Antiviral effects of PNA in duck hepatitis B virus infection model1
Introduction
Hepatitis B virus (HBV) is the causative agent of acute and chronic hepatitis B in humans. More than 350 million people worldwide are chronic virus carriers and face a significantly increased risk of developing liver cirrhosis and primary hepatocellular carcinoma. Despite the availability of a safe and effective vaccine against hepatitis B, chronic infection with HBV remains a major health problem worldwide[1–4]. The duck model of HBV (DHBV) infection represents a suitable system for the study of the activity of anti-HBV agents. It provides relevant tools for the detailed molecular studies of hepadnavirus replication and its inhibition[5–7].
To date, conventional therapy for chronic HBV infection has involved the use of either α-interferon or 3-thiacytidine (3TC, lamivudine). α-Interferon therapy is only partially effective and is frequently limited by adverse effects and is also expensive. Although 3TC efficiently inhibits HBV replication, the emergence of drug-resistant mutants which carry mutations affects the reverse transcriptase domain.
In order to control viral replication and delay the emergence of virus-resistant mutants, it is critical to look for new valid medicines and develop new antiviral strategies[8,9]. Only a few antiviral drugs that are currently under clinical trial for the treatment of persistent HBV infection are Entecavir (ETV)[10–12], Chinese medicinal herbs etc.
The present study was designed to test the efficacy of PNA, a new potent inhibitor of the hepadnaviral polymerases. PNA is a 2-amine-9-(2,3-dideoxy-2,3-dihydro-β-D-arabino-furanosyl)-6-methoxy-9H-purine. It was conducted to evaluate the antiviral activities of PNA in this alternative HBV animal model by measuring the levels of DHBV DNA in infected duck serum and livers.The changes of duck hepatitis B surface antigen (DHBsAg) and liver pathological changes were also observed.
Materials and methods
Animals One-day-old Sichuan Mallard ducklings (Anas platyrhynchos domesticus) were purchased from Sichuan Agriculture University’s hatchery (Yaan, China) and held in the animal house facilities of the Institute of Veterinary Science, Sichuan Agriculture University. Congenitally DHBV-infected ducklings detected by PCR were excluded from the experiments.
Inoculum preparation A pooled, sera-derived DHBV containing 9.6×1010 viral genomes (vge)/mL that was collected from the congenitally-infected Peking ducks during a period of 2 weeks (6–20 d old) was used as the inoculum. The viral inoculum was prepared by diluting DHBV-infected sera with uninfected duck sera to obtain the appropriate dose of 1.6×107 DHBV genome equivalents in 100 µL and was inoculated via the vena cruralis.
Drug source, preparation, and uptake PNA was synthesized and supplied in powder form by Nanjing Changao Pharmaceutical Technology (Nanjing, China).
100 mg PNA was added to 1 mL distilled water. 100 mg/mL stock solutions of PNA were stored at 4 °C for up to 1 d and allowed to reach room temperature before use. A stock solution of 3TC was prepared as earlier mentioned. PNA, 3TC, or distilled water was administered twice a day to the ducks in each group.
Ninety ducklings were divided into 6 groups. Fifteen ducklings from each group started daily treatment at d 7 after inoculum preparation. Control group I received a placebo of 2 mL distilled water and control group II was administered perorally (po) with 3TC at the dose of 50 mg/kg. Treatment group I was administered with PNA at a dose of 40 mg/kg and treatment group II was administered with PNA at a dose of 80 mg/kg by po. Treatment group III was administered with PNA at a dose of 20 mg/kg and treatment group IV was administered with PNA at a dose of 40 mg/kg PNA intravenously. The administration lasted 10 d. The experimental plan is summarized in Figure 1.
