Synthesis and antitussive evaluation of verticinone-cholic acid salt, a novel and potential cough therapeutic agent1
Introduction
Cough is one of the most common complaints from patients[1]. The present therapies were limited for lack of effective medications. Moreover, most existed medicine for treatment could bring inevitable or intolerable side-effects. The most pressing and unmet current need is to seek more effective and safer antitussive agents[2]. Traditional medicines represent a promising new trend for developing new antitussive drugs.
Shedan–Chuanbei powder, a complex of traditional Chinese medicine preparation, which consists of snake bile (Chinese name “Shedan”) and Fritillariae cirrhosae (Chinese name “Chuanbei”), is the most popular antitussive and expectorant formulation in Chinese communities. It has been officially listed in the Chinese Pharmacopoeia (1995, 2000, and 2005) due to the positive potent therapeutic effects, low toxicity, and minimal side-effects. Snake biles are the bile acids of Naja naja Linnaeus, Krait, zaocys, and Fritillariae cirrhosae among others, and are the bulbs of Fritillariae cirrhosae, Fritillariae unibracteata, Fritillariae przewalskii, and Fritillariae delavayi. However, the clinical application of Shedan–Chuanbei powder is now stringently limited because of the shortage of the 2 crude medicinal materials, especially for the sake of animal protection, therefore, it prompts us to search for new bioactive substitutes for Shedan–Chuanbei powder.
Most of the complex of traditional Chinese medicine formulations have sound scientific basis through modern pharmacological evaluation[3]. For example, many combination formulations showed significantly better pharmacological effects than individual herbal medicines in the formulations through synergistic interaction and attenuation of their toxicity. Significant chemical changes may occur during the preparation (decoction) process of a prescribed herbal formulation[4]. Verticinone and cholic acid were identified as major bioactive components in Fritillariae and snake bile[5–7], both of which can be determined with high concentrations by advanced analytical technology in Shedan–Chuanbei powder[8,9]. Enlightened with “combination principles” in drug discovery and hypothesizing that the 2 ingredients may have a synergism, in the present study, we made a salifying reaction of the 2 ingredients considering their distinct properties in acidity or alkalinity. We then obtained verticinone–cholic acid salt from the reaction. Furthermore, we also elucidated the antitussive activity, the acute toxicity, and the antitussive mechanism involved in guinea pigs.
Materials and methods
Equipment Melting points were measured with a Fisher Scientific instrument (New Jersey, USA) and were uncorrected. Infra-red (IR) spectra were recorded on a Shimadzu IR2460 spectrometer. The NMR spectra in D2O were recorded with Bruker AM-500 spectrometers (Bruker, Germany). FAB-MS was measured on a VG Auto Spec-3000 mass spectrometer (Micromass, England). An elemental analysis were performed on a CarloErba-1106 autoanalyzer (CarloErba, Milan, Italy). The compressor nebulizer (Medel ap112U, Polo di Torrile, Italy) was used to produce an aerosol with a particle median diameter of 1.87–3.54 µm. The cough sound was amplified via a clip-on microphone (Takstar DA-141, Guangdong Takstar Electronic, Dongguan, Guang-dong province, China).
Herbal materials, antitussive agents and drugs Verticinone was isolated from Fritillariae hupehensis Hsiao et KC Hsia, which is commercially available from the Hubei Institute of Chinese Materia Medica (Wuhan, China) and was identified by Prof De-tai PENG (Lichuan Institute of Chinese Materia Medica, Lichuan, Hubei province, China). A voucher specimen (F030823) was deposited at the Faculty of Pharmaceutical Science, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China).
Cholic acid was purchased from Asia Talent Enterprise (Shenzhen, Guangdong province, China). Its identity was confirmed with IR, 1H-NMR, 13C-NMR, and MS analyses. Codeine phosphate was purchased from Qinghai Pharmaceutical Factory (Qinghai, China). Glybenclamide was purchased from ABCR Gmbh (Karlsruhe, Germany). The naloxone hydrochloride injection was purchased from Beijing Four-Ring Pharmaceutical Factory (Beijing, China). Moguisteine was purchased from Boehringer Mannheim Italia (Monza, Italy).
