Polypeptide from Chlamys farreri attenuates murine thymocytes damage induced by ultraviolet B1
Introduction
Ultraviolet B (UVB) irradiation has been shown to be a particularly potent inducer of apoptosis[1]. UV-induced lesions appear in cellular DNA. Such lesions can probably appear in the lymphocytes during the exposure of skin to sunlight[2].
Irradiation of cells with UV light changes the program of gene expression. As the immediate-early genes in response to UV, the expression of the c-fos and c-jun is upregulated[3]. C-fos and c-jun are major components of active protein-1(AP-1), which is commonly activated in cell death[4].
Caspase activation is generally considered to be a key hallmark of apoptosis. Fernandez et al suggested that the synthesis of the c-Fos protein favors cell death pathway activation in which caspase-3 is involved[5]. Caspase-3 has been considered to play an important role in the terminal phase of apoptosis[6]. UV-induced activation of the c-Jun NH2-terminal kinase (JNK) results in an increase in c-jun gene expression and the activation of the c-Jun protein[7]. The activation of the JNK pathway is necessary for caspase activation and apoptosis induction[8]. During apoptosis, cytochrome c is released from mitochondrion and accumulated in the cytoplasm, where it is required for the activation of caspase-3. Caspase-3 is an effective caspase, which has been implicated in the execution phase of apoptosis. Caspase-3 activates specific nucleases and results in a characteristic pattern of nuclear DNA fragmentation[9].
From previous investigations, we have studied the protective effect of polypeptide from Chlamys farreri (PCF) against UV-irradiation on dermal fibroblasts[10,11]. As an octapeptide, PCF consists of 8 amino acids, namely, Pro, Asn, Ser, Thr, Arg, Hyl, Cys, and Gly. PCF could stimulate enzymes to remove free radicals. The DNA of the thymocytes damaged by UVB was reduced by pretreatment with PCF. PCF also exerts its protective function by modulating the mitogen-activated protein kinase (MAPK) cascade[12]. How-ever, the effect of PCF on c-fos and c-jun expression, cytochrome c release, and caspase-3 activity are unclear. In the present study, we aimed to investigate the possible protective role of PCF against UVB-induced apoptosis in murine thymocytes.
Materials and methods
Cell cultures and treatment The thymocytes were prepared from 3-week-old Kunming murine (Medical College of Qingdao University, Qingdao, China) and cultured in flasks with RPMI-1640 culture medium containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37 °C in a humidified atmosphere with 95% air and 5% CO2. The cell culture was maintained in continuous logarithmic growth at a concentration of 2×106 cells/L. The thymocytes were then divided into 6 groups including the control group, model group, 5.69 mmol/L PCF group, 2.84 mmol/L PCF group, 1.42 mmol/L PCF group, and the 5.69 mmol/L vitamin C group. The thymocytes were placed in triplicate wells per group and incubated for 24 h. Vitamin C was then added to the thymocyte suspension at a final concentration of 5.69 mmol/L and PCF was also added to the thymocyte suspension at a final concentration of 5.69, 2.84, and 1.42 mmol/L, respectively. After incubation for 2 h at 37 °C, the thymocytes were irradiated with UVB. During irradiation, the medium was discarded. The control cells were sham treated.
UVB irradiation Thymocytes at a concentration of 2×106/mL were irradiated with a battery of UVB lamps (Beijing University, Beijing China), with a peak intensity of 45 mJ/cm2 at the filter surface and a peak emission of 313 nm.
