M, Reusch U, Cochlovius B, Le Gall F, et al. Effect of domain order on the activity of bacterially produced bispecific single-chain Fv anti-bodies. J Mol Biol 2003;330:99-111.
- Korn T, Nettelbeck DM, Völkel T, Müller R, Kontermann RE. Recombinant bispecific antibodies for the targeting of adenoviruses to CEA-expressing tumour cells: a comparative analysis of bacterially expressed single-chain diabody and tandem scFv. J Gene Med 2004;26:642-51.
- Holliger P, Prospero T, Winter G. “Diabodies”: small bivalent and bispecific antibody fragments. Proc Natl Acad Sci USA 1993;90:6444-8.
- Lu D, Jimenez X, Witte L, Zhu Z. The effect of variable domain orientation and arrangement on the antigen-binding activity of a recombinant bispecific diabody. Biochem Biophys Res Commun 2004;318:507-13.
- Zhu Z, Presta LG, Zapata G, Carter P. Remodeling domain interfaces to enhance heterodimer formation. Protein Sci 1997;26:781-8.
- Brüsselbach S, Korn T, Völkel T, Müller R, Kontermann RE. Enzyme recruitment and tumor cell killing in vitro by a secreted bispecific single-chain diabody. Tumor Targeting 1999;4:115-23.
- Kipriyanov SM, Moldenhauer G, Schuhmacher J, Cochlovius B, Von der Lieth CW, Matys ER, et al. Bispecific tandem diabody for tumor therapy with improved antigen binding and pharmacokinetics. J Mol Biol 1999;293:41-56.
- Völkel T, Korn T, Bach M, Müller R, Kontermann R. Optimized linker sequences for the expression of monomeric and dimeric bispecific single-chain diabodies. Protein Eng 2001;14:815-23.
- Korn T, Müller R, Kontermann RE. Bispecific single-chain diabody-mediated killing of endoglin-expressing endothelial cells by cytotoxic T lymphocytes. J Immunother 2004;27:99-106.
- Willuda J, Honegger A, Waibel R, Schubiger PA, Stahel R, Zangemeister-Wittke U, et al. High thermal stability is essential for tumor targeting of antibody fragments: engineering of a humanized anti-epithelial glycoprotein-2 (epithelial cell adhesion molecule) single-chain Fv fragment. Cancer Res 1999;59:5758-67.
- FitzGerald K, Holliger P, Winter G. Improved tumour targeting by disulphide stabilized diabodies expressed in Pichia pastoris. Protein Eng 1997;10:1221-5.
- Glockshuber R, Malia M, Pfitzinger I, Plückthun A. A comparison of strategies to stabilize immunoglobulin Fv-fragments. Biochemistry 1990;29:1362-7.
- Le Gall F, Reusch U, Little M, Kipriyanov SM. Effect of linker sequences between the antibody variable domains on the formation, stability and biological activity of a bispecific tandem diabody. Protein Eng Des Sel 2004;17:357-66.
- Alt M, Müller R, Kontermann R. Novel tetravalent and bispecific IgG-like antibody molecules combining single-chain diabodies with the immunoglobulin g1 Fc or CH3 region. FEBS Lett 1999;454:90-4.
- Lu D, Jimenez X, Zhang H, Atkins A, Brennan L, Balderes P, et al. Di-diabody: a novel tetravalent bispecific antibody molecule by design. J Immunol Meth 2003;279:219-32.
- Shahied LS, Tang Y, Alpaugh RK, Somer R, Greenspon D, Weiner LM. Bispecific minibodies targeting HER2/neu and CD16 exhibit improved tumor lysis when placed in a divalent tumor antigen-binding format. J Biol Chem 2004. Epub ahead of print.
- Cochlovius B, Kipriyanov SM, Stassar MKKG, Schuhmacher J, Benner A, Moldenhauer G, et al. Cure of Burkitt’s lymphoma in severe combined immunodeficiency mice by T cells, tetravalent CD3 x CD19 tandem diabody, and CD28 costimulation. Cancer Res 2000;60:4336-41.
- Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov 2003;2:214-21.
