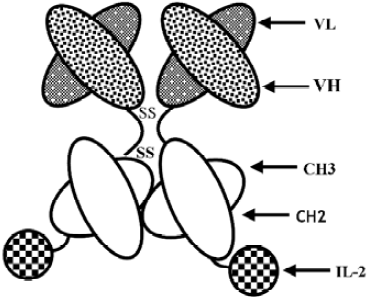
Efficient growth inhibition of ErbB2-overexpressing tumor cells by anti-ErbB2 ScFv–Fc–IL-2 fusion protein in vitro and in vivo
Introduction
The administration of high-dose recombinant interleukin 2 (IL-2) to patients has been reported to mediate the regression of tumors in selected patients with metastatic melanoma, kidney cancer, and non-Hodgkin’s lymphoma[1–5]. However, the potent antitumor activity of IL-2 is often achieved at the expense of unacceptable toxicity[6,7]. Minimizing IL-2 toxicity requires both the prevention of the systemic side-effects of IL-2 and the locally restricted enrichment of IL-2 activity to the tumor sites[8,9]. Direct injection into tumors[10–13] and cytokine gene therapy[7,8,14–16] were considered as approaches to solve this problem. However, direct injection requires the localization of the tumor but it seems impossible to directly inject IL-2 into micrometastases. Gene therapy approach requires that tumor cells be removed from the patients, transduced and reintroduced into patients, which is sophisticated to manipulate. Tumor-specific antibodies genetically fused to IL-2 provide an alternative approach to improve the therapeutic index of IL-2[17–19]. The underlying principle of antibody-IL-2 molecules is based on the targeting of potent cytokines to the tumor microenvironment, where they elicit an immune response and thus destroy tumors[1,6,20]. In the past decade, several groups have reported different antibody–IL-2 fusions[17,21,22]. These novel proteins were shown to retain both antibody and cytokine functions and to exhibit superior anticancer activities as compared with equivalent amounts of free IL-2 and antibodies.
A humanized anti-ErbB2 antibody, Herceptin has succeeded in clinical trials for the treatment of ErbB2-expressing metastatic breast cancer[23–25]. The fusion of IL-2 to an ErbB2-specific antibody may enhance its efficacy. Fusion proteins of intact antibodies and IL-2 have been well investigated and have been shown to mediate the eradication of established metastatic tumors in several animal models[17–19,22]. In previous studies, we constructed a different type of fusion protein (HFI) consisting of an anti-ErbB2 single chain antibody (scFv), Fc of human IgG1 and IL-2[26]. IL-2 was fused to the C terminus of anti-ErbB2 scFv–Fc. The molecular weight of the fusion protein is about two-third of the whole antibody–IL-2 fusion protein, which may confer the fusion protein better penetration property and higher expression level[27]. In the present study, our in vitro and in vivo data demonstrate that the fusion protein exhibits high efficiency, both in mediating antibody-dependent cell-mediated cytotoxicity (ADCC) activity in vitro and significant antitumor activity in tumor xenograft models.
Materials and methods
Tumor cell lines and animals The tumor cell lines used were human ovarian carcinoma cell line SKOV3 and human breast carcinoma cell lines SKBR3 and MCF7. The cells were maintained in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) or RPMI-1640 supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA) until ready for use.
Female Balb/C athymic nude mice were purchased from the Institute of Laboratory Animal Science, Chinese Academy of Medical Science (Beijing, China) and housed in specific, pathogen-free conditions in the Good Laboratory Practice facility. For all the studies, the mice were allowed to acclimate at least 3 d.
Construction of anti-ErbB2 scFv–Fc–IL-2 fusion protein Anti-ErbB2 scFv, human IgG1 Fc region, and human IL-2 genes were cloned into plasmid pCI-dhfr (Promega, San Luis Obispo, CA, USA) as previously described[26]. The schematic construct of HFI is shown in Figure 1. The plasmid containing the anti-ErbB2 scFv–Fc fusion gene (named scFv–Fc) was also constructed. Chinese hamster ovary (CHO) cells stably expressing HFI were generated by transfection of the plasmids and selection for dhfr+transformats in DMEM supplemented with 10% dialyzed FBS (Gibco, Carlsbad, CA, USA) and methotrexate. The fusion proteins from the cell culture medium were purified by affinity chromatography as described previously[26]. The size of the fusion proteins was analyzed in reducing conditions on SDS–PAGE and in native conditions, in which the SDS and reducing agent (DL-Dithiothreitol, DTT) were omitted from the standard Laemmli SDS protocol and in the sample buffer, by laser desorption time-of-flight mass spectrometry (MALDI–TOF–MS; Bruker–Franzen, Bremen, Germany).
Cytotoxicity assays The efficacy of the fusion protein in mediating tumor cell lysis was determined by colorimetric lactate dehydrogenase (LDH) release assays. 4×103 SKBR3, SKOV3, and MCF7 cells in 100 µL LDH as the target cells were added to 96-well flat-bottom plates, respectively, and incubated overnight. Subsequently, peripheral blood mononuclear cells (PBMC) isolated from the blood of healthy donors by Ficoll density gradient centrifugation as the effector cells were added to each well at effector-to-target (E:T) ratios of 5:1, 10:1, 20:1, and 40:1 in a final volume of 100 µL/well with the anti-ErbB2 scFv–Fc–IL-2 fusion protein (HFI) at the final concentration of 15 ng/mL. Spontaneous release (SR) was set by adding only target or effector cells; maximum release (MR) was set by adding 1% Triton X-100 to the target cells. After 4–8 h of incubation at 37 ºC in 5% CO2, the assays were centrifuged. The supernatant was transferred to 96-well flat-bottom plates and incubated with LDH reaction mixture (LDH Detection Kit, Roche, Mannheim, Germany) for 30 min at 25 ºC. The samples were measured at 492 nm with a reference wavelength of 620 nm. The percentage of cytotoxicity was calculated as ([Sample release–SReffector–SRtarget]/[MRtarget–SRtarget])×100. RPMI-1640 medium was used as the control.
