Subconjunctival sustained release 5-fluorouracil for glaucoma filtration surgery1
Introduction
Modulation of the wound-healing process to prevent excessive fibroblast proliferation and scar formation can play a major role in improving the outcome of glaucoma filtration surgery[1–3]. One potent antimetabolic agent, 5-fluorouracil (5-FU), a fluorinated pyrimidine which competitively inhibits thymidylate synthetase, resulting in an inhibition of deoxyribonucleic acid synthesis, has been found to improve the success of glaucoma filtration surgery in glaucoma patients with poor surgical prognoses[4–8]. However, frequent postoperative subconjunctival injections or intraoperative exposure to a high concentration are always associated with corneal epithelial defects, prolonged hypotony, blebitis, endophthalmitis, and cataracts[4,7,8]. To solve these problems, controlled drug delivery systems of 5-FU have been evaluated, including sustained drug delivery from liposomes[9,10] and chitosan[11], slow release from bioerodible polymers[12–21], and collagen implants[22]. This may allow low, therapeutic drug levels to be maintained at the site of action, but most of these systems could release drug for nearly 10 days only [9–11,13–19].
Using a method of microspheres accumulated by excessive carriers, we produced a 5-FU impregnated poly (D,L-lactic acid) disc (5-FU-PLA-DS) for long-term release. This paper presents the results of the characteristics of this bio-erodible disc and a series of experiments in a rabbit model of glaucoma filtration surgery.
Materials and methods
Drugs and reagents The poly (lactic acid) (PLA, molecular weight of 20 000) was obtained from Shan Dong Medical Apparatus Institute (Jinan, China). The standard 5-FU (batch N
Apparatus A Shimadzu fluorescence spectrophotometry (UV-1601PC, Tokyo, Japan) was used to determine the released characteristics in vitro. The HPLC system consisted of a LC-6A pump (Shimadzu, Tokyo, Japan), a SPD-6AV UV detector (Shimadzu, Tokyo, Japan) set at 266 nm, a SIL-9A autosampler (Shimadzu, Kyoto, Japan), a SLC-6B system controller (Shimadzu, Tokyo, Japan), and a CLASS-VP software integrator (Shimadzu, Tokyo, Japan). A C18 column (150 mm×4.6 mm; internal diameter, 5 μm; Beckman, Fullerton, CA, USA) was used to determinate the 5-FU concentration in the aqueous humor.
Preparation of discs In total, 390 mg 5-FU was suspended in 3 mL dichloromethane dissolving 350 mg of PLA and 20 mg of span-80. Fifteen mililitres of petroleum benzine was dropwised slowly (10–15 drops/min) by high-speed agitation. The redundant carrier resulted in the accumulation of the microspheres which precipitated gradually. Another 15 mL of petroleum benzine was administered to solidify for 10 min. The deposition was collected after centrifugation (1400×g, 15 min), and compressed gently into a vitric container of 300 mm×250 mm×1 mm. A hollow steel tube was used to cut it into discs of 3 mm in diameter and 1 mm thick. Each disc contained 3 mg 5-FU. The polymer control disc was made in an identical manner in the absence of 5-FU. All discs were dried under a vacuum for 24 h until the solvent was evaporated thoroughly, and then stored desiccated in a bottle.
Determination of 5-FU content and encapsulation efficiency A 5-FU-PLA-DS was chosen randomly, exactly weighed with a balance, and dissolved in 2 mL dichloromethane. In total, 5 mL of 0.1 mol/L hydrochloric acid (HCl) was administered 3 times to extract 5-FU entirely by agitation and centrifugation (1400×g, 15 min×3). The supernatant was collected and measured by the fluorescence spectrophotometry (266 nm). Repeated determinations were taken 6 times (n=6). The 5-FU content in a disc was calculated according to the following equation:
5-FU content (%)=WFU/WDS ×100%, (1)
where WFU represents the weight of 5-FU found in the disc, and WDS is the weight of the disc.
To determine the encapsulation efficiency, the extraction of 5-FU was performed in the mixture of petroleum benzine and dichloromethane after the accumulated microspheres was carried out by adding 20 mL of 0.1 mol/L HCl 3 times. The concentration of 5-FU in the HCl supernatant was analyzed by FS. Encapsulation efficiency was calculated according to the following equation:
where Wt represents the total 5-FU added, Cs is the concentration of 5-FU in the supernatant, and Vs means the total volume of the 3 portions of the HCl supernatants.