- Yang K, Basu A, Wang M, Chintala R, Hsieh MC, Liu S, et al. Tailoring structure-function and pharmacokinetic properties of single-chain Fv proteins by site-specific PEGylation. Protein Eng 2003;16:761-70.
- Bakker JM, Bleeker WK, Parren PWHI. Therapeutic antibody gene transfer: An active approach to passive immunity. Mol Ther 2004;10:411-6.
- Kontermann RE, Korn T, Jérôme V. Recombinant adenoviruses for expression of antibody fragments. In: Recombinant antibody technology for cancer therapy: reviews and protocols. Welschof M, Krauss J (Eds). Methods in Molecular Medicine. Totowa: Humana Press; 2002. p 421–33.
- Blanco B, Holliger P, Vile RG, Álvarez-Vallina L. Induction of human T lymphocyte cytotoxicity and inhibition of tumor growth by tumor-specific diabody-based molecules secreted from gene-modified bystander cells. J Immunol 2003;171:1070-7.
- Kontermann RE, Müller R. Intracellular and cell surface displayed single-chain diabodies. J Immunol Meth 1999;226:179-88.
- Jendreyko N, Popkov M, Beerli RR, Chung J, McGavern DB, Rader C, et al. Intradiabodies, bispecific, tetravalent antibodies for the simultaneous functional knockout of two cell surface receptors. J Biol Chem 2003;278:47812-9.
- Weisbart RH, Wakelin R, Chan G, Miller CW, Koeffler PH. Construction and expression of a bispecific single-chain antibody that penetrates mutant p53 colon cancer cells and binds p53. Int J Oncol 2004;25:1113-8.
- Augustin HG. Antiangiogenic tumour therapy: will it work? Trends Pharmacol Sci 1998;19:216-22.
- Nettelbeck DM, Miller DW, Jerome V, Zuzarte M, Watkins SJ, Hawkins RE, et al. Targeting of adenovirus to endothelial cells by a bispecific single-chain diabody directed against the adenovirus fiber knob domain and human endoglin (CD105). Mol Ther 2001;3:882-91.
- Wüst T, Moosmayer D, Pfizenmaier K. Construction of a bispecific single chain antibody for recruitment of cytotoxic T cells to the tumour stroma associated antigen fibroblast activation protein. J Biotechnol 2001;92:159-68.
- Thorpe PE. Vascular targeting agents as cancer therapeutics. Clin Cancer Res 2004;10:415-27.
- De Jonge J, Heirman C, de Veerman M, Van Meirvenne S, Moser M, Leo O, et al. In vivo retargeting of T cell effector function by recombinant bispecific single-chain Fv (anti-CD3 x anti-idiotype) induces long-term survival in the murine BCL1 lymphoma model. J Immunol 1998;161:1454-61.
- Cochlovius B., Kipriyanov SM. Treatment of huma B cell lymphoma xenografts with a CD3 x CD19 diabody and T cells. J Immunol 2000;165:888-95.
- Dreier T, Baeuerle PA, Fichtner I, Grun M, Schlereth B, Lorenczewski G, et al. T cell costimulus-independent and very efficacious inhibition of tumor growth in mice bearing subcutaneous or leukemic human B cell lymphoma xenografts by a CD19-/CD3- bispecific single-chain antibody construct. J Immunol 2003;170:4397-402.
- Holliger P, Manzke O, Span M, Hawkins R, Fleischmann B, Qianghua L, et al. Carcinoembryonic antigen (CEA)-specific T-cell activation in colon carcinoma induced by anti-CD3 x anti-CEA bispecific diabodies and B7 x anti-CEA bispecific fusion proteins. Cancer Res 1999;59:2909-16.
- Löffler A, Kufer P, Lutterbüse R, Zettl F, Daniel PT, Schwenk-enbecher JM, et al. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood 2000;15:2098-103.
- Dreier T, Lorenczewski G, Brandl C, Hoffmann P, Syring U, Hanakam F, et al. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer 2002;100:690-7.
- Kipriyanov SM, Cochlovius B, Schäfer HJ, Moldenhauer G, Bähre A, Le Gall F, et al. Synergistic antitumor effects of bispecific CD19 x CD3 and CD19 x CD16 diabodies in a preclinical model of Non-Hodgkin’s lymphoma. J Immunol 2002;169:137-44.