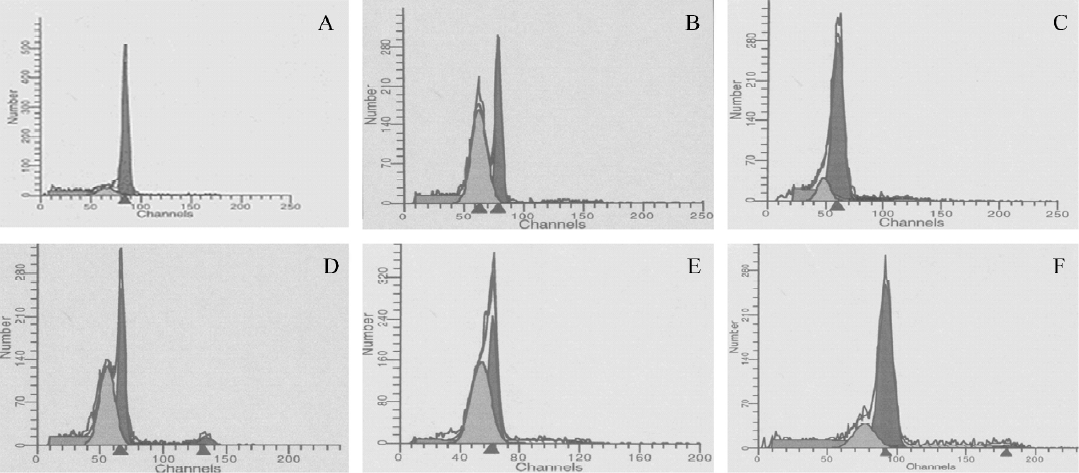
Apoptotic analysis by flow cytometry Flow cytometric analysis was performed for the quantification of cell death by apoptosis. Due to DNA degradation and subsequent leakage from cells, apoptotic cells can be detected via their diminished staining with DNA-specific fluorochromes, such as propidium iodide (Sigma, St Louis, Missouri, USA). The thymocytes (1×106 cells) were harvested and then fixed using 70% ethanol at 4 °C for 1 h. The fixed cells were washed with phosphate-buffered solution (PBS) and incubated with 1 mL hypotonic fluorochrome solution (50 mg/mL PI, 0.1% sodium citrate, and 0.1% Triton X-100), and RNase-A (50 mg/mL; Boehringer Mannheim, Penzberg, Germany) for 15 min at room temperature in the dark and then kept on ice. DNA fluorescence was analyzed by quantitative flow cytometry using CellQuest software (Becton Dickinson, Mountain View, CA, USA). The percentage of apoptotic cells was identified by analyzing the hypodiploid areas.
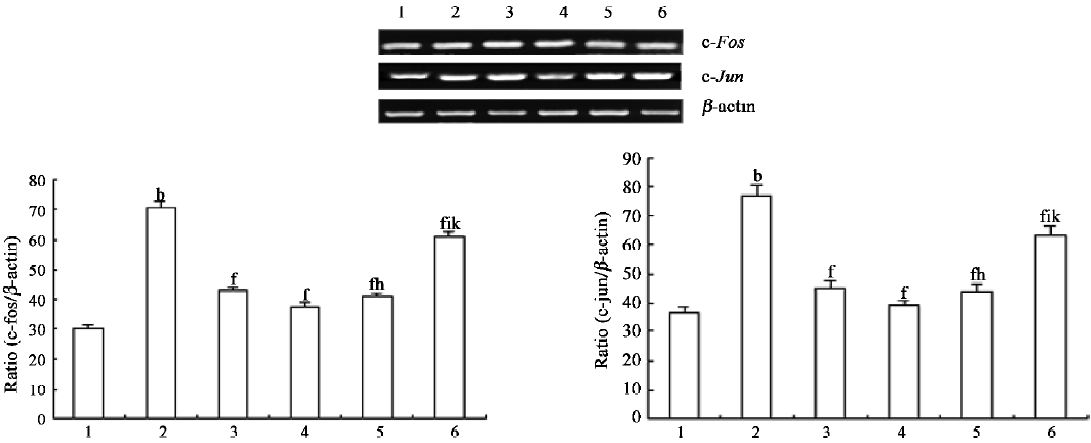
RT–PCR studies[13] Total RNA was isolated from murine thymocytes with Trizol reagent (Promega, Madison, WI, USA) according to the protocol of the manufacturer. First-strand cDNA was prepared by incubation of 1 µg total RNA with reverse transcriptase and oligo(dT) at 42 °C for 15 min. Then 2 µL of the reaction products was amplified by PCR with 2.5 U of Taq polymerase (Promega, Madison, WI, USA). PCR amplification consisted of denaturation at 95 °C for 30 s, annealing at 60 °C for 40 s, and extension at 72 °C for 2 min from 25 to 35 cycles in the linear range of the amplification number. The primers used were c-fos forward (5'-AGG AGG GAG CTG ACA GAT ACA CTC-3'), and c-fos reverse (5'-AGG CCA CAG ACA TCT CCT CTG-3'), c-jun forward (5'-CAT GGA GTC TCA GGA GCG GAT CA -3'), c-jun reverse (5'-TGA GTA CGA TTG CGT CGT CAA CG-3'), β-actin forward (5'-ACG