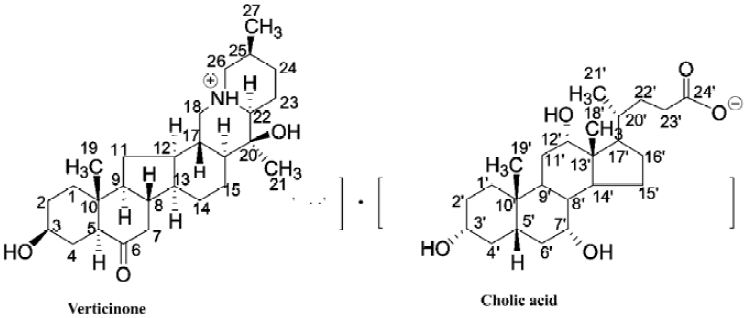
Synthesis and identification of Ver–CA The powdered bulbs of Fritillariae hupehensis Hsiao et K C. Hsia (3.5 kg) were extracted with 95% EtOH (ethanol) and the solvent was removed to give an EtOH extract. Then the extract was dissolved in 2% HCl and filtered. After filtration, the water layer was basified with ammonia (pH=12) and continuously extracted with chloroform to give a crude alkaloid fraction (20 g, 0.6% yield). The crude alkaloid fraction was then subjected to silica gel column chromatography with a discontinuous gradient elusion using the solvent system consisting of petroleum ethyl acetate-ethylene diamine. The elution was monitored by TLC and the fractions only containing verticinone were combined and dried. Then the residue was crystallized from ethyl acetate to give verticinone (10 g, 0.3% yield), and its identify was further confirmed by 1H-NMR, 13C-NMR, and MS analyses (Figure 1).
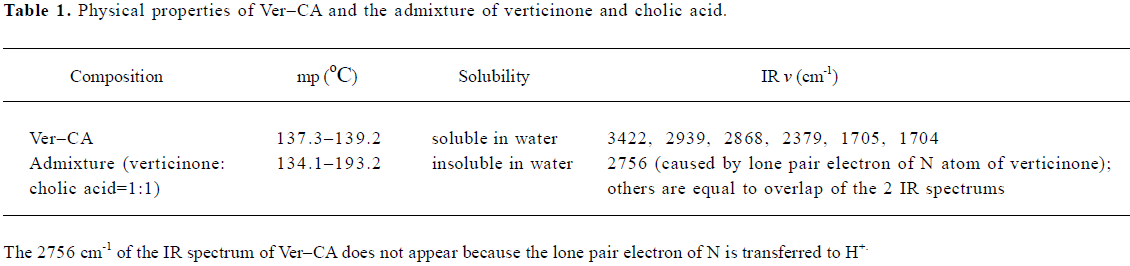
Verticinone (1 g) and cholic acid (1 g) were dissolved together in acetone and stirred for 48 h at room temperature under reflux. The organic solvent was evaporated to dryness under reduced pressure at 40 oC to afford a white residue. The residue was then further purified with distilled water. The processes of purification were as follows: first, the residue was dissolved in distilled water and then filtered in order to remove the insoluble substances; second, the water was evaporated to dryness under reduced pressure at 80 oC. We then got a white compound (1.8 g, 90% yield), which was confirmed as a salifying derivative of verticinone and cholic acid by comparing its IR, 1H-NMR, 13C-NMR, and MS with that of verticinone and cholic acid. As shown in Table 1, the difference between Ver–CA and the admixture of verticinone and cholic acid also proved that it was a salifying derivative.
Full table
Animals Dunkin–Hartley guinea pigs of both sexes (Experimental Animal Center, Tongji Medical College, Huazhong University of Science and Technology, China), weighing about 250–350 g, were used. The animals were housed in groups of 6 per cage under a 12 h light–dark cycle with food and water available continuously. Kunming mice of both sexes were used in acute toxicity experiment. This study was carried out in accordance with the “Regulation for the Administration of Affairs Concerning Experimental Animals” (State Council of China, 1988).
Drug administration Codeine phosphate and Ver–CA were dissolved in 0.9% saline. Verticinone and cholic acid were suspended in 0.5% carboxyl methylcellulose solution. Glybenclamide (3 mg/kg), also suspended in 0.5% carboxyl methylcellulose solution, was injected 30 min after the administration of the antitussive agents. Naloxone hydrochloride (0.8 mg/kg) was injected 45 min after the administration of the antitussive agents.
