Antigen-binding characteristics of AbCD71 and its inhibitory effect on PHA-induced lymphoproliferation1
Introduction
Cluster of differentiation(CD) 71, human transferrin receptor, is abundantly expressed in rapidly dividing cells as well as on many tumor cells, which makes CD71 a relatively specific marker of these cells[1]. Laboratory investigations and clinical studies have demonstrated that anti-CD71 mAb or CD71 binding factors have antiproliferative effects on CD71-positive cells by blocking the engagement of transferrin with its receptor and interfering in the intake of iron into cell[2,3].
However, murine mAb can induce the production of human antimouse antibodies when administered repeatedly in the human body, which may render the antibody ineffective and may also harm patients[4]. Furthermore, the administered murine mAb could not bind to the Fc receptor expressed on human phagocytes, natural killer cells, B cells etc. There-fore, murine mAb could not exert opsonization and phagocytosis effects and could not mediate antibody-dependent, cell-mediated cytotoxicity[5]. Hence, murine mAb showed no effective suppression on lymphoproliferation in vivo and had limited effect on lessening transplantation rejection, which would restrain their further applications in clinic practice[6]. At present, efforts have been directed at the engineering of antibodies to improve their utility, lessen unwanted effects, and alter the effector functions through the modification of Ig genes by using recombinant expression techniques[7]. The chimeric human/murine antibody containing murine V regions combined with human C regions retained the ability of mAb to recognize specific antigen, but had low antigenicity to humans[8].
In the present study, starting with a previously established hybridoma 7579 producing a monoclonal antibody to CD71, we prepared the chimeric human/murine anti-CD71 mAb (AbCD71), confirmed its biological characteristics, and exploited its effect on lymphoproliferation.
Materials and methods
Plasmid construction and transfection Total RNA prepared from the anti-CD71 mAb-secreting hybridoma cell line 7579 (preserved by our laboratory) was reverse-transcripted into cDNA. Then PCR was performed using the following primers: variable domain of light chain(VL) sense P1:5'-GGG
ELISA The sandwich ELISA was used to determine the expression of AbCD71 in the transfectoma supernatant. The plate was coated with 10 µg/mL goat antihuman immunoglobulin G (IgG, γ chain specific, Sigma, St Louis, MO, USA). The secondary antibody was 1:800 diluted mouse antihuman κ chain mAb (Sigma, USA). 1:1000 diluted horseradish peroxidase (HRP)-conjugated goat antimouse IgG (Kirkegaard Perry Labs Inc, Gaithersburg MD, USA) served as the third antibody. After exposure to the HRP substrate (Pierce, Rockford, IL, USA), optical density (OD) values were read at a 490 nm wavelength.
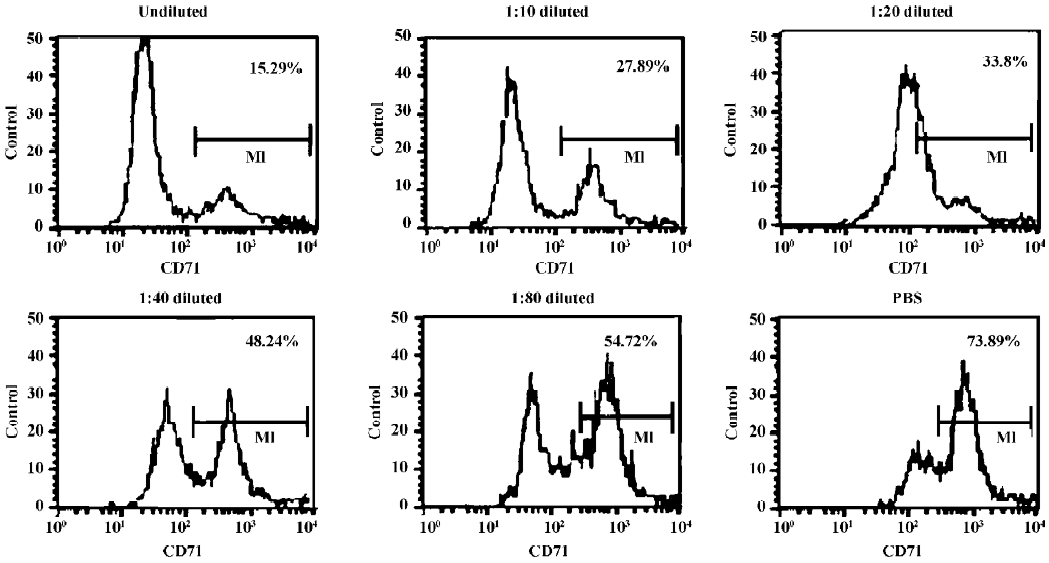
Indirect immunofluorescence assay The antigen-binding characteristic of AbCD71 was measured by indirect immunofluorescence assay. CD71-positive CEM cells were cocultured with original murine anti-CD71 mAb hybridoma supernatant at different dilutions (undiluted, 1:10, 1:20, 1:40, and 1:80) or PBS. Then the transfectoma supernatant was added. After goat antihuman IgG–fluorescent isothiocyanate (FITC) (BD Biosciences, San Diego, CA, USA) was supple-mented, the percentage of the FITC-positive cells was evaluated by FACSCalibur flow cytometer (BD Biosciences, USA).