- Schlenzka J, Moehler TM, Kipriyanov SM, Kornacker M, Benner A, Bähre A, et al. Combined effect of recombinant CC19 x CD16 diabody and thalidomide in a preclinical model of human B cell lymphoma. Anticancer Drug 2004;15:915-9.
- Löffler A, Gruen M, Wuchter C, Schriever F, Kufer P, Dreier T, et al. Leukemia 2003;17:900-9.
- Gruen M, Bommert K, Bargou RC. T-cell-mediated lysis of B cells induced by a CD19xCD3 bispecific single-chain antibody is perforin dependent and death receptor independent. Cancer Immunol Immunother 2004;53:625-32.
- Kipriyanov SM, Moldenhauer G, Strauss G, Little M. Bispecific CD3 x CD19 diabody for T cell-mediated lysis of malignant human B cells. Int J Cancer 1998;77:763-72.
- Mack M, Gruber R, Schmidt S, Riethmüller G, Kufer P. Biological properties of a bispecific single-chain antibody directed against 17-1A (EpCAM) and CD3. J Immunol 1997;158:3965-70.
- Maletz K, Kufer P, Mack M, Raum T, Pantel K, Riethmüller G, et al. Bispecific single-chain antibodies as effective tools for eliminating epithelial cancer cells from human stem cell preparations by redirected cell cytotoxicity. Int J Cancer 2001;93:409-16.
- Wimberger P, Xiang W, Mayr D, Diebold J, Dreier T, Baeuerle PA, et al. Efficient tumor cell lysis by autologous, tumor-resident T lymphocytes in primary ovarian cancer samples by an EP-CAM-/CD3-bispecific antibody. Int J Cancer 2003;105:241-8.
- Jost CR, Titus JA, Kurucz I, Segal DM. A single-chain bispecific Fv2 molecule produced in mammalian cells redirects lysis by activated CTL. Mol Immunol 1996;33:211-9.
- Hayden MS, Linsley PS, Gayle MA, Bajorath J, Brady WA, Norris NA, et al. Single-chain mono- and bispecific antibody derivatives with novel biological properties and antitumour activity from a COS cell transient expression system. Ther Immunol 1994;1:3-15.
- Honemann D, Kufer P, Rimpler MM, Chatterjee M, Friedl S, Riecher F, et al. A novel recombinant bispecific single-chain antibody, bscWue-1 x CD3, induces T-cell-mediated cytotoxicity towards human multiple myeloma cells. Leukemia 2004;18:636-44.
- Bruenke J, Fischer B, Barbin K, Schreiter K, Wachter Y, Mahr K, et al. A recombinant bispecific single-chain Fv antibody against HLA class II and FcgammaRIII (CD16) triggers effective lysis of lymphoma cells. Br J Haematol 2004;125:167-79.
- McCall AM, Shahied L, Amoroso AR, Horak EM, Simmons HH, Nielsen U, et al. Increasing the affinity for tumor antigen enhances bispecific antibody cytotoxicity. J Immunol 2001;166:6112-7.
- Haisma HJ, Grill J, Curiel DT, Hoogeland S, van Beusechem VW, Pinedo HM, et al. Targeting of adenoviral vectors through a bispecific single-chain antibody. Cancer Gene Ther 2000;7:901-4.
- Grill J, van Beusechem VW, van der Valk P, Dirven CMF, Leonhart A, Pherai DS, et al. Combined targeting of adenoviruses to integrins and epidermal growth factor receptors increased gene transfer into primary glioma cells and spheroids. Clin Cancer Res 2001;7:641-50.
- Witlox MA, van Beusechem VW, Grill J, Haisma HJ, Schaap G, Bras J, et al. Epidermal growth factor receptor targeting enhances adenoviral vector based suicide gene therapy of osteo-sarcoma. J Gene Med 2002;4:510-6.
- Dirven CMF, Grill J, Lamfers MLM, van der Valk P, Leonhart AM, van Beusechem VW, et al. Gene therapy for meningioma: improved gene delivery with targeted adenoviruses. J Neurosurg 2002;97:441-9.