TCT GCT GGA AGG TGG AC -3'), and β-actin reverse (5'-GGT ACC ACC ATG TAC CCA GG-3'). The PCR products of c-fos, c-jun, and β-actin were187, 248, and 163 bp, respectively. The expression of housekeeping gene β-actin was used as the control and checked for each set of RT–PCR experiment, as shown in lower panel (Figure 2). Aliquots of 10 μL of the PCR products were electrophoresed in a 1.6% agarose gel containing ethidium bromide. c-fos and c-jun were analyzed by a gel image analysis system and the level of c-fos and c-jun was reflected with the ratio of c-fos/β-actin or c-jun/β-actin.
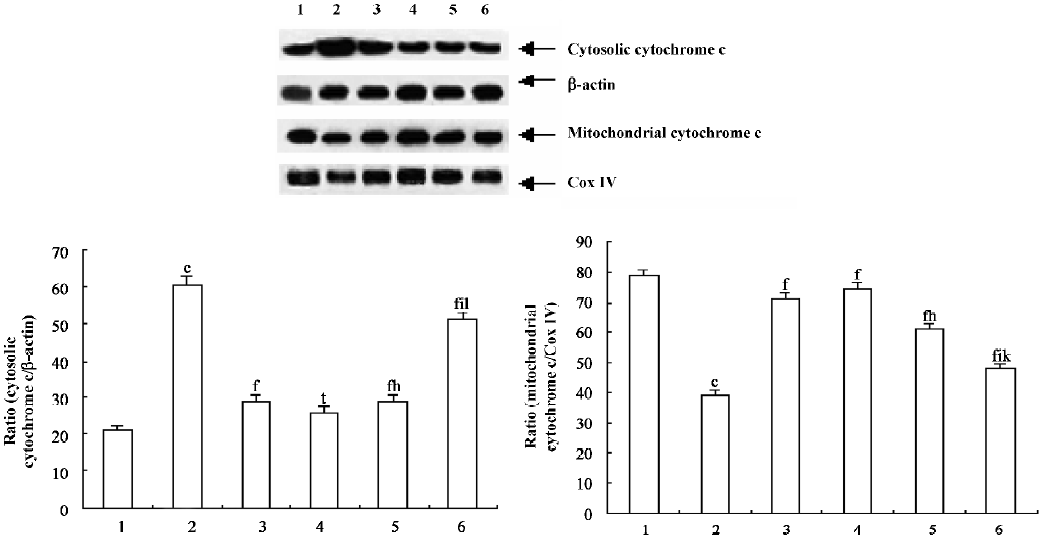
Purification of mitochondria and cytosol[14] The thymocytes were immediately washed twice with ice-cold PBS (pH 7.4) and centrifuged at 1000×g for 5 min at 4 °C. The pellets were then resuspended in 2 volumes of buffer A (250 mmol/L sucrose [Sigma, St Louis, Missouri, USA], 20 mmol/L N-2-hydroxyethylpiperazine-N-ethane-sulphonicacid [Sigma, St Louis, Missouri, USA]/potassium hydroxide [pH 7.5], 10 mmol/L KCl, 1.5 mmol/L MgCl2, 1 mmol/L EDTA, 1 mmol/L ethylene glycol-bis (β−aminoethyl ether) N,N,N’,N’-tetraacetic acid [EGTA], 1 mmol/L dithiothreitol, and 1 mmol/L phenylmethylsulfonyl fluoride with 10 µg/mL aprotinin, 10 µg/mL leupeptin, and 1.8 mg/mL iodoacetamide) and lysed at 4 °C for 30 min. The thymocytes were centrifuged at 1000×g for 10 min at 4 °C to remove the nuclei. The supernatant was centrifuged at 1×104 g for 15 min at 4 °C, and the pellets were the mitochondrial fractions. The pellet fractions were resuspended in buffer A. The supernatant of the 1×104 g spin was further centrifuged at 1×105 g for 1 h at 4 °C, and the resulting supernatant contained the soluble cytosolic fractions. The cytosolic fractions and mitochondrial fractions were divided into multiple samples and frozen at -80 °C. The protein concentrations were then determined.