This methodology was also used to analyze the percentage of CD71-expressed PBMC activated by phytohemagglutinin (PHA). After being isolated by Ficoll–Hypaque density centrifugation, the PBMC were cocultured with PHA (final concentration, 25 µg/mL; Sigma, USA). At different time-points (12, 36, and 60 h) after activation, the cells were collected in order to analyze the CD71 expression by flow cyto-meter (FCM).
Preparation and purification of antibodies The transfectoma was intraperitoneally inoculated into Balb/c (nu/nu) nude mice to induce the ascites for the preparation of AbCD71. Hybridoma 7579 was inoculated into Balb/c mice to induce the ascites for the preparation of anti-CD71 mAb. The antibodies were purified from the ascites via diethyl-aminoethyl (DEAE)-Sephadex A-50 chromatography (Pharmacia, Piscataway, NJ, USA). Then the purified antibody was identified by SDS–PAGE and indirectly elevated in the immunofluorescence assay as mentioned before.
Inhibitory effect of AbCD71 on PHA-induced PBMC proliferation The PBMC were cocultured with PHA at a final concentration of 0 and 25 µg/mL, respectively, and AbCD71 or murine anti-CD71 mAb (final concentration, 1, 10, and 100 µg/mL, respectively) for 5 d. Then PBMC proliferation was measured by methyl thiazolyl tetrazolium(MTT) method. The untreated PBMC group was set as negative control and the isotype antibody-treated group was set as another negative control. The PBMC group, induced with 25 µg/mL PHA, but untreated with antibodies, was set as the positive control. Triplicates were set for each group. After reading the OD values (A) at 570 nm, the inhibitory rates were calculated as the following formula: the inhibitory rate (%) of cell proliferation=(1–[the mean A of the experimental group/the mean A of the positive control group])×100.
In another experiment, at different time-points after PHA stimulation (0, 12, 36, and 60 h), AbCD71 or murine anti-CD71 mAb (final concentration, 0, 1, 10, and 100 µg/mL, respectively) was supplemented into the PBMC culture system. After 5 d of culture, the MTT assay was performed with the same controls as before. Then the inhibitory rates were calculated.
Statistical analysis The t-test was performed to compare the inhibitory rates. Differences were regarded as statistically significant when P<0.05.
Results
Confirmation of AbCD71 constant domains After G418 screening, the cells in the 4 wells showed transfectoma cells growth (numbered as C1–4).
The mean OD values of the 4 G418-resistant clones were higher than those of the blank control and the negative control (Table 1). The results indicated that the heavy chain of AbCD71 in the transfectoma supernatant could bind to goat antihuman IgG (γ specific) and its light chain could bind to mouse antihuman κ chain mAb.