- van Beusechem VW, Grill J, Mastenbroek DCJ, Wickham TJ, Roelvink PW, Haisma HJ, et al. Efficient and selective gene transfer into primary human brain tumors by using single-chain antibody-targeted adenovrial vectors with native tropism abolished. J Virol 2002;76:2753-62.
- Heideman DA, van Beusechem VW, Offerhaus GJ, Wickham TJ, Roelvink PW, Craanen ME, et al. Selective gene transfer into primary human gastric tumors using epithelial cell adhesion molecule-targeted adenoviral vectors with ablated native tropism. Hum Gene Ther 2002;13:1677-85.
- Holliger P, Brissinck J, Williams RL, Thielemans K, Winter G. Specific killing of lymphoma cells by cytotoxic T-cells mediated by a bispecific diabody. Protein Eng 1996;9:299-305.
- Zhu Z, Zapata G, Shalaby R, Snedecor B, Chen H, Carter P. High level secretion of a humanized bispecific diabody from Escherichia coli. Biotechnology 1996;14:192-6.
- Helfrich W, Kroesen BJ, Roovers RC, Westers L, Molema G, Hoogenboom HR, et al. Construction and characterization of a bispecific diabody for retargeting T cells to human carcinomas. Int J Cancer 1998;76:232-9.
- Krebs B, Griffin H, Winter G, Rose-John S. Recombinant human single chain Fv antibodies recognizing human interleukin-6. Specific targeting of cytokine-secreting cells. J Biol Chem 1998;273:2858-65.
- Xiong D, Xu Y, Liu H, Peng H, Shao X, Lai Z, et al. Efficient inhibition of human B-cell lymphoma xenografts with an anti-CD20 x anti-CD3 bispecific diabody. Cancer Lett 2002;177:29-39.
- Hayashi H, Asano R, Tsumoto K. A highly effective and stable bispecific diabody for cancer immunotherapy: cure of xenografted tumors by bispecific diabody and T-LAK cells. Cancer Immunol Immunother 2004;53:497-509.
- Takemura S, Kudo T, Asano R, Suzuki M, Tsumoto K, Sakurai N, et al. A mutated superantigen SEA D227A fusion diabody specific to MUC1 and CD3 in targeted cancer immunotherapy for bile duct carcinoma. Cancer Immunol Immunother 2002;51:33-44.
- Gao Y, Xiong D, Yang M, Liu H, Peng H, Shao X, et al. Efficient inhibition of multidrug-resistant human tumors with a recombinant bispecific anti-P-glycoprotein x anti-CD3 diabody. Leukemia 2004;18:513-20.
- Arndt MA, Krauss J, Kipriyanov SM, Pfreundschuh M, Little M. A bispecific diabody that mediates natural killer cell cytotoxicity against xenotransplantated human Hodgkin’s tumors. Blood 1999;94:2562-8.
- Kontermann RE, Wing MG, Winter G. Complement recruitment using bispecific diabodies. Nat Biotechnol 1997;15:629-31.
- DeNardo SJ, DeNardo GL, DeNardo DG, Xiong CY, Shi XB, Winthrop MD, et al. Antibody phage libraries for the next generation of tumor targeting radioimmunotherapeutics. Clin Cancer Res 1999;5:3213s-3218s.
- DeNardo DG, Xiong CY, Shi XB, DeNardo GL, DeNardo SJ. Anti-HLA-DR/anti-DOTA diabody construction in a modular gene design platform: bispecific antibodies for pretargeted radio-immunotherapy. Cancer Biother Radiopharm 2001;16:525-35.
- Nettelbeck DM, Rivera AA, Kupsch J, Dieckmann D, Douglas JT, Kontermann RE, et al. Retargeting of adenoviral infection to melanoma: combining genetic ablation of native tropism with a recombinant bispecific single-chain diabody (scDb) adapter that binds to fiber knob and HMWMAA. Int J Cancer 2004;108:136-45.
- Reusch U, Le Gall F, Hensel M, Moldenhauer G, Ho AD, Little M, et al. Effect of tetravalent bispecific CD19xCD3 recombinant antibody construct and CD28 costimulation on lysis of malignant B cells from patients with chronic lymphocytic leukemia by autologous T cells. Int J Cancer 2004;112:509.