Western blot analysis Cytochrome c was analyzed in both the mitochondrial and cytosolic fractions. Equal quantities (40 µg) of lysates were electrophoresed in a SDS–PAGE and then electrotransferred onto a nitrocellulose membrane (Trans-Blot, Bio-Rad, Richmond, CA, USA). After blocking with PBS containing 0.1% Tween 20 (Sigma, St Louis, Missouri, USA) and 5% skim milk at 4 °C overnight, the membrane was incubated with a rabbit polyclonal antibody against cytochrome c (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Antigen–antibody complexes were detected with horseradish peroxidase-conjugated anti-rabbit Immunoglobulin G (1:1000; Santa Cruz Biotechnology, USA). The accuracy of protein loading in this way was verified by comparing Western blot intensities of constant basic proteins, these being β-actin in the case of cytosolic fractions, and cytochrome oxidase IV in the case of mitochondrial fractions. The immunoreactive band was quantified by a densitometer with ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Determination of caspase-3 activity[15] To determine the activation of caspase-3 in the thymocytes, apoptosis was assessed in the permeabilized cells by means of a phycoerythrin (PE)-labeled, anti-activated caspase-3 polyclonal antibody (BD Pharmingen, San Diego, CA, USA), which is a specific antibody against the activated enzyme. At checking time, the cells were washed twice in cold PBS and fixed for 20 min with cold Cytofix/Cytoperm solution (BD Pharmingen, USA). The cells were then washed twice with Perm/Wash buffer (BD Pharmingen, USA) and then stained with the PE-labeled, anti-activated caspase-3 antibody for 30 min at room temperature. After a final wash, the cells were analyzed by flow cytometry.
Statistical analysis Data were expressed as mean±SD. One-way ANOVA was performed. The dose-response data for PCF were analyzed with Student–Newman–Keuls test when a significant F-value was obtained, and P<0.05 was considered statistically significant.
Results
PCF decreased the apoptotic rate in thymocytes induced by UVB Exposure to UVB increased the apoptotic rate in the thymocytes (Figure 1 and Table 1). However, the cell viability was markedly increased by pretreatment with PCF, dose dependently. PCF was found to block the cell apoptosis induced by UVB. The inhibitory effect of PCF on UVB-induced apoptosis in the thymocytes was observed at the highest dose of 5.69 mmol/L among the explored concentrations of PCF.
