Organic anion transporting polypeptide-1B1 haplotypes in Chinese patients1
Introduction
The members of the organic anion-transporting polypeptides (OATP) represent a family of important proteins involved in the membrane transport of endogenous and xenobiotic compounds. OATP are expressed in a wide variety of tissues, including the liver, kidney, brain, and small intestine[1–2]. Human OATP1B1 (also known as OATP-C, OATP2, SLCO1B1 gene), a sodium-independent bile acid transporter, is specifically expressed on the basolateral membrane of hepatocytes and translocate a broad range of compounds, such as bile acids, bilirubin, sulfate and glucuronide conjugates, thyroid hormones, peptides, and drugs like 3-hydroxy-3-methylglutaryl-co-enzymeA (HMG CoA)-reductase inhibitors (pravastatin, rosuvastatin, pitavastatin) and methotrexate[3–5]. Recent studies have proven that OATP1B1 plays an important role in the hepatocellular uptake and consequently the elimination of numerous chemicals.
A number of single nucleotide polymorphisms (SNP) have been identified in the encoding and regulating regions of the OATP1B1 gene among different populations. The frequency of the SLCO1B1*5 (521T>C, Val174Ala) variant frequency is 14% in European Americans and the SLCO1B1*9 (1463G>C, Gly488Ala) variant frequency is 9% in African Americans[6], but these 2 common polymorphisms are extremely low in Japanese populations, which exhibit significant ethnic difference. In Orientals, SLCO1B1*1b (388G>A, Asn130Asp) and SLCO1B1*15 (a haplotype of SLCO1B1*1b and SLCO1B1*5) are 2 common SNP with relatively high frequencies of 66% and 16%, respectively. Recent studies have elucidated that both SLCO1B1*1a and SLCO1B1*15 exhibit reduced transport function and play an important role in pravastatin, pitavastatin, rosuvastatin (a substrate for OATP1B1) systemic exposure and elimination[7–10]. SLCO1B1*17 (–11187G>A, 388G>A, and 521T>C) was found to be associated with increased plasma concentrations of pravastatin in humans. However, there are no published studies concerning the functional significance of the –11187G>A promoter SNP in vitro until now[9,10].
Therefore, this study was carried out to determine the SLCO1B1*1b and *15 polymorphisms in the Chinese population.
Materials and methods
Chemicals and reagents All primers for the PCR were synthesized by Bioasia Company (Shanghai, China). Taq DNA polymerase, dNTP mixture, PCR buffer, GeneRuler 500 bp DNA ladder, and the restriction endonuclease ClaI were all purchased from MBI Fermentas (Vilnius, Lithuania). All other chemicals were of highest grade and available from commercial sources.
Patients One hundred and eleven unrelated, healthy, Chinese Han male volunteers were recruited in this study. They were medical students at the Central South University, Xiangya Medical College (Hunan, China) and their mean age was 20±2 years. This research was approved by the Ethics Committee of the Central South University and written informed consent was obtained from each participant. The participants were assessed by medical history, physical examination, and clinical laboratory test of hematological, liver and kidney function, blood tests for human hepatitis B or C, and blood glucose. They were non-smokers who did not take any medication or alcohol in the 14 d before the study.
Blood acquisition and DNA isolation Five milliliters of venous blood was collected in a sterile tube containing EDTA and stored at -80 °C. Genomic DNA was isolated from leukocytes through a standard manual chloroform–phenol extraction procedure and stored at 4 °C until use.
PCR–restriction fragment length polymorphism assay for 388G>A genotyping The 388G>A genotype was determined by means of PCR–restriction fragment length polymorphism (RFLP) analysis according to Torina et al[6] with some modifications. The PCR reaction was carried out in a total volume of 25 µL consisting of 2.5 µL 10×PCR buffer (with MgCl2), 0.2 mmol/L of each dNTP, 60 pmol/L of each primer, 100 ng of genomic DNA as a template, and 2.5 U Taq polymerase. The sequences of the primers used for detection of 388G>A were: forward primer, 5'-GCAAATAAA-GGGGAATATTTCTC-3' and reverse primer, 5'-AGAGATGT-AATTAAA TGTATAC-3'. PCR amplification to detect 388G>A was performed using the Gene Amplification PCR System 2400 (Perkin Elmer, Foster City, CA, USA) with an initial denaturation at 94 °C for 5 min, followed by 37 cycles of denaturation at 94 °C for 30 s, annealing at 46 °C for 30 s, and extension at 72 °C for 30 s. A final 5 min extension at 72 °C was adopted. After amplification, the PCR products (274 bp) were digested with the CLaI restriction endonuclease at 30 °C for at least 6 h. Digested products were analyzed by electrophoresis on a 2.5% agarose gel in the presence of ethidium bromide.