In vitro release studies A disc was suspended in a cuvette with 1 mL simulated body fluid (SBF), and the cuvette was immersed in a water bath kept at 37 °C. The release medium was periodically removed and replaced with fresh SBF. The amount of 5-FU released in SBF was measured by FS. The determination was stopped when the 5-FU concentration was less than 5 μg/mL per day at 2 consecutive time points. Ten samples were tested simultaneously.
Morphological observation The external morphology of the 5-FU-PLA-DS was observed by scanning electron microscopy (SEM; Model JSM-840; Jeol, Tokyo, Japan). This was also used to follow the degradation of the discs in vitro. The microspheres suspended in the supernatant were observed by an optical microscopy (Olympus CHT-001, Lake Success, NY, USA).
Experimental animals A prospective, randomized, placebo-controlled, masked observer study was performed using 40 male and female New Zealand White rabbits initially weighing 2.2–3.4 kg. The experiment was performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. The project was also approved by the local animal research review committee of the authors’ institution. All animals were maintained in a 12 h day and 12 h night cycle. They were fed and had access to water ad libitum.
A posterior lip sclerectomy was performed on both eyes of each animal as described previously[17]. The animals were divided into 2 groups. The left/right eyes of group 1 (25 rabbits) were randomly chosen to implant a sterile 5-FU-PLA-DS at the filtration site in the subconjunctival space, and the contralateral eyes received a polymer disc. For group 2 (15 rabbits), before entering the anterior chamber of unilateral eyes selected randomly, a 5×3 mm sponge soaked in 5-FU (dissolved in distilled water) at a concentration of 25 mg/mL was placed over the planned filtration site for 5 min and irrigated thoroughly. The fellow eyes underwent trabeculectomy alone. At each end of the surgery, 1 drop of 1% of atropine sulfate and dexamethasone and 0.5% erythromycin ointment were applied into the conjunctival sac, and 0.3% ofloxacin and 0.5% cortisone acetate eye drops were instilled 3 times per day for 3 d.
Clinical observations Evaluations were performed on postoperative d 1–3, 7, and every week up to d 90, or more often when required. The evaluations consisted of observation of general health, Seidal test, red reflex, intraocular pressure measurement by a Perkins handheld applanation tonometer (HL-2; Kowa, Nagoya, Japan), and slit lamp biomicroscopy of the anterior segments. Ultrasound biomicroscopy (UBM) detection under topical anesthesia was performed from postoperative d 7.
The two-tailed, Student t-test was used to compare the intraocular pressure of the experimental eyes with that of each control group. Survival analyses were carried out for bleb failure. Log-rank tests were used to test for overall survival differences among the 4 groups. Kaplan Meier survival plots illustrated these data. A level of P<0.05 was considered statistically significant.
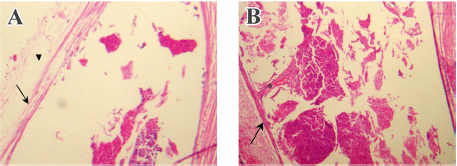
Conjunctival biopsy Two animals from group 1 were killed at postoperative d 90 by an intravenous injection of a lethal dose of pentobarbital. Their eyes were enucleated, with care taken not to disturb the filtration bleb. After immersion in 10% formalin for 48 h, the bleb tissue and the conjunctiva covering the disc was dehydrated, infiltrated, and embedded in paraffin. Six micron step sections were cut and stained with hematoxylin-eosin. They were studied by conventional optical microscopy.
Quantitative determination of 5-FU in the aqueous humor Concentrations of 5-FU were determined by the HPLC system. The mobile phases were solvent A (10 mmol/L sodium phosphate buffer, pH 4.8) and solvent B (80% methanol) (A:B=9:1), and the flow rate was 1 mL/min. After clinical observation, a corneal paracentesis was made under local anesthesia with two 5-FU-PLA-DS-implanted eyes and one 5-FU-exposed eye, and 30 μL of aqueous humor was collected for quantitative analysis. Samples and quantitative standards were homogenized and directly injected into the HPLC system with an injection volume of 20 μL.