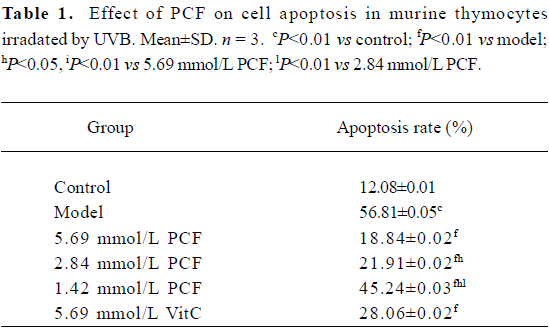
Full table
Transcription of early response genes c-fos and c-jun in thymocytes exposed to UVB irradiation were decreased by PCF The RT–PCR results demonstrated a significant enhancement in the transcription levels of c-fos and c-jun mRNA 30 min after UVB exposure (Figure 2, lane 2). In the PCF groups, the transcription level of the c-fos and c-jun genes was decreased (Figure 2, lanes 4–6).
PCF suppressed cytochrome c release into the cytosol of the irradiated thymocytes To evaluate the contribution of the mitochondrial pathway to the process of UVB-induced apoptosis and to examine the possibility that PCF affects cytochrome c release, the cytosolic fraction was prepared and the kinetics of the release of cytochrome c into the cytosol of the irradiated thymocytes was detected by Western blotting. Treatment of the cells with UVB light induced the release of cytochrome c into the cytosolic fraction (Figure 3, lane 2). Pre-incubation with PCF suppressed the release of cytochrome c (Figure 3, lanes 4–6).
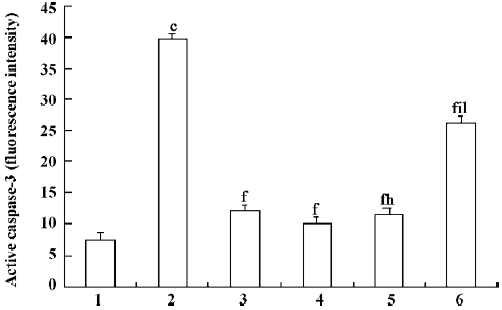
Effect of PCF on UVB-induced caspase-3 activation To investigate the activation of caspase-3 in UVB-induced apoptosis, the thymocytes were exposed to 45 mJ/cm2 UVB in the absence or presence of PCF. Irradiation with UVB resulted in increased activated caspase-3 (Figure 4), suggesting an important role for caspase-3 in the process. The cells pretreated with PCF, activating caspase-3, were reduced.
Discussion
This study reveals that PCF inhibits apoptotic-like cell death induced by UVB in thymocytes. These functions are associated with the prevention of the transcription of the c-fos and c-jun genes, cytochrome c release, and caspase-3 activation. The concentrations found to inhibit cell death and molecule expression in this study were 1.42–5.69 mmol/L. The concentration of PCF (2.84–5.69 mmol/L) in the thymocytes during UVB irradiation seems to be effective in inducing cytoprotection. Therefore, this finding may contribute to understanding the function of PCF during UVB irradiation on thymocytes.
UVA can activate MAPK signaling pathways leading to AP-1 activation and c-fos expression in human keratinocytic cells[16]. Previous studies have found that the MAPK pathway in thymocytes is activated in response to UVB. UV response includes changes in the expression of AP-1 transcription factors c-Fos and c-Jun[17]. In the present study, PCF against the enhancement of the transcription of c-fos and c-jun in the UVB-irradiated thymocytes is confirmed by RT–PCR. C-Fos, a nuclear proto-oncogene protein, forms linkages with the c-Jun/AP-1 transcription factor. After translation, these proteins regulate gene transcription by binding to the AP-1 sites of target genes[18]. Transcription of the members of the fos, jun, and amino-terminal fragment (ATF) gene families is highly induced in response to UV irradiation[19]. The aberrant expression of these proto-oncogenes may transform a normal cell into a tumor cell and plays an important role in tumorigenesis. In the present study, the constitutive expression of c-fos and c-jun in thymocytes induces apoptosis after UVB radiation. C-fos and c-jun transcription is increased more than 2-fold at 30 min after UVB irradiation when compared with the control. PCF could attenuate c-fos and c-jun transcription after radiation, which is affected by varying the PCF doses (Figure 2). This phenomenon suggested that the inhibition of the transcription of c-fos and c-jun by PCF may prevent the development of neoplasms. Some studies find that interference with c-jun activity reduces apoptosis in lung epithelial cells upon induction of stress, indicating that c-jun is required for programmed cell death[20]. This also suggests a critical role played by c-Fos and c-Jun as early-response molecules activated in cell death.