AbCD71 competed with its parental murine mAb to bind to CD71-positive CEM cells It was confirmed that AbCD71 contained human IgG heavy chain and light chain constant domains. Did AbCD71 still remain the murine variable domain and retain antigen-binding specificity? Indirect immunofluorescence assays showed that as the concentration of the hybridoma supernatant decreased from undiluted to a dilution of 1:80, the percentage of FITC-positive cells increased from 15.16%±0.73% to 56.22%±2.65%. When the CEM cells were precultured with PBS, the percentage rose to 71.49%±3.60% (Figure 1). These figures suggested that the less the murine mAb combined with CD71 molecules, the more AbCD71 bound to the CEM cells, hence an increase in the CEM cells emitting green fluorescence.
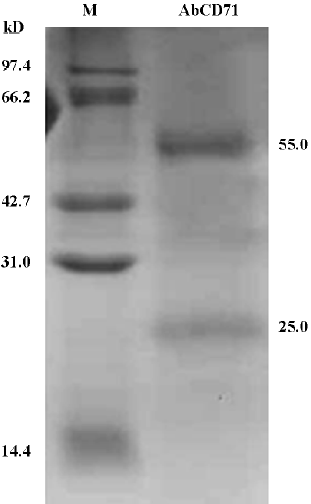
Identification of purified AbCD71 by SDS–PAGE After the transfectoma clone secreting AbCD71 was identified, the clone cells were inoculated into mice and the resultant ascites fluid was used to prepare purified AbCD71. The yield was about 4–8 mg of the antibody in 3–5 mL of the ascites. SDS–PAGE displayed 2 specific protein bands with molecular weights of about 55 and 25 kDa, which presumably represented the heavy chain and light chain of AbCD71, respectively (Figure 2). The purity of the antibody was more than 95%. The specificity of purified AbCD71 was identified by indirect immunofluorescence assay. The same results (data not shown) were obtained as those of the transfectoma supernatant.
AbCD71 could inhibit PHA-induced lymphoproliferation Data showed that AbCD71 could inhibit PHA-induced lymphocyte proliferation obviously (Figure 3A). When the concentration of the antibody varied between 1–100 µg/mL, the inhibitory rate increased as the concentration of AbCD71 rose. There was no statistical difference in the inhibitory rates between AbCD71 and original murine anti-CD71 mAb at the corresponding concentrations (P>0.05).
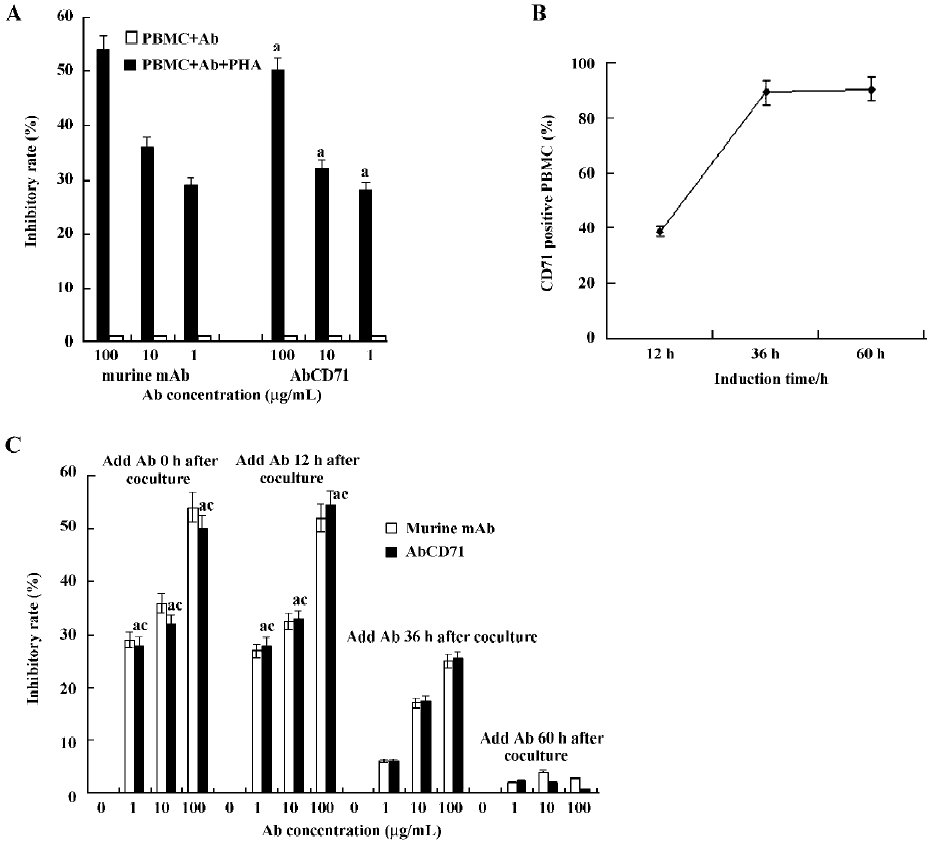
The PBMC failed to proliferate in the absent of PHA. Hence, AbCD71 or the murine antibody showed no effect on PBMC proliferation (inhibitory rate <1%).