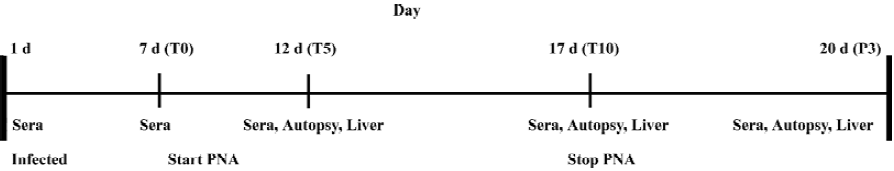
Sample collection The serum samples were collected daily at 1 d before treatment, 5 and 10 d after treatment, and at 3 d withdrawal for monitoring viremia in each animal. The blood samples were collected from the jugular vein, and serum was collected following the incubation of the blood samples at 37 °C for 90 min and then stored at -20 °C.
An autopsy was performed in 5 ducks in each group at the same time for determining the amount of inoculum virus bound in the liver. The liver samples from all the ducks were divided into 2 parts. Part I was snap-frozen in liquid nitrogen and then stored at -80 °C and part II was fixed in 4% paraformaldehyde buffered with phosphate-buffered solution (PBS). The experimental plan is summarized in Figure 1.
Analysis of serum samples
Isolation of DNA from sera From each serum sample, 300 µL was incubated at 65 °C for 4 h in lysis buffer (20 mmol/L Tris-HCl [pH 8.0] 10 mmol/L EDTA, 0.1% SDS, and 0.8% proteinase K). DNA was then extracted with phenol-chloroform and precipitated with ethanol. The pellet was dissolved in 50 µL of RNase- and DNase-free ddH2O.
PCR was employed to exclude congenitally DHBV-infected ducklings. The serum samples were tested by PCR for the presence of DHBV DNA. The PCR mixture also contained 5 µL of 10×reaction buffer (TaKaRa, Dalian, China), 4 µL of 25 mmol/L MgCl2, 1 µL of 10×dNTP, 1 µL Taq polymerase (TaKaRa, Dalian, China), 1 µL of both forward and reverse primers, and 50 µL ddH2O. The PCR primers were designed according to the sequence in GenBank (Accession N
Oligonucleotide primers and probe for real-time PCR Real-time PCR was performed using the BioRad Lightcycler (BioRad, Idaho, USA) on extracted DHBV DNA samples and quantitated using fluorescence probe. Each 25 µL reaction mixture contained 50 ng DNA, 2 µL probes (Genecore, Shanghai, China), 2.5 µL MgCl2 (at a final concentration of 2.5 mmol/L), 0.25 µL TaKaRa Ex Taq HS (5 U/µL) (TaKaRa, Dalian, China), and 0.5 µL of 10 µmol/L primers. The probe sequence (5'-CGGGCTCCCCTCTCCCACG-3') was labeled with 6-carboxy-fluorescein dye at the 5'-end and with the quencher dye 6-carboxy-tetramethyl-rhodamine at the 3'-end. The sense primer sequence was 5'-GAGCCCCTTCACCCC-AAC-3' and antisense primer sequence was 5'-ATCTATGGT-GGCTGCTCGAACT-3'.The primers and probe were synthesized by Shanghai Genecore (TaqMan minor groove binder, Shanghai, China). The PCR protocol required initial incubation for 5 min at 95 °C and then 40 cycles of 5 s at 95 °C, 10 s at 55 °C, and 15 s at 72 °C. Each sample was detected in triplicate.
Preparation of standards The 998 bp PCR product of the S gene was inserted into the vector of a pMD18-T(TaKaRa, Dalian, China). It resulted in plasmid and named pDHBV (kept by the Key Laboratory of Animal Disease and Human Health of Sichuan Province, China). Plasmid DNA was then isolated with the DNA purification system (Beijing SaiBeiSeng Genetech, Beijing, China) and quantified by spectrophotometry (BioRad SmartSpec3000, BioRad, USA) . Concentrations were converted to copies/µL by use of the Avogadro constant. Serial dilutions of plasmids were used for the construction of the standard curve and for the validation of the real-time PCR assay[13–16]. Plasmids were detected in triplicate. The correlation between the quantification of DHBV and pDHBV plasmids was evaluated by the regression analysis.