Results
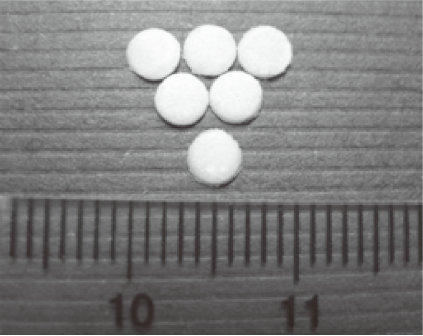
Characterization of 5-FU-loading disc The 5-FU-PLA-DS (Figure 1), with an average weight of 6.267±0.082 mg (mean±SD), contained 3.066±0.077 mg 5-FU. The mean 5-FU content and encapsulation efficiency were 48.92%±1.95% and 83.50%±2.63%, respectively.
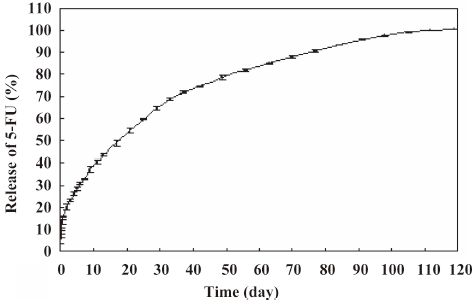
In vitro release of 5-FU-PLA-DS The release profile of 5-FU from the disc is shown in Figure 2. The drug was released for at least 110 d, with the concentration of > 10 μg/mL per day in the first 91 d. Although approximately 15.6% of the drug was released on the first day, the delivery rate slowed down markedly on d 2. The mean 5-FU concentration was 60 μg/mL 6 d after release. It was satisfactory that the 5-FU was released steadily at the average rate of 19–35 μg per day on the second month and 10–19 μg per day on the third month.
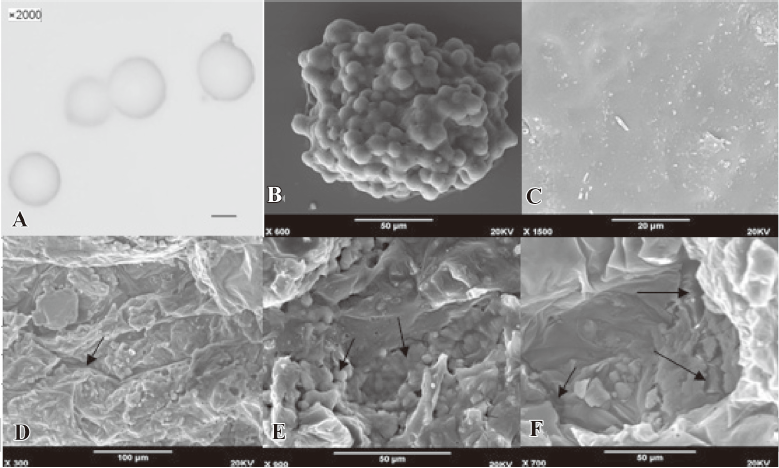
The diameter of the 5-FU-PLA-MS was approximately 10 μm (Figure 3A). Figure 3C-3F showed the degeneration process of the 5-FU-PLA-DS. It is clear that the external morphology of the disc underwent great change during the release experiment.
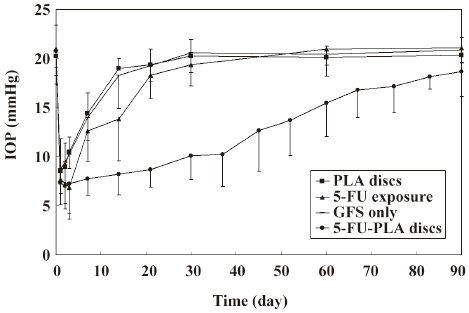
Intraocular pressure As is shown in Figure 4, the postoperative intraocular pressure in the experimental eyes were significantly lower than the polymer controls and trabeculectomies from d 3 to 83 after surgery, and the eyes with 5-FU intraoperatively from d 7 to 90 (P<0.05). All the control eyes returned to their preoperative intraocular pressure levels before postoperative d 30. The mean intraocular pressure of the experimental eyes (18.65±2.52 mmHg) was still lower than the controls on postoperative d 90, but the difference was not statistically significant (P>0.05).