It was demonstrated that when AbCD71 and PHA were added together, proliferation of resting lymphocytes could be inhibited by AbCD71. It remained unclear whether stimulated lymphocyte proliferation could be suppressed. For that reason, first, the percentage of activated PMBC, on which membrane CD71 expression was upregulated, was measured. We found that 38.6%±1.91%, 89.6%±4.38%, and 90.8%±4.12% PBMC were activated and expressed CD71 on their membranes after 12, 36, and 60 h of PHA stimulation, respectively (Figure 3B). Second, following stimulation for 12, 36, and 60 h, the PBMC were incubated with AbCD71. The results showed that AbCD71 inhibited proliferation of induced PBMC in a dose-dependent manner when the concentration of the antibody varied between 1 and 100 µg/mL. Proliferations were suppressed strongly at the time-points of 0 and 12 h. PBMC proliferation at 60 h was not evidently inhibited. There were statistical differences (P<0.01) between the groups treated with the same concentration, but induced for different time periods (Figure 3C). All these data showed no statistical difference (P>0.05) when compared with the corresponding concentration of murine mAb-treated groups.
Discussion
In the present study, the light chain gene and the heavy chain gene of AbCD71 were constructed into 2 expression vectors, respectively. Only when those 2 vectors were cotransfected into the same recipient cell could an intact antibody be produced. Using ELISA, the coated goat antihuman IgG γ could be combined when the transfectoma supernatant contained the human IgG heavy chain. Sub-sequently, the mouse antihuman Ig κ was supplemented. After exposure to the substrate, only those wells containing intact human IgG (κ specific) could be developed into yellow. Hence, the species specificity of AbCD71 constant regions was confirmed. The following indirect immunofluorescence assay demonstrated that the chimeric Ab could compete with their original murine mAb to bind to the target antigen. This means that AbCD71 retained the affinity and antigen-binding specificity of its parental murine mAb. The above 2 assays verified that AbCD71 not only reserved the variable region of its original murine mAb, but also possessed of the constant region of human IgG.
CD71 expression is essential for continued growth and is closely linked to the proliferative status of a cell. In addition, the CD71 molecule is recognized as a lymphocyte activation marker[9,10]. Therefore, CD71 may be a potential therapeutic target for transplantation rejection. After the biological characteristics of AbCD71 were identified, its antiproliferative effect on human PBMC was investigated. PBMC could proliferate and express CD71 on their membranes when induced by mitogen[11], which could mimic post-transplantation lymphoproliferative responses. Our experiment also verified that the expression of CD71 on PBMC increased in response to 25 µg/mL PHA stimulation. After exposure to PHA for 36 h, most PBMC were activated and proliferated efficiently because the percentage of CD71-expressed PBMC showed no evident alteration in the following hours.