Analysis of the virus in liver tissues Aliquots of 0.1 g (fidelity 0.0001) liver tissue of each sample were extracted using a DNeasy tissue kit (Tiangen, Beijing, China). DNA was eluted in 50 µL TE buffer (50 mmol/L EDTA, 20 mmol/L Tris-HCl [pH 8.0]). Viral load was quantified by real-time PCR as earlier mentioned.
The fixed samples were sectioned and examined for DHBsAg by immunoperoxidase staining as a marker of infected cells[17,18]. The expression of DHBsAg was detected by a monclonal anti-DHB antibody (kindly provided by the Institute for Viral Hepatitis, Chongqing Medical University, Chongqing, China). The specimens collected from control group I, positive for DHBV, served as positive controls. The negative control was established by omitting the primary antibody incubation. The assessment criterion for IHC was that cells bearing a brown-yellow stain in the nuclei were considered positive. Immunoreactivity was quantified by drawing around the whole section using Image Pro Plus software (Media Cybernetics, Silver Spring, MD, USA) and determining the mean intensity. Tissues were also sectioned and stained with hematoxylineosin (HE) for the histological analysis[19,20].
Statistics The data were expressed as mean±SD and analyzed by t-test. P<0.05 was considered statistically significant.
Results
Detection of DHBV-related avihepadnaviruses in serum samples In total, 4 of 100 ducklings detected were found to be congenitally-infected birds by PCR and were excluded from experiments (Figure 2).
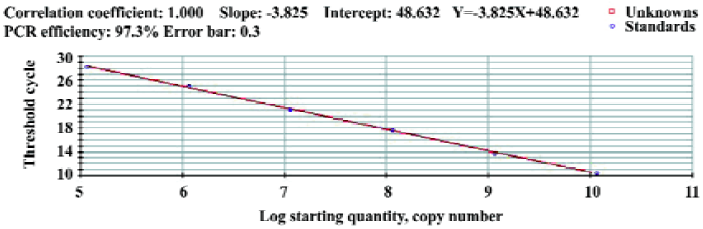
Standard curve The concentration of plasmid DNA was 221.72 µg/L. Concentrations were converted to 1.14×1010 copies/µL by use of the Avogadro constant. The amplification lines of reaction templates were 1.14×1010copies/L diluting as 10×grad (10-1, 10-2, 10-3, 10-4, 10-5, 10-6). The standard curve was drawn by 40 cycles of amplification of the plasmid samples and we obtained a linear quantification with a slope of -3.825 and an R2 of >0.996 (Figure 3). According to the standard curve, the sample copies were equal to lg (48.632-Y)/3.825, where Y is the Ct (cycle threshold). The sensitivity limit was 5 copies/µL (100% positive results).
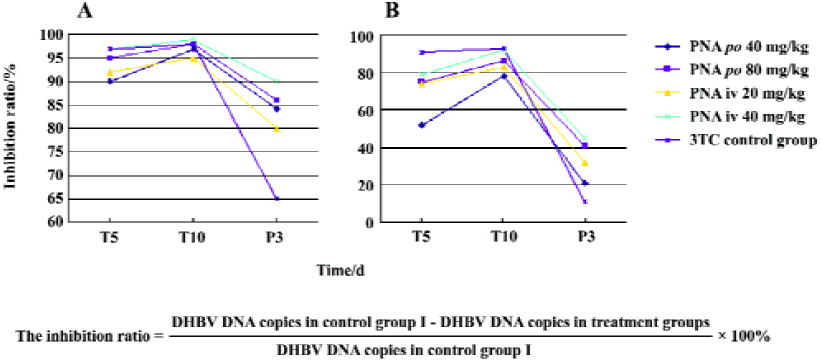
Effect of treatment with PNA on DHBV replication level in duck serum and liver tissues The DHBV replication level in the duck serum and liver was markedly decreased in the PNA- and 3TC-treatment groups. PNA treatment significantly downregulated the DHBV replication levels in the serum and liver tissue (Figure 4). Compared with control group II, there was no significant difference in inhibiting efficacy in treatment groups I and III (P>0.05), but the inhibiting efficacy of treatment groups II and IV were better than that of control group II (P<0.05). Interestingly, significant differences were seen at 3 d withdrawal. The DHBV replication levels slightly increased at 3 d withdrawal, but rebounded slightly in the PNA-treatment groups than in control group II (P<0.05). The DHBV replication levels in the treated groups were lower than that of control group I (Figure 4 and Table 1). The DHBV replication levels in sera were in a positive relationship with that in the livers, but the DHBV replication levels in the livers were lower than that in the sera.