Clinical appearance of the eyes There were no intraoperative complications of conjunctival button holes or vitreous loss. The Seidel test was positive in 2 eyes (8%) in the experimental group on postoperative d 3, and one of them also accompanied with hypotony. Bleb leakage was also found simultaneously in 4 of the eyes (26.7%) exposed to 5-FU intraoperatively, 3 eyes (12%) in the polymer group, and 2 eyes (13.3%) with trabeculectomy alone. The discs were devoid of extrusion in both experimental eyes and polymer controls throughout the experiment. There were no postoperative complications of pigmentation, hemorrhage, or endophthalmitis in the eyes implanted with 5-FU-PLA-DS.
Trabeculectomy triggered slight conjunctival hyperemia resolved after approximately 1 week. Hyperemia, triggered by the presence of 5-FU-PLA-DS and 5-FU applied intraoperatively, increased obviously than trabeculectomy alone, especially by intraoperative 5-FU on postoperative d 2–14 (Figure 5). It disappeared 1 month after surgery in the 5-FU-exposed eyes, while both the 5-FU-PLA-DS and the polymer implanted eyes still had 1 case on postoperative d 60.
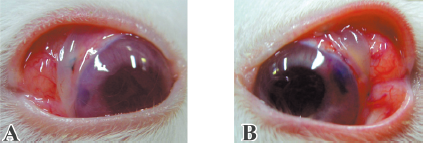
At the site of surgery, trabeculectomy triggered reversible edema of the cornea in 5 eyes, which reached its apex at d 3. The presence of polymer alone triggered edema of the same intensity, which was also reversible. The group that received 5-FU-PLA-DS also showed some edema (Figure 5), but it remained localized at the surgical site. In contrast, the frequency and the severity of corneal edema in the 5-FU intraoperative exposed eyes were significantly higher than in any other group. Two eyes had diffuse corneal edema extending to half the surface of the cornea on d 3. There was no difference between trabeculectomy and PLA control eyes on anterior segment reaction.
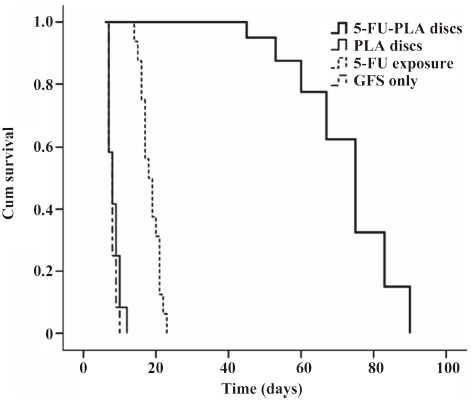
Survival curves and bleb appearance The experimental eyes showed a longer duration of filtering blebs, with averages of 72.8±12.5 d compared with 8.3±1.7 d of the polymer controls, 7.8±1.1 d of the trabeculectomy alone, and 18.5±2.7 d of 5-FU intraoperative exposure, respectively (P<0.01; Figure 6).
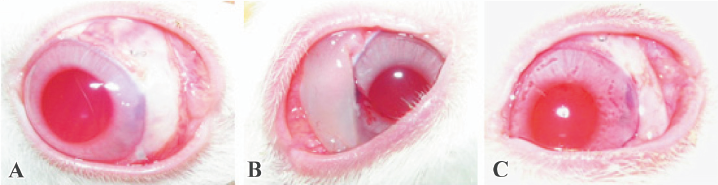
Compared with the huge, high, and thin bleb of the 5-FU-exposed eyes, the dispersed filtration bleb of the 5-FU-PLA-DS-implanted eyes was similar to that of the polymer controls and trabeculectomy alone on postoperative d 3 (Figure 7). Avascular blebs were formed gradually in most of the experimental eyes, but they were not as huge and transparent as that of the eyes treated with 5-FU intraoperatively. The filtration blebs in the eyes received polymer disc and trabeculectomy alone formed on postoperative d 1; however, that of the other 2 groups were not obvious at that time. The blebs in the polymer control and trabeculectomy alone achieved their developing peaks on d 3, while that of the 5-FU intraoperative exposure and 5-FU-PLA-DS eyes, on postoperative d 7 and 14, respectively. The blebs shrank soon after the developing peak in the 3 control groups, and all of them disappeared thoroughly 1 month after surgery, but the experimental eyes showed a significantly long persistence. Two blebs still existed on postoperative d 83.