Assay of antitussive activity in guinea pigs The unanesthetized and unrestrained animals were placed individually in a transparent perspex chamber (dimensions: 16 cm×16 cm× 16 cm) and exposed to a nebulized aqueous solution of 17.5% citric acid saline solution for 5 min with a flow rate of 0.5 mL/min. The same nebulizer was used throughout the experiment. During the 5 min observation period, the animals were continuously watched by a trained observer and the frequency of coughs was recorded. The coughs could easily be distinguished from sneezes since there is a clear difference in sound as well as in the behavior of the ani-mals[10]. The animals were selected by the number of outbursts of coughing observed during an aerosol exposure 24 h before the test; animals with more than 30 or fewer than 10 outbursts in 5 min were not used for the studies. After 24 h recovery, the selected guinea pigs were randomly divided into several groups with at least 6 animals in each group for different treatments. The number of coughs produced 60 min after the administration of antitussive agents (Ct) was compared with the number of control coughs (Cc) which was tested at the first day. The antitussive effect was expressed as the percentage of inhibition of the number of control coughs [(Cc–Ct)/Cc×100%].
Assay of acute toxicity of Ver–CA and verticinone in mice Test solutions of various concentrations were administrated (po) to mice. The animals were observed continuously for gross effects and then at 24 h intervals for up to 7 d. Behavioral, toxic effects, and mortality response were recorded.
Statistical analysis The LD50 values and their 95% confidence intervals were determined by the Bliss method[11] using Lanzhou Pharmaceutical software (version 1.01, Lan-zhou Biopharmaceutics, Shenyang, Liaoning Province, China). Data of antitussive effect were expressed as mean± SEM. The statistical significance of difference was assessed by t-test. A P-value of 0.05 or less was considered significant.
Results
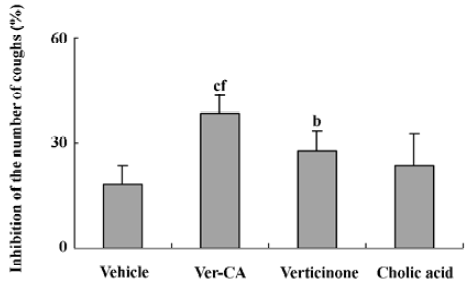
Antitussive effect of Ver–CA, verticinone, and cholic acid The guinea pigs produced 23.20±4.44 coughs in 5 min of exposure to the aerosol of 17.5% citric acid. On the next day, 60 min after administration of the vehicle, the guinea pigs were treated with the same aerosol again and produced 19.00±4.06 coughs in 5 min, which suggested that the effect of the vehicle on the number of citric acid-induced coughs was not significant (P>0.05).
The antitussive effects of Ver–CA, verticinone, and cholic acid are shown in Figure 2. At the same dose, Ver–CA, but not cholic acid and verticinone, showed potent antitussive effects in the guinea pigs. Furthermore, compared with verticinone and cholic acid, the cough reduction of the effects produced by Ver–CA were significantly different (P<0.01 vs verticinone and cholic acid).
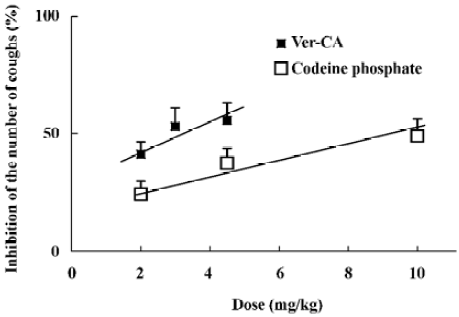
Moreover, as shown in Figure 3, the dose–response relationship studies suggest that at dosages ranging from 2 to 4.5 mg/kg (po), Ver-CA showed potent antitussive activity in a dose-dependent manner. In a parallel study, codeine phosphate, one of the most commonly used potent antitussive agents[12], was used as the positive control. A dose–response inhibitory effect of codeine on the citric acid-induced cough was also monitored at dosages ranging from 2 to 10 mg/kg (po). It was found to be less effective in comparison to Ver–CA. The values for the 50% inhibition of cough (ID50) of Ver–CA and codeine phosphate were determined as 2.9 and 10 mg/kg, respectively.
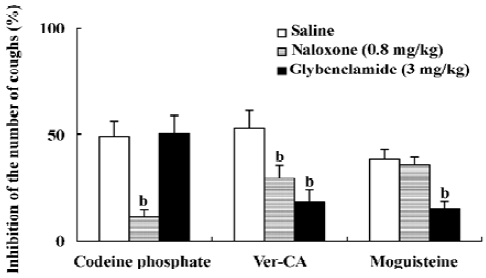
Effects of naloxone and glybenclamide on the antitussive effect of Ver–CA As shown in Figure 4, pretreatment with naloxone (0.8 mg/kg, ip), a non-selective opioid receptor antagonist, significantly (P<0.05) reduced the antitussive effect of codeine phosphate, a centrally-acting, narcotic antitussive drug (with saline, 49.04%±7.32%, n=6; with naloxone, 11.12%±3.80%, n=6). The antitussive effect of Ver–CA, but not moguisteine, was partially antagonized by naloxone. On the other hand, pretreatment with glyben-clamide (3 mg/kg, ip), an ATP-sensitive K+ channel blocker, significantly reduced the antitussive effect both of Ver–CA and moguisteine (a peripheral non-narcotic antitussive drug), but not that of codeine phosphate.