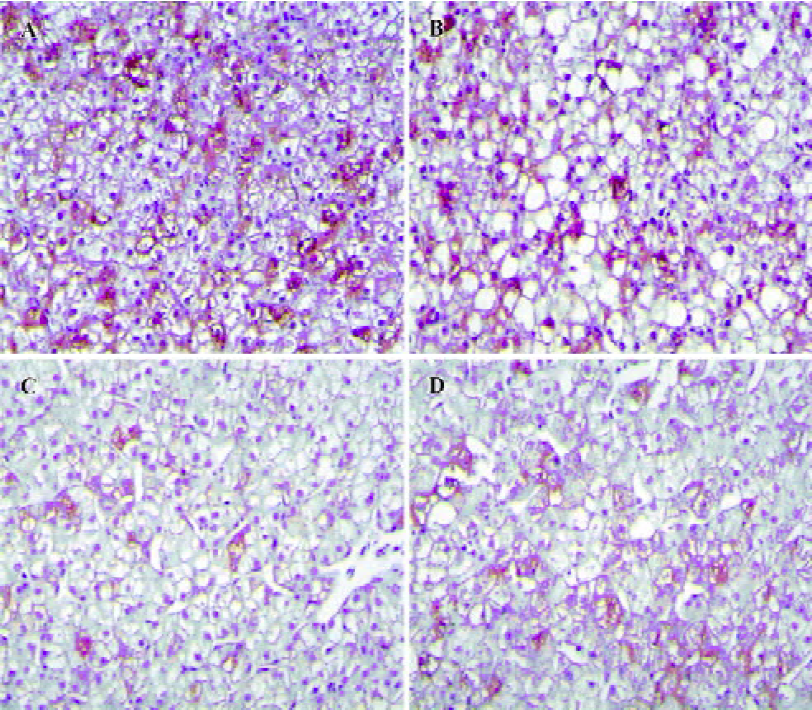
Histopathological features Positive DHBsAg immu-nostaining showed a yellow-brown color localized in the liver cells. The intensity of the DHBsAg stain varied with the samples (Figure 5). The proportion of positive liver cells ranged from 5% to 85%. The majority of liver cells showed negative DHBsAg staining and was observed as blue with a hematoxylin counterstain. The negative control was not immunostained.