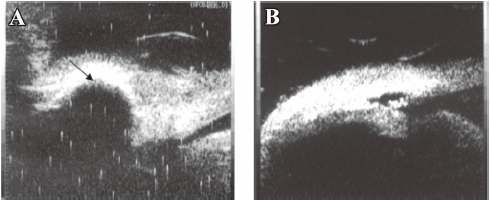
UBM The results of the UBM detection could determine success or failure of the surgery. It showed good correlation to the bleb observation. A diffused, high bleb was accompanied with a subconjunctival patent fistula, and if the bleb became flat and localized, some lamellar or punctiform hyperechoic substances would be found in the deep sclerectomy area (Figure 8). Ciliary body detachment with suprachoroidal hemorrhage was found in 1 eye treated with 5-FU intraoperatively, which was also accompanied with hemorrhage and flat anterior chamber.
Conjunctival biopsy Neither the 5-FU-PLA-DS or the polymer control disc was degraded completely 90 d after implantation. Due to the difficulty in separating the implant from conjunctiva, they underwent histological examination together. As is shown in Figure 9, no evidence of a toxic or inflammatory reaction to the disc was found. A thin membrane of connective tissue (3–5 cells thick) was seen surrounding the disc. It seemed that the 5-FU-PLA-DS was bio-eroded faster than the control disc without 5-FU.
Quantitative determination of 5-FU In the eyes that received 5-FU-PLA-DS, a quantitative determination in the aqueous humor showed detectable amounts of 5-FU for 10.2±1.3 weeks. The apex of 8.7 μg/mL on average appeared on the first day after surgery. The concentration decreased with time. It was lower than 1 μg/mL at 1 week after surgery. The lowest detectable amounts of 5-FU was 25 ng/mL. This indicated that PLA was still present in the subconjunctival space for that period and released 5-FU in a continuous fashion. In contrast with the in vitro release profile, no burst release of 5-FU was observed in the aqueous humor. The 5-FU concentration in the aqueous humor was undetectable in the eyes treated with 5-FU intraoperatively.
Discussion
As known, lipophilic drugs can distribute more homogeneously in the matrix of PLA, which is also lipophilic, and can be released for a longer time without a “burst” effect. The physical incorporation of PLA and 5-FU can not afford a homogeneous distribution, as 5-FU is not lipophilic. This explains why the pattern of drug delivery systems composed of PLA and 5-FU reported previously is always microspheric[14–16]. Microspheres may have good shapes and steady delivery behaviors, but the drug loading and the duration is not enough to keep the filtration fistula open for a long period. The subconjunctival delivery systems are usually designed to provide a sustained release over an extended period (approximately 2 weeks). This 2 week period is critical with respect to inflammatory and fibrotic reactions[19]. However, 5-FU inhibits the proliferation of fibroblasts, but has no effect on fibroblast migration or attachment at concentrations that inhibit proliferation[23]. Fibroblasts moved from other places could still proliferate to repair the filtration wound after the 2 week drug release. Therefore, trabeculectomy treated with these 2 week drug release systems in the rabbit eyes[17–19] failed within 1 month eventually, marked with intraocular pressure returned to normal and bleb disappeared.
To circumvent this difficulty, the microsphere-accumulated disc with higher drug loading and longer duration of release is thought to be helpful. In this study, the gradual accumulation of 5-FU-loaded microspheres, with a diameter of 10 μm, could provide a well distribution of the drug in the polymer. 5-FU is released by diffusion through the matrix of PLA, and degradation of the matrix within and between microspheres occurs simultaneously. As is shown in Figure 2, the release procedure took place stepwise inward from the surface of the disc. The microspheres in the inner layer would be exposed to release 5-FU after the ones of outer layer were degraded. This asynchronous delivery from different layers of microspheres may lead to a sustained, steady, and low level release over a long period. As expected, it is clear that 5-FU was delivered from the 5-FU-PLA-DS at a relatively constant rate for at least 110 d in the in vitro release profile. The initial burst of 15.6% of total loading may have been due to the rapid release of the drug deposited on the implant surface and to the outer microspheres broken by the compression process during the preparation.