For light microscopy, SKBR3 cells were incubated with PBMC in the medium containing HFI at a final concentration of 15 ng/mL in 96-well plates at 37 °C for 12 h. The cells were then directly viewed under a light microscope (Nikon, Yokohama, Kanagawa, Japan).
Lymphokine-activated killer cell generation Human PBMC at a cell concentration of 2.5×106–5×106/mL in 50 mL flasks was stimulated with recombinant interleukin 2 (rIL-2) at 500 IU/mL in RPMI-1640 medium supplemented with 2 mmol/L glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin, and 20% FBS. The cells were cultured for 5–7 d in a humidified incubator with 5% CO2 at 37 °C. The cells were harvested and viability was determined by the trypan blue dye exclusion method.
In vivo antitumor activity Six to seven-week-old female Balb/C athymic nude mice were sc injected into the right flank with 1×107 SKOV3 cells. The mice were treated intravenously with recombinant IL-2 (10 000 IU), HFI (5, 2.5, and 1.25 µg, respectively), scFv–Fc (5 µg) in combination with 1×106 lymphokine-activated killer (LAK) cells, or phosphate-buffered saline (PBS) twice weekly. In each group, 7 or 8 mice were used. The mice were monitored daily and tumor volume was measured with a caliper twice a week, using the formula volume=length×width2/2. At the end of the experiments (after treatment for 21–35 d), the mice were killed. The organs (spleen, heart, lung, liver, and kidney) and primary tumors were removed, fixed in formalin, and examined histologically. Flow cytometry (FACS) was used to examine the effector cell profile, the blood was collected from the mice by retro-orbital bleeding in heparinized Eppendorf microcentrifuge tubes. FITC conjugated anti-human CD3 and anti-human CD16 antibodies were added at a final concentration of 2 µg/mL in 100 µL volume and incubated on ice for 20 min. The stained cells were washed 3 times with ice-cold PBA (PBS, 2% BSA, 0.1% NaN3) and analyzed on a FACSCalibur (Becton Dickinson, Franklin Lakes, NJ, USA), using CELLQUEST software (BD Biosciences, San Jose, CA, USA).
Histology and immunohistochemistry Tissue samples were fixed in 4% buffered paraformaldehyde and embedded in paraffin. Tumor tissues were analyzed by hematoxylin–eosin (HE) staining and immunohistochemistry. Briefly, for the immunohistochemical analysis, the paraffin-embedded sections were dewaxed and rehydrated. Endogenous peroxidase activity was quenched by 3% hydrogen peroxide in distilled water for 5–10 min and then washed in PBS before immunohistochemical staining. The Streptavidin–Biotin method was used to detect ErbB2 expression in the tumor tissues. Primary antibodies (mouse monoclonal antibodies against ErbB2), the biotinylated antimouse antibody, and the streptavidin peroxidase reagent were purchased from Beijing Zhongshan Golden Bridge Biotechnology (Beijing, China). Antigen retrieval was achieved by microwave treatment. Sections were sequentially incubated with the primary antibody, the biotinylated antimouse antibody, and the streptavidin peroxidase reagent. Peroxidase activity was detected with 3,3'-diaminobenzidine (DAB) solution, and the sections were weakly counterstained with hematoxylin, washed, dehydrated with alcohol and xylene, and mounted with coverslips. Omission of the primary antibody was used as negative controls.
Toxicity studies Six-week-old female Balb/C athymic nude mice (n=4) were given 1 single iv injection of 0, 5, 10, or 20 µg HFI diluted in a volume of 100 µL PBS. The mice were monitored daily and toxicity was measured in terms of weight loss and mortality.
Statistical analysis Data were expressed as mean±SD. For the cytotoxicity assay, the data were compared by t-test using standard statistical software, SPSS version 11.0 (Chicago, IL, USA). For comparisons among the groups in the experiments in vivo, an ANOVA test was performed. P<0.05 was considered statistically significant.
Results
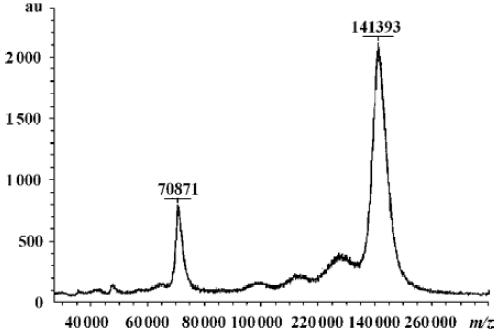
Characterization of fusion proteins The antigen-binding and IL-2 activities of the fusion proteins were determined by FACS and ELISA as described previously (data not shown)[26]. HFI specifically and efficiently bound to SKBR3 cells expressing high levels of ErbB2, but bound less efficiently to MCF7 cells expressing low levels of ErbB2[28], indicating that the fusion of anti-ErbB2 scFv–Fc to the amino terminus of IL-2 did not interfere with the ability of the antibody to recognize the ErbB2 antigen. To determine the molecular mass and assembly of the fusion proteins, the purified proteins were analyzed by SDS–PAGE and MALDI–TOF–MS. In the presence of reducing agents, the fusion proteins migrated with an apparent molecular mass of ~70 kDa, the expected molecular mass of the fusion protein, as previously determined (data not shown)[26]. The MALDI–TOF–MS indicated the purified HFI (Figure 2). The molecular weight of HFI in a monovalent form was found to be 70 871 m/z and that in a bivalent form was 141393 m/z. The results indicated that the fusion of IL-2 to anti-ErbB2 scFv–Fc did not appear to alter the assembly and secretion of the bivalent form of the scFv–Fc–IL-2 fusion protein.