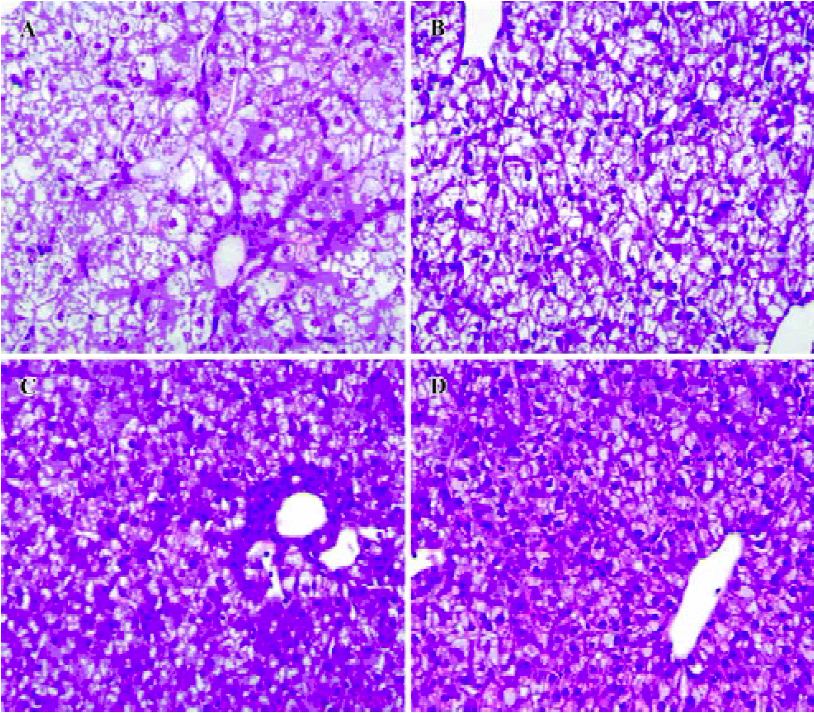
Necrosis, steatosis, and often swelling of the hepatic cytoplasm were found in control group, as well as focal necrosis and vacuolar degeneration. In contrast, pathological changes in the treatment groups were obviously improved. A significant improvement of the hepatocellular architecture and a considerable reduction in necrosis and vacuolation were found. Pathological changes were associated with DHBsAg and DHBV replication levels (Figure 5, 6 and Table 1).
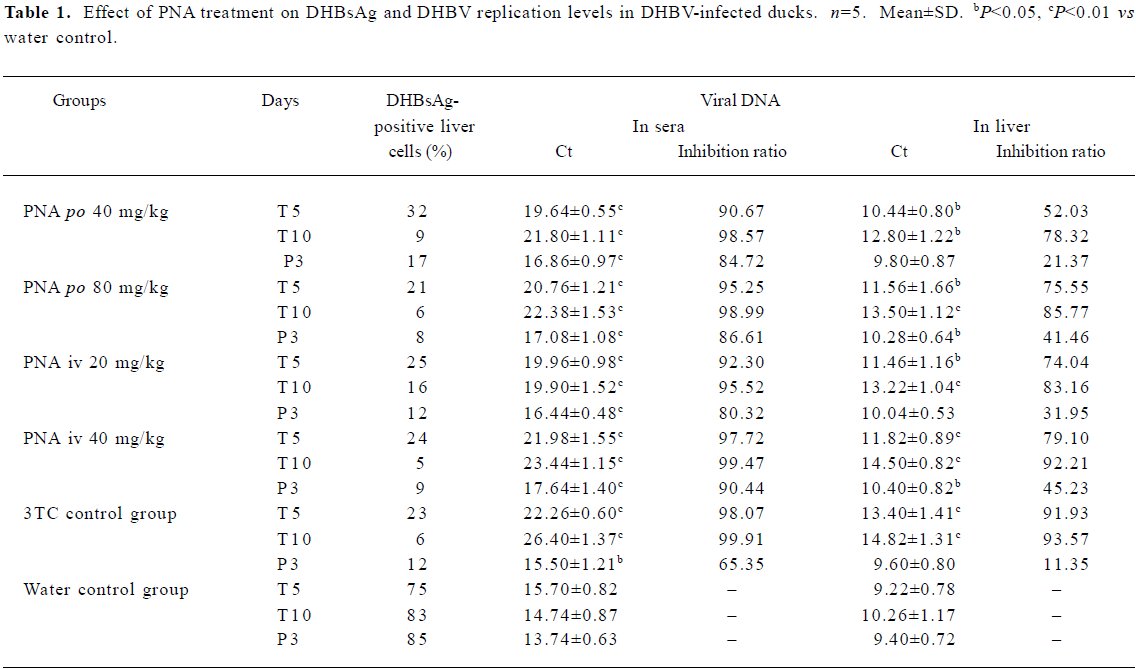
Full table
Discussion
In total, 4 of 100 ducklings detected were found as congenitally-infected birds, but 10%–50% of congenital infection was reported. An epidemiological survey of the prevalence of DHBV in different duck varieties and areas should be conducted. Analysis of data from the reports can supply some useful information for DHBV or HBV morphogenesis and will help to define their epidemiology more precisely[21].