It was demonstrated that there is a significant difference in intraocular pressure reduction and bleb duration between the experimental eyes and the controls. Eyes that were treated with 5-FU-PLA-DS after trabeculectomy had lower intraocular pressure for almost 90 d and longer persistence of filtration blebs and patent fistulas, as supported by the UBM findings. This improvement in the duration of lowered intraocular pressure in the experimental eyes was due to the slow and continuous release of 5-FU. Khaw et al[24] reported the cell proliferation of cultured human tenon fibroblasts treated by 5-FU. At a concentration of 10 μg/mL, treatment periods of 3 d or more resulted in no increase in cell numbers up to d 30. Although the 5-FU-PLA-DS released 5-FU for at least 110 d at >10 μg/mL lasting at least 90 d in vitro, therapeutic effects lasted less than 90 d in the animal models. This was due to the enhancement of the degradation of the disc in vivo and to inadequate drug concentrations to inhibit fibrosis at the filtering site. Moreover, the wound-healing ability in rabbits is strong than primates[21,22]. The conjunctiva biopsy showed that the 5-FU-PLA-DS was bio-eroded faster than the control disc, which also indicated that the tiny space left by drug diffusion can accelerate the polymer degradation.
Intraoperative 5-FU also had a marked effect on intraocular pressure and bleb persistence, as found in this research, but it triggered severe complications, such as bleb leaks, corneal edema, and epithelial defects. When incorporated in PLA, 5-FU also appeared to trigger toxic reactions. There were some conjunctival hyperemia and corneal edema, but the intensity was lower than the intraoperative 5-FU application. The slow release of 5-FU from the 5-FU-PLA-DS prevented the focal attainment of high and toxic drug levels. The quantitative determination of 5-FU concentrations in the aqueous humor is particularly important to ensure that the levels reached are not toxic to the corneal endothelium. The concentration of 5-FU at each time point was far below the threshold concentration for 5-FU toxicity to the corneal endothelium (1–10 mg/mL) reported by Mannis et al [25]. The undetectable 5-FU in intraoperative exposed eyes may have been due to the tamponade which was performed before entering the anterior chamber, while the drug had diffused into the ocular tissues before being thoroughly irrigated[26].
In our studies, the PLA implant appeared to be biocompatible by the histological study. There was no significant difference between PLA control eyes and trabeculectomy alone on intraocular pressure reduction and anterior segment reaction. In conclusion, our results suggest this new drug delivery system, using the accumulation of 5-FU-PLA-DS, may have potential usefulness for glaucoma filtration surgery. It would be ideal if we could find a solution to decrease the rate of the initial release in future studies.
References
- Collignon NJ. Wound healing after glaucoma surgery: how to manage it? Bull Soc Belge Ophtalmol 2005;295:55-9.
- Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol 2003;48:314-46.
- Tahery MM, Lee DA. Pharmacologic control of wound healing in glaucoma filtration surgery. J Ocul Pharmacol 1989;5:155-79.
- The Fluorouracil Filtering Surgery Study Group. Five-year follow-up of the fluorouracil filtering surgery study. Am J Ophthalmol 1996;121:349-66.
- Joshi AB, Parrish RK 2nd, Feuer WF. 2002 survey of the American Glaucoma Society: practice preferences for glaucoma surgery and antifibrotic use. J Glaucoma 2005;14:172-4.
- Abraham LM, Selva D, Casson R, Leibovitch I. The clinical applications of fluorouracil in ophthalmic practice. Drugs 2007;67:237-55.
- Sisto D, Vetrugno M, Trabucco T, Cantatore F, Ruggeri G, Sborgia C. The role of antimetabolites in filtration surgery for neovascular glaucoma: intermediate-term follow-up. Acta Ophthalmol Scand 2007;85:267-71.
- Fraser S. Trabeculectomy and antimetabolites. Br J Ophthalmol 2004;88:855-6.