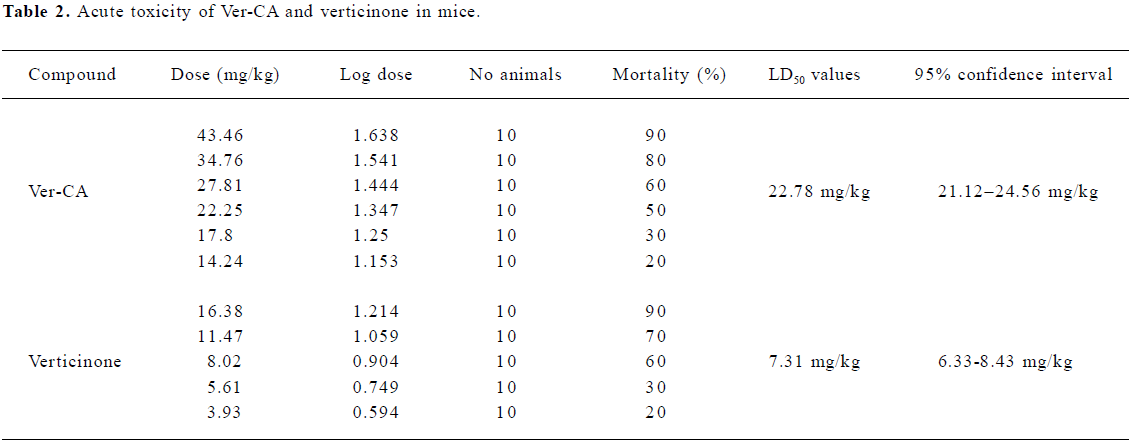
Acute toxicity of Ver–CA and verticinone After oral administration of more than 22.8 mg/kg of Ver–CA, fidgety and tachypnea were observed within a few minutes and the belly of the mouse touched the floor of the cage. One or 2 min before death, sudden jumps and convulsions were observed. All deaths occurred within 60 min after drug administration (po). Similar behavioral changes were observed after the administration of lethal doses of verticinone. Table 2 shows the LD50 values (po) of Ver–CA and verticinone. The toxicity of Ver–CA was only one-third that of verticinone.
Full table
Discussion
Shedan–Chuanbei powder, a complex traditional Chinese medicine formulation, is used for coughs, expectorant, and asthma therapy in clinics in China. Its potent antitussive effect was previously reported in experimental models of coughs[13]. Substitutes for Shedan–Chuanbei powder are already available in markets, such as an artificial snake bile substitute of natural snake bile or other Fritillariae species to substitute Fritillariae cirrhosae[13,14], but all of these substitutes still confront many daunting challenges, such as standardization problems due to the natural variability of the crude materials and the chemical complexity of the prepara-tion. However, Ver–CA, a salifying derivative of verticinone and cholic acid, would make standardization and preparation problems simpler because it is a single compound.
Further bioactivity studies showed that Ver–CA had much more potent antitussive effects than the monomer verticinone or cholic acid at the same doses. We compared the effects of the free state and inorganic salty state of the total alkaloids from Fritillariae hupehensis in a previous study and found that the antitussive effect of free-state alkaloids was a little more potent than the alkaloids’ hydrochloride and much more potent than the alkaloids’ sulfate[15]. So the antitussive effect of the alkaloids from Fritillariae hupehensis may not be increased because of the increased solubility in water. Therefore, the much more potent antitussive effect of Ver–CA salt rather than verticinone may be due to the synergistic effect between verticinone and cholic acid.
Moreover, the antitussive effect of Ver–CA was also higher than codeine phosphate, which could be proven by comparison of their antitussive ID50 values. In addition, the antitussive effect of Ver–CA was only partially antagonized by pretreatment with naloxone, although naloxone completely antagonized the antitussive effect of codeine phosphate, which indicated that the potent antitussive effect of Ver–CA was partially through the opioid receptor.