The development of new antiviral strategies has created a need for methods that are able to monitor the low viremic levels reached during therapy, both for the early detection of drug-resistant mutants and for the evaluation of the treatment outcome. This study was carried out with a simplified system for real-time PCR by TaqMan MGB technology. The method for the detection of DHBV was accurate and had a high degree of sensitivity. This result was consistent with previous reports using similar approaches[16,22]. It showed that this system allowed reliable quantification of the viral genome in biological samples, and therefore, may be a valuable tool for real-time quantification when the detection of multiple targets in a single well is not required. In conclusion, we developed a sensitive, specific, and reproducible test for the quantification of DHBV DNA. This assay potentially allowed for the analysis of sera DHBV DNA without the need for concentration or dilution of samples, independent of treatment administration. The same approach could also be used to distinguish and quantify viral variants emerging during therapy.
Our results showed that PNA exhibited a potent inhibitory effect on DHBV replication in vivo. The originality of our study relied on the association of compounds that study the efficacy of antiviral treatment in a complete set of experimental systems with a real-time PCR, IHC, pathology and in vivo studies in infected ducklings on treatment pathway- and dose-related outcomes[11,12]. The DHBV replication levels appeared to be directly related to the amount of DHBsAg-positive liver cells.
The results of our study showed that PNA was effective in suppressing DHBV replication of DHBV in models po and iv. Administered perorally or intravenously at concentrations as little as 20 or 40 mg/kg can reduce serum viral DNA levels. Perhaps the favorable pharmacokinetic properties of PNA and the relatively long intracellular half-life of PNA, a strategy of dosing regimens, was undertaken to enable a long-term study to be more easily conducted.
In the immunohistochemical study, the reported intensity and rate of DHBsAg positivity could be influenced by the experimental procedures. IHC can assess the relative content of a special protein in degeneration cells and reliably evaluate the level of DHBV DNA expression.
This study highlights a number of significant gaps in our understanding of hepadnavirus persistence during treatment. What is the mechanism of continued virus production during treatment in control group II? Are some producer cell types less sensitive to PNA or is there an ongoing breakthrough of the suppression of replication? In the stable state of reduced production of viruses during PNA treatment, how are the clearance mechanisms recalibrated to balance production and result in steady serum levels of DHBV DNA and DHBsAg?
In summary, PNA, a potent inhibitor of the hepadnaviral polymerases, prevents the development of infection when administered in the early stages of DHBV infection. It is a potent and rapid-acting suppressor of DHBV replication. The mechanism which allows DNA to rebound withdrawal and the spontaneous viral genome variability leading to the emergence of drug-resistant mutants requires further study. A long-term study and the toxicity of PNA also require further study. It is likely that dosage form is important for PNA. This study has opened the way for further studies, including an optimal dosing regimen and effective treatment approaches.
Acknowledgements
We thank Yu-fei GUO and Xu CHAO for excellent technical assistance.
References
- Kane MA. Global status of hepatitis B immunisation. Lancet 1996;348:696.
- Blumberg BS. Hepatitis B virus, the vaccine, and the control of primary cancer of the liver. PNAS 1997;94:7121-5.
- Lee WM. Hepatitis B virus infection. N Engl J Med 1997;337:1733-45.
- Lin KW, Kirchner JT, Hepatitis B. Am Fam Physician 2004;69:75-82.
- Mason WS, Halpern MS, England JM, Seal G, Egan J, Coates L, et al. Experimental transmission of duck hepatitis B virus. Virology 1983;131:375-84.