Amplification refractory mutation system–PCR assay for 521T>C genotyping The amplification refractory mutation system (ARMS) was introduced, avoiding the use of restriction enzymes[10]. The 4 primers used in ARMS–PCR were according to Torina et al[6] with slight modifications. PCR amplification was performed in a Perkin Elmer DNA Model PJ2000 thermal cycler (USA) in a total volume of 25 µL solution containing 2.5 µL 10×buffer (with MgCl2), 0.4 mmol/L of each dNTP, 40 pmol/L of each primer, 2 U LA–Taq enzyme and approximately 200 ng genomic DNA as a template. The PCR conditions involved an initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 48 °C for 30 s, and extension at 72 °C for 30 s with a final extension at 72 °C for 7 min. Because the restriction endonuclease was unnecessary, the PCR products (totally 260 bp) were detected by means of 2% agarose gel electrophoresis and were detected by ethidium bromide staining. Two samples of 521T>C of each genotype were directly sequenced to confirm our genotyping result. The primers and conditions are listed in detail in Table 1.

Full table
Data analysis Haplotypes were reconstructed on the basis of the phase-unknown genotype data using PHASE version 2 software (Stephens, et al, Seattle, Washington, USA), a computer-assisted statistical analysis based on Bayesian statistics. Haplotypes, as well as the genotype frequency deviation from the Hardy–Weinberg equilibrium, were evaluated by appropriate χ2-test. The SPSS software package version 11.2 (Chicago, IL, USA) was used to perform the statistical analysis. A P-value of less than 0.05 was accepted as significant.
Results
The PCR product in different 388G>A genotypes are shown in Figure 1. Patients with 388GG produced 155 and 119 bp fragments, whereas those from 388AA homozygotes generated an additional 274 bp fragment. Heterozygous 388G>A-mutated genotypes produced 3 fragments of 155, 119, and 274 bp.

Four specific primers were used to amplify 3 fragments in different 521T>C genotypes (Figure 2). The wild-type allele yielded 2 fragments of 260 and 179 bp in length, while the variant 521T>C homozygotes resulted in 2 fragments of 260 and 123 bp in length. The heterozygotes resulted in 3 fragments of 260, 123, and 179 bp.
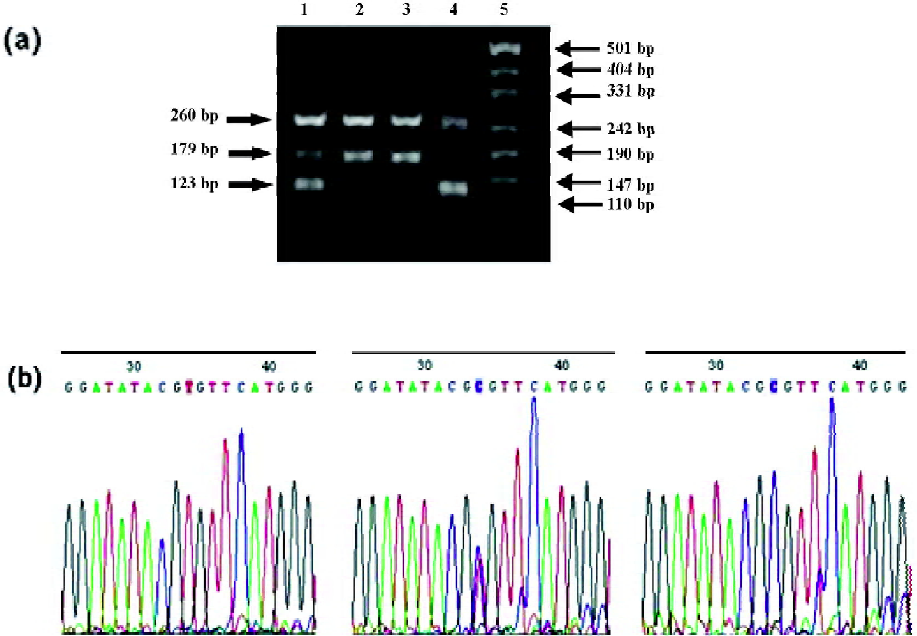
Of the 111 Chinese patients, 39 patients (35.1%) were heterozygotes and 62 (55.9%) were homozygotes for the 388G allele and 27 (24.3%) were heterozygotes and 2 (1.8%) were homozygotes for 521T>C mutation. The frequencies of the alleles and genotypes were calculated and are listed in Table 2. The distribution of the 3 genotypes of the 388G>A and 521T>C polymorphisms conformed well to the predictions of Hardy–Weinberg equilibrium (P>0.05).