Cytotoxicity studies HFI was evaluated for its ability to mediate ADCC by LDH assays against ErbB2-expressing cells. Examples of killing assays are shown in Figure 3A–3C. At a concentration of 15 ng/mL and all E:T ratios, the fusion protein mediated the efficient lysis to SKBR3 and SKOV3 cells, which expressed high levels of ErbB2[29,30] (P<0.01). Both cells were killed to a greater extent by HFI-mediated lysis than MCF7 cells expressing low levels of ErbB2, demonstrating that HFI was effective against the ErbB2-overexpressing cell lines in vitro.
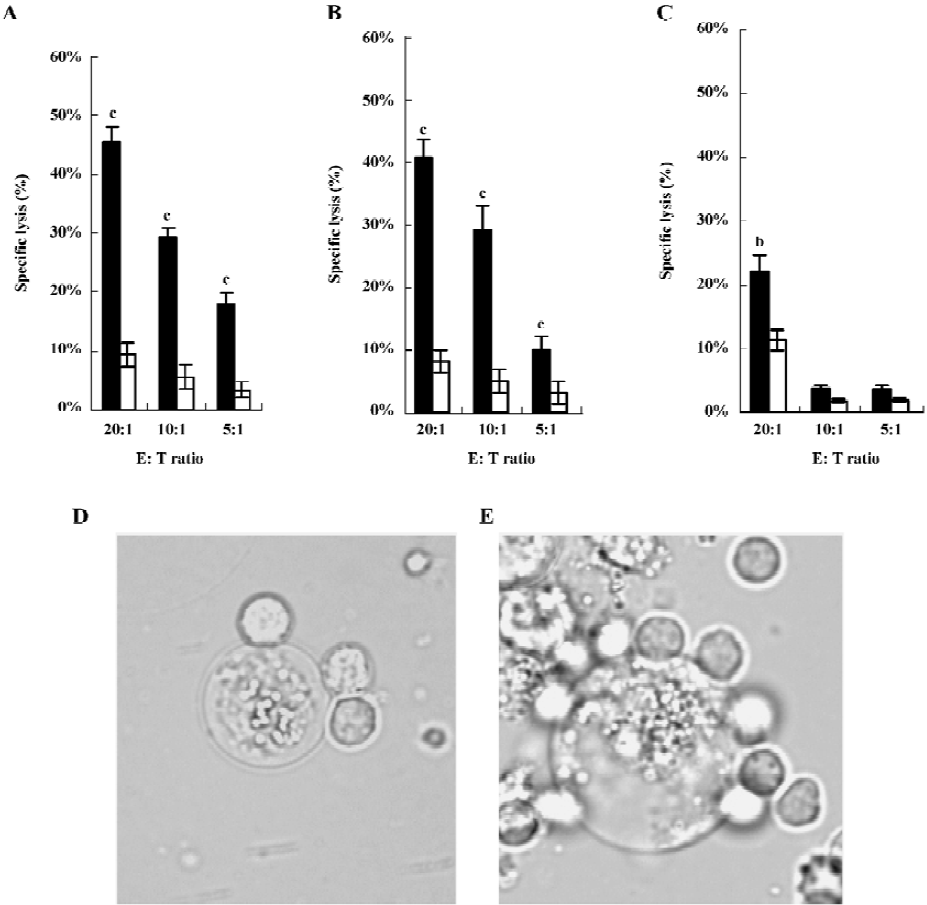
Morphological observations clearly revealed the binding and lysis of effector cells to tumor cells mediated by HFI (Figure 3D and 3E). An extremely swollen SKBR3 cell was tightly surrounded by the effector cells after 12 h incubation (Figure 3E). The dying or dead tumor cells were easily observed in the incubation system. In contrast, the effector cells largely appeared healthy with intact cell membranes.
In vivo antitumor activity After demonstrating that HFI had in vitro biological activity, the in vivo antitumor activity was investigated using a SKOV3 animal model. A total of 1×107 SKOV3 cells in 0.15 mL PBS were injected sc into the right flank of the mice on d 1. A direct comparison between free IL-2 and HFI was performed on d 3 after sc tumor cell implantation. The therapy groups consisted of mice (8 animals/group) receiving iv injections of PBS, IL-2 (10 000 IU), and HFI (5 µg) in combination with LAK cells twice weekly. 1 µg HFI contains ~2000 IU of IL-2 activity as determined by in vitro proliferative assays with IL-2-dependent T-cells[26]. The data demonstrated a dramatic improvement in efficacy with complete regression in all mice treated with 5 µg HFI twice weekly for 21–25 d (P<0.01) (Figure 4A). The improved efficacy is likely attributable to the increased half-life and improved targeting of IL-2.