A subsequent inhibitory rate analysis suggested that the administration of AbCD71 at the time of PHA stimulation could strongly inhibit proliferation and clonal expansion of PBMC in vitro. In evaluating the optimal timing of AbCD71 addition to the culture medium, we could see that there was a distinct inhibition when AbCD71 was added at 0 and 12 h after PHA induction, compared with 36 and 60 h timepoints. AbCD71 administration at the early stage of mitogen presentation was more effective than at the time of maximal receptor expression, suggesting an early role for AbCD71 in antirejection. The possible explanation is that AbCD71 exerts growth suppression by blocking the CD71 molecules distributed on PBMC, and thus blocking iron uptake in activated lymphocytes. Cellular iron is crucial for cell survival[1,12]. At the early stage of mitogen stimulation, PBMC needed iron for continued growth and proliferation. CD71 blockade at this period resulted in the lack of sufficient iron availability. Hence, PBMC proliferated poorly in response to mitogen stimulation[13]. At the late stage, when PBMC already underwent clonal expansion, their particular needs for high levels of iron ceased. CD71 blockade and iron starvation at this time would have had a limited effect on the total PBMC number. Hence, the inhibitory rate at 36 h decreased and the data reached nadir at 60 h. Future studies will improve our understanding of AbCD71 in the activation of lymphocytes and will provide the knowledge necessary for utilizing AbCD71 blockade as a novel therapeutic strategy for clinical transplantation.
The above data manifested that AbCD71 could specifically bind to the CD71 molecules expressed on the membranes of activated lymphocytes and could inhibit the lymphoproliferation. It seems that AbCD71 administration would lower the incidence of transplantation rejection and not produce human antimouse antibodies. Thus, the use of AbCD71 may result in more effective immunosuppression in transplantation. AbCD71 looks to be a promising immuno-suppressant. Our approach to blocking the transferrin receptor using chimeric human/murine mAb provides a novel strategy for prolonging allograft survival.
References
- Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML. The transferrin receptor part I: biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol 2006;121:144-58.
- White S, Taetle R, Seligman PA, Rutherford M, Trowbridge IS. Combinations of anti-transferrin receptor monoclonal antibodies inhibit human tumor cell growth in vitro and in vivo: evidence for synergistic antiproliferative effects. Cancer Res 1990;50:6295-301.
- Kreitman RJ, Pastan I. Recombinant toxins containing human granulocyte-macrophage colony-stimulating factor and either pseudomonas exotoxin or diphtheria toxin kill gastrointestinal cancer and leukemia cells. Blood 1997;90:252-9.
- Mirick GR, Bradt BM, Denardo SJ, Denardo GL. A review of human anti-globulin antibody (HAGA, HAMA, HACA, HAHA) responses to monoclonal antibodies. Not four letter words. Q J Nucl Med Mol Imaging 2004;48:251-7.
- Presta LG. Engineering of therapeutic antibodies to minimize immunogenicity and optimize function. Adv Drug Deliv Rev 2006;58:640-56.
- Klee GG. Human anti-mouse antibodies. Arch Pathol Lab Med 2000;124:921-3.
- Gonzales NR, De Pascalis R, Schlom J, Kashmiri SV. Minimizing the immunogenicity of antibodies for clinical application. Tumour Biol 2005;26:31-43.
- Hwang WY, Foote J. Immunogenicity of engineered antibodies. Methods 2005;36:3-10.
- Woodward JE, Bayer AL, Chavin KD, Boleza KA, Baliga P. Anti-transferrin receptor monoclonal antibody: a novel immuno-suppressant. Transplantation 1998;65:6-9.
- Jason J, Archibald LK, Nwanyanwu OC, Bell M, Jensen RJ, Gunter E, et al. The effects of iron deficiency on lymphocyte cytokine production and activation: preservation of hepatic iron but not at all cost. Clin Exp Immunol 2001;126:466-73.
- Lio D, Candore G, Cigna D, D’Anna C, Di Lorenzo G, Giordano C, et al. In vitro T cell activation in elderly individuals: failure in CD69 and CD71 expression. Mech Ageing Dev 1996;89:51-8.
- Daniels TR, Delgado T, Helguera G, Penichet ML. The transferrin receptor part II: targeted delivery of therapeutic agents into cancer cells. Clin Immunol 2006;121:159-76.
- Keyna U, Nusslein I, Rohwer P, Kalden JR, Manger B. The role of the transferrin receptor for the activation of human lym-phocytes. Cell Immunol 1991;132:411-22.