Full table
A haplotype comprising of the 2 common SNP was constructed for these 111 individuals (222 sequences). The results of the haplotype analysis using a pseudo-Bayesian algorithm revealed 3 types of haplotypes in our Chinese population. These haplotypes were A-T (*1a), G-T (*1b), and G-C (*15). The haplotype frequencies were 26.1%, 59.9%, and 14%, respectively (Table 2). Of the 4 (2×2) possible combinations of the SNP, haplotype A-C (in order 388–521) was not present in our patients. In the population in our present study, the diplotypes of the individuals were determined by PHASE 2.0 software consisting of 6 types in all: *1a/*1a, *1a/*1b, *1a/*15, *1b/*1b, *1b/*15, and *15/*15. The detailed haplotypes and their frequencies are shown in Table 3.

Full table
Discussion
Drug metabolic enzymes and transporters have long been determined as major determinants of drug metabolism and disposition. However, accumulating evidence has proved that membrane transporters are also critical factors to the drug disposition process both in vitro and in vivo. Human OATP1B1 belongs to the OATP family of drug uptake transporters. It is specifically localized at the basolateral membrane of hepatocytes and is responsible for the hepatic uptake of a series of structurally-divergent compounds, including some endogenous chemicals and many clinically-used drugs.
More than 20 functionally-relevant SNP in the SLCO1B1 gene have been identified in different populations[6,12–14]. The common SNP with impaired transport activity appeared to be 521T>C (Val174Ala) in European Americans and Japanese and G1463>C (Gly488Ala) in African Americans. However, the clinical significance of these commonly seen mutations for large numbers of endogenous and xenobiotic substrates transported by OATP1B1 remains to be further studied.
We detected both the SLCO1B1*1b and SLCO1B1*15 variants in this study to characterize OATP1B1 genetic polymorphisms in the Chinese population. The frequency of the SLCO1B1*1b haplotype was 59.9% in our study, which is similar to previous reports of Japanese (62.9%)[7] and African Americans (74%), but higher than that of European Americans (30%)[6]. The frequency of the SLCO1B1*15 haplotype was 14% in Chinese, similar to that in Japanese (15.8%), but greater than that of Caucasians (2.4%) and African Americans (0%)[15].
The allele frequencies of drug transporter polymorphisms among different ethnic groups may contribute to drug therapeutic effects as well as toxicity. For example, pravastatin is one of the HMG-CoA reductase inhibitors (statins) widely used in the treatment of hypercholesterolemia[16], which experienced no obvious metabolism by cytochrome P450s, but could be efficiently taken up from circulation by the liver through OATP1B1[6]. It has been reported that increased systemic exposure and decreased non-renal elimination to pravastatin in vivo was significantly associated with the 521T>C variant in both Europeans and Japanese. The 388G>A site and novel mutation –11187G>A were also proven to be of pharmacokinetic significance[7–9]. In a recent study, patients with the SLCO1B1*15 allele were proven to be associated with higher serum bilirubin levels[17–19]. Thus, the determination of OATP1B1 gene polymorphisms in specific ethnic groups is very important for contributing to individualized drug therapeutics and the pathogenesis of hereditary diseases. However, the effect of the 521T>C genotype on the pharmacokinetics of rosuvastatin was not observed significantly in the Chinese, Malay, and Asian–Indian populations[20]. Our study indicated that the SLCO1B1 polymorphisms could not fully explain the interindividual difference of rosuvastatin disposition. The functional polymorphisms of CYP2C9 and some certain drug transporters, for instance breast cancer resistance protein (BCRP), may also contribute to the disposition of rosuvastatin[21], whereas pravastatin is not obviously metabolized by CYP450.
In summary, the present study has shown that the SLCO1B1*1b and SLCO1B1*15 variants represent common genetic polymorphisms in the Chinese population. There were remarkable ethnic differences in the frequencies of these 2 haplotypes. Our findings suggest that about 26.1% of the Chinese population carrying the SLCO1B1*15 variant might exhibit impaired transport activity of OATP1B1.