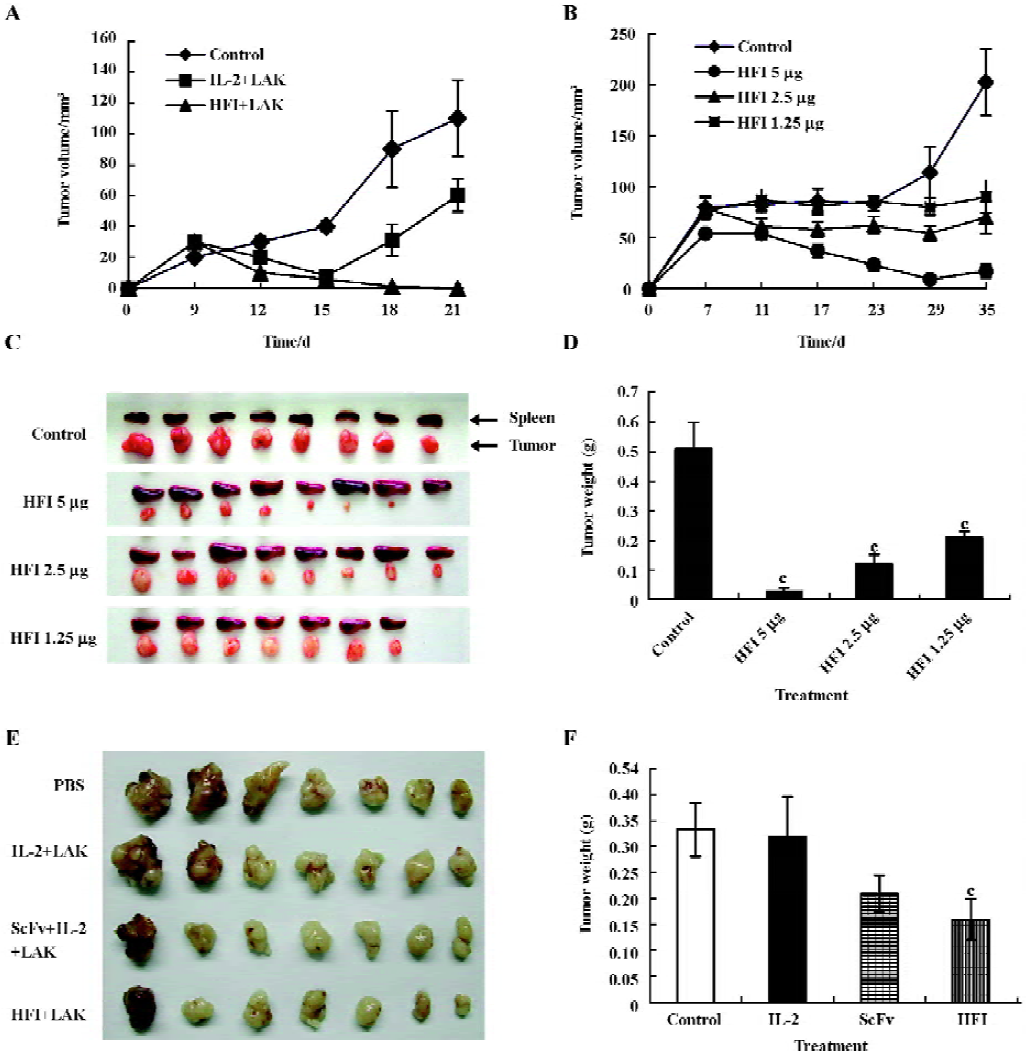
To determine the antitumor activity of HFI at the decreased doses and prolonged treatment, therapy was started in groups of mice treated with PBS or with 5, 2.5, or 1.25 µg HFI, respectively. Injections were repeated twice weekly for 35 d. After 29 d treatment, tumors started to regrow (Figure 4B–4D). Only 1 of the tumors in the group treated with 5 µg HFI was regressed. One mouse in the group treated with 1.25 µg HFI died. The therapeutic effect observed in the mice treated with the 5 µg dose was considerably better than that observed with the 2.5 (P<0.01) and 1.25 µg doses (P<0.01).
To compare the therapeutic efficacy of HFI with that of scFv–Fc and to investigate the effect of HFI on larger tumors, injections of PBS, scFv–Fc (5 µg), or HFI (5 µg) were started when the tumors reached ~285 mm3 in size on d 6 after tumor cell implantation. Treatment with HFI arrested the growth of tumors approximately 6 d after the beginning of the treatment compared with the controls (P<0.05), whereas scFv–Fc exhibited no significant effect compared with PBS and free IL-2 (P>0.05); the tumors developed quickly after treatment with scFv–Fc for 15 d. (Figure 4E and 4F).
Effector cell profile The effector cell population was derived from normal human PBMC after culturing for 7 d in medium containing IL-2. Both T cell and natural killer cell (NK) populations are expected to be activated by this procedure. Human CD3+ and CD16+ cells were detected from the peripheral blood of mice in all doses of the HFI treated groups (Table 1).
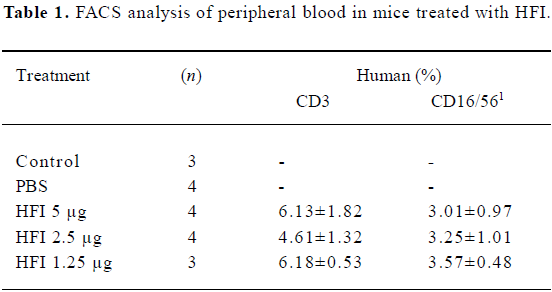
Full table
Toxicity Treatment with HFI was well tolerated at the 5 µg dose and no significant weight loss was apparent. A transient weight loss (<5%) was observed, but the toxicity appeared to be moderate. The greater weight loss (~6%) occurred at the 20 µg dose, which was 4-fold greater than the one used in the therapy experiments. All the mice survived the experiment.