The major upstream regulator of AP-1 is JNK. After the exposure of cells to several cytokines or extracellular stresses, JNK is activated and subsequently phosphorylates its major target c-Jun and related molecules. This in turn leads to the enhanced transcriptional regulation of c-Jun and its dimerization partners with subsequent effects on the promoter regions of downstream target genes[21]. It has been suggested that the JNK pathway mediates apoptosis following UVB irradiation by inducing the release of mitochondrial cytochrome c into the cytosol, which is followed by the activation of procaspase-3[22]. In agreement with these results, our studies show that UVB irradiation led to the release of mitochondrial cytochrome c into the cytosol, leading to the activation of caspase-3, which plays a major role in the execution of the cell death process. Pretreatment of PCF can reduce the cytochrome c release and attenuate the subsequent activation of caspase-3 in the thymocytes. The inhibition of caspases generally inhibits apoptosis. There is evidence that inhibiting apoptosis may alleviate certain harmful effects on cells irradiated by UVB. Therefore, pretreatment with PCF provides prevention of UVB-induced apoptosis. The inhibition of apoptosis by PCF correlated with the prevention of cytochrome c release and caspase-3 activation, which are essential components of the UV-induced apoptotic pathway.
A previous study[12] has testified that the DNA of thymocytes damaged by UVB is reduced by pretreatment with PCF. In the present study, all 3 doses of PCF have the ability to protect thymocytes from UVB-induced cytotoxicity. PCF averts the expression of c-fos and c-jun in the UVB-irradiated thymocytes; this modulation may interfere with the activity of transcription factors which is related to apoptosis. The anti-apoptotic activity of PCF is also associated with the prevention of mitochondrial cytochrome c release and the suppression of subsequent caspase-3 activation. We find that PCF exerted its protective effects through the mitochondrial pathway.
This paper reports new findings about the additional functions of the PCF in response to UVB irradiation. In conclusion, UVB caused apoptotic cell death by increasing c-fos and c-jun expression and cytochrome c release, and induced caspase-3 activation. These are regarded as the characteristics of cell death and all are significantly attenuated by co-incubation with a major constituent of the Chlamys Farreri extract, PCF. Present observations provide new insight into the protective functions of PCF involved in the process of apoptosis in thymocytes induced by UVB. These results show that PCF is a good preventive regimen against UVB radiation.
Acknowledgement
The authors are very grateful to Dr Yao-ru YONG for technical assistance.
References
- Novak Z, Bonis B, Baltas E, Ocsovszki I. Xenon chloride ultraviolet B laser is more effective in treating psoriasis and in inducing T cell apoptosis than narrow-band ultraviolet B. J Photochem Photobiol B 2002;67:32-8.
- Snopov SA, de Gruijl FR, Roza L, van der Leun JC. Immunochemical study of DNA modifications in the nuclei of UV-damaged lymphocytes. Photochem Photobiol Sci 2004;3:85-90.
- Blattner C, Kannouche P, Litfin M. UV-induced stabilization of c-fos and other short-lived mRNAs. Mol Cell Biol 2000;20:3616-25.
- Wagner EF, Matsuo K. Signalling in osteoclasts and the role of Fos/AP1 proteins. Ann Rheum Dis 2003;62:83-5.
- Fernandez M, Pirondi S, Antonelli T, Ferraro L, Giardino L, Calza L. Role of c-Fos protein on glutamate toxicity in primary neural hippocampal cells. J Neurosci Res 2005;82:115-25.