- Mason WS, Seal G, Summers J. Virus of Peking ducks with structural and biological relatedness to human hepatitis B virus. J Virol 1980;36:829-36.
- Ling Z. Research of JMS inhibitory effect on Duck HBV in vivo. Acta Pharmacol Sin 2003;24:379-80.
- Seigneres B, Martin P, Werle B, Schorr O, Jamard C, Rimsky L, et al. Effects of pyrimidine and purine analog combinations in the duck hepatitis B virus infection model. Antimicrob Agents Chemother 2003;47:1842-52.
- Luca GG, Amber M, Heike M, Rick K, Robert H, Silverman BR, et al. Interferon-regulated pathways that control hepatitis B virus replication in transgenic mice. J Virol 2002;76:2617-21.
- Innaimo SF, Seifer M, Bisacchi GS, Standring DN, Zahler R, Colonno RJ. Identification of BMS-200475 as a potent and selective inhibitor of hepatitis B virus. Antimicrob Agents Chemother 2002;46:82-8.
- Foster WK, Miller DS, Marion PL, Colonno RJ, Kotlarski I, Jilbert AR. Entecavir therapy combined with DNA vaccination for persistent duck hepatitis B virus infection. Antimicrob Agents Chemother 2003;47:2624-35.
- Foster WK, Miller DS, Scougall CA, Kotlarski I, Colonno RJ, Jilbert AR. Effect of antiviral treatment with entecavir on age- and dose-related outcomes of duck hepatitis B virus infection. J Virol 2005;79:5819-32.
- Abe A, Inoue K, Tanaka T, Kato J, Kajiyama N, Kawaguchi R, et al. Quantitation of hepatitis B virus genomic DNA by real-rime detection PCR. J Clin Microbiol 1999;37:2899-903.
- Garson JA, Grant PR, Ayliffe U, Ferns RB, Tedder RS. Real-time PCR quantitation of hepatitis B virus DNA using automated sample preparation and murine cytomegalovirus internal control. J Virol Methods 2005;126:207-13.
- Jardi R, Rodriguez F, Buti M, Costa X, Cotrina M, Valdes A, et al. Quantitative detection of hepatitis B virus DNA in sera by a new rapid real-time fluorescence PCR assay. J Viral Hepatitis 2001;8:465-71.
- Zanella AR, Domenighini D, Albertini A, Cariani E. Quantitative analysis of hepatitis B virus DNA by real-time amplification. Eur J Clin Microbiol Infect Dis 2002;21:22-6.
- Cullen JM, Marion PL, Newbold JE. A sequential histologic and immunohistochemical study of duck hepatitis B virus infection in Pekin ducks. Vet Pathol 1989;26:164-72.
- Jilbert AR, Botten JA, Miller DS, Bertram EM, Hall PDLM, Kotlarski I, et al. Characterization of age- and dose-related outcomes of duck hepatitis B virus infection. Virology 1998;243:273-82.
- George GC, Paul BS, Ernest CW, Chak HX, Lee KM, Lau WY. Immunohistochemical analysis of pro-apoptotic Bid level in chronic hepatitis, hepatocellular carcinoma and liver metastases. Cancer letters 2001;172:75-82.
- Kangmin Z, Robert SL, Edward AB, Marianna KB. The relationship of hepatitis history and pathological diagnosis of primary liver cancer. J Clinical Epidemiol 1997;50:297-301.
- Ku T, Ha W, Zhang LZ, Ha R, Ha Y, Cheng Q, et al. Epidemiological survey of HBsAg carriers in the Uighur and Han populations in the Khotan area of Xinjiang autonomous region. Di Yi Jun Yi Da Xue Xue Bao 2004;24:1287-8.
- Beck IA, Drennan KD, Melvin AJ, Mohan KM, Herz AM, Alarcon J, et al. Simple, sensitive, and specific detection of human immunodeficiency virus type 1 subtype B DNA in dried blood samples for diagnosis in infants in the field. J Clin Microbiol 2001;39:29-33.