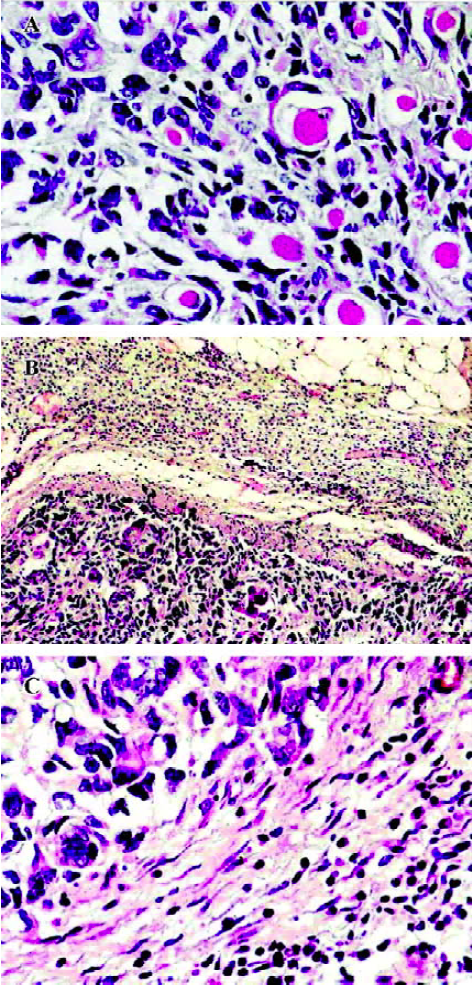
Histology and immunohistochemistry Histologically, the PBS-treated tumors consisted of large pleomorphic cells that had large dark nuclei and increased basophilic cytoplasm with some mitoses. Many infiltrations of the tumor cells into the adjacent muscle tissues were often observed (Figure 5A). It is noteworthy that the tumors treated with HFI were surrounded by the thin fibrous capsule and a large amount of infiltrating lymphocytes (Figure 5B and 5C). This is in contrast to the tumors, where no lymphocyte infiltrate was observed from mice when treated with either PBS or free IL-2. In the mice of all the groups, no pathological findings were seen in the spleen, kidney, lung, and heart. A histological examination of livers from the mice treated with HFI revealed the presence of inflammatory foci and focal hepatocyte necrosis. Similar characteristics were found in the study of antitumor activity of targeted IL-12 and have been described as IL-12-induced hepatotoxicity[32].
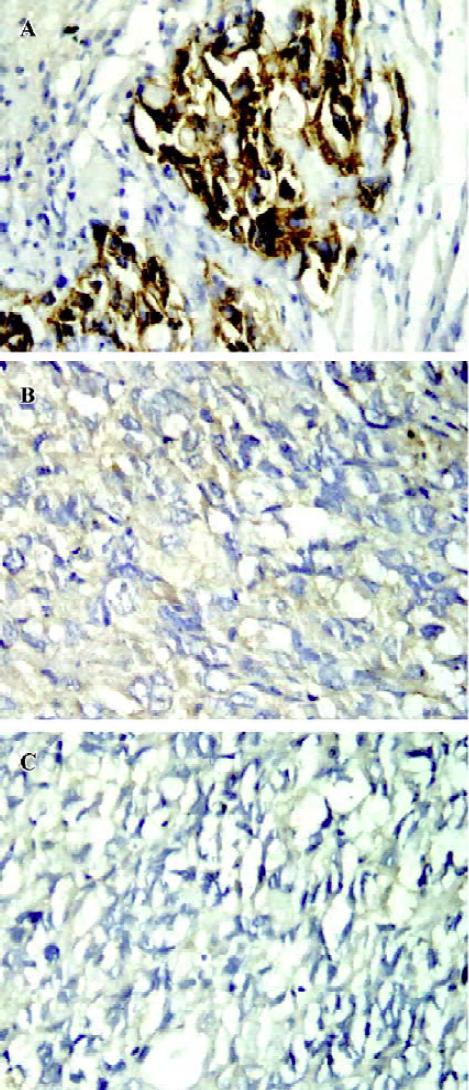
The tumors from the mice (6 or 7 for each group) were analyzed by immunohistochemistry for ErbB2 expression. The results demonstrated that ErbB2 was strongly expressed in the control tumors (Figure 6A). However, the expression of ErbB2 in the tumor tissues was remarkably decreased or even disappeared after 5 µg HFI treatment (Figure 6B and 6C).
Discussion
IL-2 is an important pleiotrophic cytokine and exhibits a wide variety of biological activities, including the stimulation of antitumor effector cells, such as cytotoxic T cells[33–35], NK cells[33,36–39], and macrophages[7,15,16]. It also influences dendritic cell interactions with various effector cell populations, which may elicit further antitumor involvement with subsequent development of tumor-specific T cell responses[16]. It has been reported that IL-2 treatment can augment the activation of pre-existing antigen-specific T cells, enhance their recognition and destruction of neoplastic tissue, and activate NK cells[1,37,40]. However, systemic cytokine therapy frequently causes severe problems with toxicity that makes it impossible to achieve an effective dose at the tumor sites. Increasing local cytokine concentration and limiting generalized toxicity have been the goals pursued by researchers and clinicians. In recent years, the antibody has become a specific delivery vehicle to selectively target IL-2 to metastatic/residual nodules. The specific targeting has been expected to elicit the local activation of NK cells at tumor sites to provide more effective tumor destruction[19–22].