- Elorza B, Elorza MA, Frutos G, Chantres JR. Characterization of 5-fluorouracil loaded liposomes prepared by reverse-phase evaporation or freezing-thawing extrusion methods: study of drug release. Biochim Biophys Acta 1993;1153:135-42.
- Hitzman CJ, Elmquist WF, Wattenberg LW, Wiedmann TS. Development of a respirable, sustained release microcarrier for 5-fluorouracil I: In vitro assessment of liposomes, microspheres, and lipid coated nanoparticles. J Pharm Sci 2006;95:1114-26.
- Aksungur P, Sungur A, Unal S, Iskit AB, Squier CA, Senel S. Chitosan delivery systems for the treatment of oral mucositis: in vitro and in vivo studies. J Control Release 2004;98:269-79.
- Gao H, Gu Y, Ping Q. The implantable 5-fluorouracil-loaded poly(l-lactic acid) fibers prepared by wet-spinning from suspension. J Control Release 2007;118:325-32.
- Pandey SK, Cochener B, Apple DJ, Colin J, Werner L, Bougaran R, et al. Intracapsular ring sustained 5-fluorouracil delivery system for the prevention of posterior capsule opacification in rabbits: a histological study. J Cataract Refract Surg 2002;28:139-48.
- Sastre RL, Olmo R, Teijón C, Muñíz E, Teijón JM, Blanco MD. 5-Fluorouracil plasma levels and biodegradation of subcutaneously injected drug-loaded microspheres prepared by spray-drying poly(D,L-lactide) and poly(D,L-lactide-co-glycolide) polymers. Int J Pharm 2007;338:180-90.
- Moritera T, Ogura Y, Honda Y, Wada R, Hyon SH, Ikada Y. Microspheres of biodegradable polymers as a drug-delivery system in the vitreous. Invest Ophthalmol Vis Sci 1991;32:1785-90.
- Huang KH, Zhu ZH, Liu JH, Chen QK, Liu XY, Chang J. Preparation and release efficiency of polylactic acid nanoparticle. Ai Zheng 2005;24:1023-6.
- Blandford DL, Smith TJ, Brown JD, Pearson PA, Ashton P. Subconjunctival sustained release 5-fluorouracil. Invest Ophthalmol Vis Sci 1992;33:3430-5.
- Lee DA, Leong KW, Panek WC, Eng CT, Glasgow BJ. The use of bioerodible polymer and 5-fluorouracil in glaucoma filtering surgery. Invest Ophthalmol Vis Sci 1988;29:1692-7.
- Einmahl S, Behar-Cohen F, D’Hermies F, Rudaz S, Tabatabay C, Renard G, et al. A new poly(ortho ester)-based drug delivery system as an adjunct treatment in filtering surgery. Invest Ophthalmol Vis Sci 2001;42:695-700.
- Heller J. Ocular delivery using poly(ortho esters). Adv Drug Deliv Rev 2005;57:2053-62.
- Jampel HD, Leong KW, Dunkelburger GR, Quigley HA. Glaucoma filtration surgery in monkeys using 5-fluorouridine in polyanhydride discs. Arch Ophthalmol 1990;108:430-5.
- Hasty B, Heuer DK, Minckler DS. Primate trabeculectomies with 5-fluorouracil collagen implants. Am J Ophthalmol 1990;109:721-5.
- Lee DA, Shapourifar TS, Kitada S. The effect of 5-fluorouracil and cytarabine on human fibroblasts from Tenon’s capsule. Invest Ophthalmol Vis Sci 1990;31:1848-55.
- Khaw PT, Word S, Porter A, Grierson I, Hitchings RA, Rice NSC. The long-term effects of 5-fluorourocil and sodium butyrate on human tenon’s fibroblasts. Invest Ophthalmol Vis Sci 1992;33:2043-52.
- Mannis MJ, Sweet EH, Lewis RA. The effect of fluorouracil on the corneal endothelium. Arch Ophthalmol 1988;106:816-7.
- Wilkins MR, Occleston NL, Kotecha A, Waters L, Khaw PT. Sponge delivery variables and tissue levels of 5-fluorouracil. Br J Ophthalmol 2000;84:92-7.