References
- Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta 2003;1609:1-18. Review.
- Kim RB. Organic anion-transporting polypeptide (OATP) transporter family and drug disposition. Eur J Clin Invest 2003;33 Suppl 2:1-5.
- Abe T, Kakyo M, Tokui T, Nakagomi R, Nishio T, Nakai D, et al. Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1. J Biol Chem 1999;120:17159-63.
- Hsiang B, Zhu Y, Wang Z, Wu Y, Sasseville V, Yang WP, et al. A novel human hepatic organic anion transporting polypeptide 2 (OATP2): identification of a liver-specific organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA-reductase inhibitor transporters. J Biol Chem 1999;274:37161-8.
- Konig J, Cui Y, Nies AT, Keppler D. A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest Liver Physiol 2000;278:G156-64.
- Tirona RG, Leake BF, Merino G, Kim RB. Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European and African–Americans. J Biol Chem 2001; 276: 35 669–75.
- Nishizato Y, Ieiri I, Suzuki H, Kimura M, Kawabata K, Hirota T, et al. Polymorphisms of OATP-C (SLC21A6) and OAT3 (SLC22A8) genes: consequences for pravastatin pharmaco-kinetics. Clin Pharmacol Ther 2003;73:554-65.
- Mwinyi J, Johne A, Bauer S, Roots I, Gerloff T. Evidence for inverse effects of OATP-C (SLC21A6) *5 and *1b haplotypes on pravastatin kinetics. Clin Pharmacol Ther 2004;75:415-21.
- Niemi M, Schaeffeler E, Langb T, Fromma MF, Neuvonen M, Kyrklund C, et al. High plasma pravastatin concentrations are associated with single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide-C (OATP-C, SLCO1B1). Pharmacogenetics 2004;14:429-40.
- Kameyama Y, Yamashita K, Kobayash K, Hosokawa M, Chiba K. Functional characterization of SLCO1B1 (OATP-C) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15+C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet Genomics 2005;15:513-22.
- Ye S, Dhillon S, Ke XY, Collins AR, Day INM. An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res 2001;29:E88-8.
- Kim RB. 3-Hydroxy-3-methylglutaryl–coenzyme A reductase inhibitors (statins) and genetic variability (single nucleotide polymorphisms) in a hepatic drug uptake transporter: What’s it all about? Clin Pharmacol Ther 2004;75:381-5.
- Tirona RG, Kim RB. Pharmacogenomics of organic anion-transporting polypeptides (OATP). Adv Drug Deliv Rev 2002;54:1343-52.
- Morimoto K, Oishi T, Ueda S, Ueda M, Hosokawa M, Chiba K. A novel variant allele of OATP-C (SLCO1B1) found in a Japanese patient with pravastatin-induced myopathy. Drug Metab Pharmacokinet 2004;19:453-5.
- Pasanen MK, Backman JT, Neuvonen PJ, Niemi M. Frequencies of single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide 1B1 SLCO1B1 gene in a Finnish population. Eur J Clin Pharmacol 2006;62:409-15.
- Hatanaka T. Clinical pharmacokinetics of pravastatin mechanisms of pharmacokinetic events. Clin Pharmacokinet 2000;39:397-412.
- Cui YH, Konig J, Leier I, Buchholz U, Keppler D. Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6*. J Biol Chem 2001;276:9626-30.
- Ieiri I, Suzuki H, Kimura M, Takane H, Nishizato Y, Irie S, et al. Influence of common variants in the pharmacokinetic genes (OATP-C, UGT1A1, and MRP2) on serum bilirubin levels in healthy subjects. Hepatol Res 2004;30:91-5.
- Nozawa T, Nakajima M, Tamai I, Noda K, Nezu JC, Sai Y, et al. Genetic polymorphisms of human organic anion transporters OATP-C (SLC21A6) and OATP-B (SLC21A9): allele frequencies in the Japanese population and functional analysis. J Pharmacol Exp Ther 2002;302:804-13.
- Lee E, Ryan S, Birmingham B, Zalikowski J, March R, Ambrose H, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther 2005;78:330-41.
- Zhang W, Yu BN, He YJ, Fan L, Li Q, Liu ZQ, et al. Role of BCRP 421C>A polymorphism on rosuvastatin pharmacokinetics in healthy Chinese males. Clin Chim Acta 2006;373:99-103.