Several antibody–cytokine fusion proteins have been developed. In the preclinical trials using murine models, antibody–cytokine fusion proteins have shown to be very effective antitumor agents. Most studies have focused on IL-2 as the effector molecule and whole antibodies were generally used as the targeting vehicles. The production of a whole antibody–cytokine fusion protein requires the co-expression of a heavy chain usually fused to IL-2 and a light chain in a single host. It does not guarantee that both heavy chain and light chain are present in equimolar concentrations. In the present study, we constructed an anti-ErbB2 scFv–Fc–IL-2 fusion protein, HFI. The recombinant human IL-2 gene is fused with the anti-ErbB2 scFv–Fc gene at the C-terminal. The fusion protein was expressed as a single peptide and folded as a homodimer formed by the covalently linking of Fc portions leading to a bivalent molecule. The biological activities of both antibody and IL-2 were well maintained. HFI was demonstrated as potent to initiate a cytotoxic activity of previously unstimulated PBMC against human breast and ovarian cancer cells at low effector-to-target ratios in experiments in vitro. It was more effective in killing SKBR3 and SKOV3 cells expressing ErbB2 highly than MCF7 cells expressing ErbB2 at lower level[29,30], suggesting that the killing effect mediated by HFI was dependent on the expression level of ErbB2 molecules on the target cells.
To determine the efficacy of the fusion protein in vivo, the xenograft models of human ovarian carcinoma tumors in nude mice were treated with engrafted human effector cells and fusion proteins. The analysis by FACS showed that both human T and NK cells were present in the peripheral blood of mice. NK cells may play the major role in this process since they are more potently activated by high doses of IL-2 than T cells cultured in the absence of their cognate antigens. Nonetheless, a role of human T cells cannot be ruled out. Potent antitumor effects mediated by both NK and T cells have been reported in a severe combined immunodeficiency mouse transplantation model[41]. In this study, significant antitumor activity of the fusion protein was demonstrated in an in vivo environment. In the mice treated with HFI, the tumor environment became infiltrated with lymphocytes. In addition, the inhibition effect on tumor growth in mice was observed when the treatment with HFI was started after large tumors had already established, although complete eradication of tumors was not achieved. No signs of overt toxicity were seen in the mice injected with twice-weekly doses of the fusion proteins. Notably, splenomegaly was observed in most of the HFI-treated mice. It has been reported that the doses required for an adjuvant effect of CD40 mAb when mixed with antigen are highly reactogenic, leading to splenomegaly and polyclonal antibody production[31]. The speculative adjuvant effect caused by HFI may still need to be determined.
ErbB2 overexpression underlies enhancement in pro-liferative, prosurvival, and metastatic signaling. ErbB2 mediated signaling also confers resistance to anticancer agents, such as cisplatin, paclitaxel, doxorubicin, etoposide, and radiation[42–44]. In breast cancer, ErbB2 overexpression is a significant negative prognostic indicator for a variety of therapies. Patients with ErbB2-overexpressing tumors have shown a significantly lower overall survival rate and shorter relapse time than those with ErbB2-negative tumors[45,46]. Therapeutic antibody Herceptin has been shown to dephosphorylate and downregulate ErbB2 levels, block receptor signaling, and exhibit clinical efficacy against breast cancer[25,47]. The downregulation of ErbB2 by Herceptin has been shown to impact cell sensitivity to chemotherapeutic agents, suppress ErbB2-induced malignant phenotypes, and results in the recruitment of the cbl protein, ubiquitination, and inducing the proteasome-dependent degradation of ErbB2[48]. It is noteworthy that ErbB2 expression in the tumors diminished after 5 µg dose HFI treatment in this study. However, the efficacy of ErbB2-targeting inhibitors is correlated with the ErbB2 overexpression level. The largest effects of these therapies are seen in the cell lines expressing the highest levels of ErbB2[28]. It was reported that the effect of Herceptin was restricted to BT474 and SKBR3 cells, which express ErbB2 highly, but not in MCF-7 cells with low levels of ErbB2. It may explain that the tumors in the mice resumed growth after treatment by HFI for approximately 1 month in the present study. The similar results were also observed by treatment with Herceptin[49]. Herceptin improved sensitization of ErbB2-overexpressing breast cancer cells to Taxol and several chemotherapeutic-induced apoptosis, cytotoxicity, and tumor growth inhibition[50–53]. Thus, pretreatment of the therapeutic antibody or antibody–cytokine fusion protein may enhance the cytotoxicity of chemotherapeutic agents and benefit the treatment of ErbB2-overexpressing breast carcinomas. The mechanisms of ErbB2 downregulation by HFI are not clear.
In conclusion, the genetically engineered anti-ErbB2 scFv–Fc–IL-2 fusion protein retains ErbB2 specificity and IL-2 biological activity. The fusion proteins mediated efficient ADCC in vitro and exhibited significant antitumor activity in the tumor model using SKOV3 ovarian cancer cells. It is simple to express such a bivalent fusion protein. It has been found that NK cells were not deficient in patients with solid and hematological malignancies, even after high-dose chemotherapy. With the efficient stimulation by targeted IL-2, NK cells may elicit an antitumor response in patients who are deficient in T cells following high-dose chemo-therapy.