- Bhojani MS, Hamstra DA, Chang DC, Coppola JM, Khan AP, Reddy GR, et al. Imaging of proteolytic activity using a conditional cell surface receptor. Mol Imaging 2006;5:129-37.
- Lo PK, Huang SZ, Chen HC, Wang FF. The prosurvival activity of p53 protects cells from UV-induced apoptosis by inhibiting c-Jun NH2-terminal kinase activity and mitochondrial death signaling. Cancer Res 2004;64:8736-45.
- Kutuk O, Poli G, Basaga H. Resveratrol protects against 4-hydroxynonenal-induced apoptosis by blocking JNK and c-JUN/AP-1 signaling. Toxicol Sci 2006;90:120-32.
- Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res 2000;45:528-37.
- Ding BX, Wang CB. Inhibitory effect of polypeptide from Chlamys farreri on UVB-induced apoptosis and DNA damage in normal human dermal fibroblasts in vitro. Acta Pharmacol Sin 2003;24:1006-10.
- Wang CB, Ding BX, Guo SB, Wang YZ, Han YT, Wang YJ. Protective effect of polypeptide from Chlamys farreri on mitochondria in human dermal fibroblasts by ultraviolet B. Acta Pharmacol Sin 2003;24:692-96.
- Chen HY, Zhu L, Zhan SM, Han ZW, Du W, Wang YJ, et al. Polypeptide from Chlamys farreri inhibits murine thymocytes apoptosis and modulates UVB induced signaling pathway activation. Life Sci 2005;77:768-79.
- Huang H, Petkova SB, Cohen AW, Bouzahzah B, Chan J, Zhou JN, et al. Activation of transcription factors AP-1 and NF-kappa B in murine Chagasic myocarditis. Infect Immun 2003;71:2859-67.
- Wu B, Ootani A, Iwakiri R, Fujise T, Tsunada S, Toda S, et al. Ischemic preconditioning attenuates ischemia-reperfusion-induced mucosal apoptosis by inhibiting the mitochondria-dependent pathway in rat small intestine. Am J Physiol Gastrointest Liver Physiol 2004;286:G580-7.
- Carracedo J, Ramirez R, Soriano S, Martin-Malo A, Rodriguez M, Aljama P. Caspase-3-dependent pathway mediates apoptosis of human mononuclear cells induced by cellulosic haemodialysis membranes. Nephrol Dial Transplant 2002;17:1971-7.
- Silvers AL, Bachelor MA, Bowden GT. The role of JNK and p38 MAPK activities in UVA-induced signaling pathways leading to AP-1 activation and c-fos expression. Neoplasia 2003;5:319-29.
- Li T, Dai W, Lu L. Ultraviolet-induced junD activation and apoptosis in myeloblastic leukemia ML-1 cells. J Biol Chem 2002; 277: 32 668–76.
- Kawasaki H, Komai K, Ouyang Z, Murata M, Hikasa M, Ohgiri M, et al. c-Fos/activator protein-1 transactivates wee1 kinase at G1/S to inhibit premature mitosis in antigen-specific Th1 cells. EMBO J 2001;20:4618-27.
- Rieger KE, Chu G. Portrait of transcriptional responses to ultraviolet and ionizing radiation in human cells. Nucleic Acids Res 2004;32:4786-803.
- Li Y, Arita Y, Koo HC, Davis JM, Kazzaz JA. Inhibition of c-Jun N-terminal kinase pathway improves cell viability in response to oxidant injury. Am J Respir Cell Mol Biol 2003;29:779-83.
- Huang Y, Keen JC, Hager E, Smith R, Hacker A, Frydman B, et al. Regulation of polyamine analogue cytotoxicity by c-Jun in human MDA-MB-435. Mol Cancer Res 2004;2:81-8.
- Sethi G, Sodhi A. Activation of c-Jun N-terminal kinase is required for ultraviolet B-induced apoptosis of murine peritoneal macrophages in vitro. J Photochem Photobiol B 2004;73:133-40.