References
- Hank JA, Robinson RR, Surfus J, Mueller BM, Reisfeld RA, Cheung NK, et al. Augmentation of antibody dependent cell mediated cytotoxicity following in vivo therapy with recombinant interleukin 2. Cancer Res 1990;50:5234-9.
- Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature 2001;411:380-4.
- Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Linehan WM, Seipp CA, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg 1989;210:474-84.
- Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med 1988;319:1676-80.
- Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst 1994;86:1159-66.
- Roth JA, Cristiano RJ. Gene therapy for cancer: What have we done and where are we going? J Natl Cancer Inst 1997;89:21-39.
- Zatloukal K, Schneeberger A, Berger M, Schmidt W, Koszik F, Kutil R, et al. Elicitation of a systemic and protective anti-melanoma immune response by an IL-2-based vaccine. Assessment of critical cellular and molecular parameters. J Immunol 1995;154:3406-19.
- Dranoff G, Mulligan RC. Gene transfer as cancer therapy. Adv Immunol 1995;58:417-54.
- Maas RA, Dullens HF, Den OW. Interleukin-2 in cancer treat-ment: disappointing or (still) promising? A review. Cancer Immunol Immunother 1993;36:141-8.
- Cortesina G, De SA, Giovarelli M, Barioglio MG, Cavallo GP, Jemma C, et al. Treatment of recurrent squamous cell carcinoma of the head and neck with low doses of interleukin-2 injected perilymphatically. Cancer 1988;62:2482-5.
- Forni G, Musso T, Santoni A, Giovarelli M. Local administration of interleukin-2 activates lymphocytes from tumor bearing mice to recruit host immunoreactivity and inhibit tumor growth. Prog Clin Biol Res 1987;244:105-14.
- Pizza G, Severini G, Menniti D, De VC, Corrado F. Tumour regression after intralesional injection of interleukin 2 (IL-2) in bladder cancer. Preliminary report. Int J Cancer 1984;34:359-67.
- Rutten VP, Klein WR, De Jong WA, Misdorp W, Den OW, Steerenberg PA, et al. Local interleukin-2 therapy in bovine ocular squamous cell carcinoma. A pilot study. Cancer Immunol Immunother 1989;30:165-9.
- Hurford RK Jr, Dranoff G, Mulligan RC, Tepper RI. Gene therapy of metastatic cancer by in vivo retroviral gene targeting. Nat Genet 1995;10:430-5.
- Schmidt W, Schweighoffer T, Herbst E, Maass G, Berger M, Schilcher F, et al. Cancer vaccines: the interleukin 2 dosage effect. Proc Natl Acad Sci USA 1995;92:4711-4.
- Soiffer R, Lynch T, Mihm M, Jung K, Rhuda C, Schmollinger JC, . Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci USA 1998; 95: 13 141–6.
- Gillies SD, Lan Y, Wesolowski JS, Qian X, Reisfeld RA, Holden S, et al. Antibody-IL-12 fusion proteins are effective in scid mouse models of prostate and colon carcinoma metastases. J Immunol 1998;160:6195-203.
- Gillies SD, Reilly EB, Lo KM, Reisfeld RA. Antibody-targeted interleukin 2 stimulates t-cell killing of autologous tumor cells. Proc Natl Acad Sci USA 1992;89:1428-32.
- Penichet ML, Morrison SL. Antibody-cytokine fusion proteins for the therapy of cancer. J Immunol Methods 2001;248:91-101.
- Lode HN, Xiang R, Dreier T, Varki NM, Gillies SD, Reisfeld RA. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood 1998;91:1706-15.
- Hornick JL, Khawli LA, Hu P, Lynch M, Anderson PM, Epstein AL. Chimeric CLL-1 antibody fusion proteins containing granulocyte-macrophage colony-stimulating factor or interleukin-2 with specificity for beta-cell malignancies exhibit enhanced effector functions while retaining tumor targeting properties. Blood 1997;89:4437-47.
- Xiang R, Lode HN, Dolman CS, Dreier T, Varki NM, Qian X, et al. Elimination of established murine colon carcinoma metastases by antibody-interleukin 2 fusion protein therapy. Cancer Res 1997;57:4948-55.
- Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-her2 receptor monoclonal antibody, inhibits basal and activated her2 ectodo-main cleavage in breast cancer cells. Cancer Res 2001;61:4744-9.
- Shak S. Overview of the trastuzumab (herceptin) anti-her2 monoclonal antibody clinical program in her2-overexpressing metastatic breast cancer. Herceptin Multinational Investigator Study Group. Semin Oncol 1999;26:71-7.
- Yip YL, Ward RL. Anti-erbb-2 monoclonal antibodies and erbb-2-directed vaccines. Cancer Immunol Immunother 2002;50:569-87.
- Shi M, Xie Z, Feng J, Sun Y, Yu M, Shen B, et al. A recombinant anti-erbb2, scfv-fc-il-2 fusion protein retains antigen specificity and cytokine function. Biotechnol Lett 2003;25:815-9.
- Shi M, Xie Z, Yu M, Shen B, Guo N. Controlled growth of Chinese hamster ovary cells and high expression of antibody-IL-2 fusion proteins by temperature manipulation. Biotechnol Lett 2005;27:1879-84.
- Emlet DR, Schwartz R, Brown KA, Pollice AA, Smith CA, Shackney SE. Her2 expression as a potential marker for response to therapy targeted to the egfr. Br J Cancer 2006;94:1144-53.
- Dean GS, Pusztai L, Xu FJ, O’Briant K, DeSombre K, Conaway M, et al. Cell surface density of p185(c-erbb-2) determines susceptibility to anti-p185(c-erbb-2)-ricin a chain (rta) immunotoxin therapy alone and in combination with anti-p170(egfr)-rta in ovarian cancer cells. Clin Cancer Res 1998;4:2545-50.
- Xu F, Yu Y, Le XF, Boyer C, Mills GB, Bast RC Jr. The outcome of heregulin-induced activation of ovarian cancer cells depends on the relative levels of her-2 and her-3 expression. Clin Cancer Res 1999;5:3653-60.
- Barr TA, McCormick AL, Carlring J, Heath AW. A potent adjuvant effect of CD40 antibody attached to antigen. Immunology 2003;109:87-92.
- Halin C, Rondini S, Nilsson F, Berndt A, Kosmehl H, Zardi L, et al. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat Biotechnol 2002;20:264-9.
- Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med 1982;155:1823-41.
- Hayes RL, Koslow M, Hiesiger EM, Hymes KB, Hochster HS, Moore EJ, et al. Improved long term survival after intracavitary interleukin-2 and lymphokine-activated killer cells for adults with recurrent malignant glioma. Cancer 1995;76:840-52.
- Maass G, Schmidt W, Berger M, Schilcher F, Koszik F, Schneeberger A, et al. Priming of tumor-specific t cells in the draining lymph nodes after immunization with interleukin 2-secreting tumor cells: three consecutive stages may be required for successful tumor vaccination. Proc Natl Acad Sci USA 1995;92:5540-4.
- Bukowski JF, Warner JF, Dennert G, Welsh RM. Adoptive transfer studies demonstrating the antiviral effect of natural killer cells in vivo. J Exp Med 1985;161:40-52.
- Favrot MC, Floret D, Negrier S, Cochat P, Bouffet E, Zhou DC, et al. Systemic interleukin-2 therapy in children with progressive neuroblastoma after high dose chemotherapy and bone marrow transplantation. Bone Marrow Transplant 1989;4:499-503.
- Koehne G, Zeller W, Stockschlaeder M, Zander AR. Phenotype of lymphocyte subsets after autologous peripheral blood stem cell transplantation. Bone Marrow Transplant 1997;19:149-56.
- Uharek L, Zeis M, Glass B, Steinmann J, Dreger P, Gassmann W, et al. High lytic activity against human leukemia cells after activation of allogeneic NK cells by IL-12 and IL-2. Leukemia 1996;10:1758-64.
- Nagayama H, Takahashi S, Takahashi T, Ogami K, Ikebuchi K, Tojo A, et al. Il-2/lak therapy for refractory acute monoblastic leukemia relapsing after unrelated allogeneic bone marrow transplantation. Bone Marrow Transplant 1999;23:183-5.
- Lustgarten J. Anti-her-2/neu-il-2 or heregulin-il-2 fusions proteins redirect non-tumor specific ctl to the tumor site for tumor eradication. Cancer Immunol Immunother 2003;52:751-60.
- Yu D, Hung MC. Role of erbb2 in breast cancer chemosensitivity. Bioessays 2000;22:673-80.
- Yu D, Jing T, Liu B, Yao J, Tan M, McDonnell TJ, et al. Overexpression of erbb2 blocks taxol-induced apoptosis by up-regulation of p21cip1, which inhibits p34cdc2 kinase. Mol Cell 1998;2:581-91.
- Yu D, Liu B, Jing T, Sun D, Price JE, Singletary SE, et al. Overexpression of both p185c-erbb2 and p170mdr-1 renders breast cancer cells highly resistant to taxol. Oncogene 1998;16:2087-94.
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the her-2/neu oncogene. Science 1987;235:177-82.
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the her-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989;244:707-12.
- Guan H, Jia SF, Zhou Z, Stewart J, Kleinerman ES. Herceptin down-regulates her-2/neu and vascular endothelial growth factor expression and enhances taxol-induced cytotoxicity of human ewing’s sarcoma cells in vitro and in vivo. Clin Cancer Res 2005;11:2008-17.
- Klapper LN, Waterman H, Sela M, Yarden Y. Tumor-inhibitory antibodies to her-2/erbb-2 may act by recruiting c-cbl and enhancing ubiquitination of her-2. Cancer Res 2000;60:3384-8.
- Spiridon CI, Ghetie MA, Uhr J, Marches R, Li JL, Shen GL, et al. Targeting multiple her-2 epitopes with monoclonal antibodies results in improved antigrowth activity of a human breast cancer cell line in vitro and in vivo. Clin Cancer Res 2002;8:1720-30.
- Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-her2 antibody (herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against her2/neu overexpressing human breast cancer xenografts. Cancer Res 1998;58:2825-31.
- Kim R, Tanabe K, Emi M, Uchida Y, Toge T. Modulation of tamoxifen sensitivity by antisense bcl-2 and trastuzumab in breast carcinoma cells. Cancer 2005;103:2199-207.
- Lee S, Yang W, Lan KH, Sellappan S, Klos K, Hortobagyi G, et al. Enhanced sensitization to taxol-induced apoptosis by herceptin pretreatment in erbb2-overexpressing breast cancer cells. Cancer Res 2002;62:5703-10.
- Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185her2/neu monoclonal antibody plus cisplatin in patients with her2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol 1998;16:2659-71.