Animal studies have demonstrated that the activation of K+ channels can inhibit airway sensory nerve activity, and the modulation of these channels can attenuate experimentally-induced coughs[2]. Morita and Kamei[18] studied the antitussive effect of moguisteine, a peripherally–acting, non-narcotic antitussive drug, and had proposed that ATP-sensitive K+ channels may be involved in the antitussive effect of peripherally-acting antitussive drugs[16–18]. In the present study, moguisteine was also used as a control. Similar to moguisteine, the antitussive effect of Ver–CA was significantly reduced by pretreatment of glybenclamide, suggesting that Ver–CA may exert its antitussive effect partially through ATP-sensitive K+ channels. Thus, the antitussive effect of Ver–CA may depend on both the peripheral (modulated by ATP-sensitive K+ channels) and central mechanisms (modulated by the opioid receptor). Moreover, the acute toxicity of Ver–CA was only one-third that of verticin-one. Thus, the salifying derivative appeared to have a synergism and attenuated toxic effects compared to single verticinone and cholic acid. Therefore, our present data, if confirmed for other animal species and for humans, may suggest a novel approach to the symptomatic treatment of coughing derived from a traditional Chinese formulation. In addition, further attention should be paid to the water solubility of the compound which would be a versatile choice in future preparations.
We had elucidated that the antitussive mechanism of the total alkaloids from Fritillariae hupehensis was central through the experimental cough model elicited in pentobarbital-anesthetized cats by electrical stimulation of the superior laryngeal nerve, which had been used to determine the site of antitussive drugs[19,20]. So the central and peripheral mechanisms of Ver–CA may be a result of the synergism of verticinone and cholic acid. Our present study could also provide evidence that Fritillariae combined with snake bile in Shedan–Chuanbei powder has a sound scientific base.
References
- Schappert SM. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 1995. Vital Health Stat 13 1997;129:1-38.
- Dicpinigaitis PV. Potential new cough therapies. Pulm Pharmacol Ther 2004;17:459-62.
- Yuan R, Lin Y. Traditional Chinese medicine: an approach to scientific proof and clinical validation. Pharmacol Ther 2000;86:191-8.
- Jia W, Gao WY, Yan YQ, Wang J, Xu ZH, Zheng WJ, et al. The rediscovery of ancient Chinese herbal formulas. Phytothe Res 2004;18:681-6.
- Lin G, Li P, Li SL, Chan SW. Chromatographic analysis of Fritillaria isosteroidal alkaloids, the active ingredients of Beimu, the antitussive traditional Chinese medicinal herb. J Chromatogr A 2001;935:321-38.
- Qian BC, Xu HJ. Studies on the antitussive and sedative activities of peimine and peiminine. Acta Pharm Sin 1985;20:306-8. Chinese..
- Yeh YH, Wang DY, Liau MY, Wu ML, Deng JF, Noguchi T, et al. Bile acid composition in snake bile juice and toxicity of snake bile acids to rats. Comp Biochem Phys C 2003;136:277-84.
- Wei XC. Determination of the contents of verticinone in Shedan-Chuanbei powder. J Chin Med Meter 1999;22:420-1. Chinese..
- Wu WY. Determination of the contents of taurocholate in Shedan-Chuanbei powder. J Chin Med Meter 1998;21:419-20. Chinese..
- Forsberg K, Karlsson JA, Theodorsson E, Lundberg JM, Persson CGA. Cough and bronchoconstriction mediated by capsaicin-sensitive sensory neurons in the guinea-pig. Pulm Pharmacol 1988;1:33-9.
- Bliss CI. The calculation of the dosage-mortality curve. Ann Appl Biol 1935;22:134-67.
- Adcock JJ, Schneider C, Smith TW. Effects of codeine, morphine and a novel opioid pentapeptide BW443C, on cough, nociception and ventilation in the unanaesthetized guinea pig. Br J Pharmacol 1988;93:93-100.
- Hu XM, Shi CZ. A preliminary pharmacodynamic study on the preparation of artificial Fel Serpentis and Bulbus Fritiliariae Cirrhosae. Tradit Chin Drug Res Clin Pharmacol 2001;12:95-7. Chinese..
- Zhao RY, Fan CF, Fan XD. Experimental pharmacodynamic study of Bear Gall and bulbs of Fritillariae Ussuriensis oral liquid. Chin J Tradit Med Sci Tech 1999;6:151-3. Chinese..
- Zhang P. A preclinical pharmaceutical research of the total alkaloids from bulbs of and its preparation [PhD Dissertation]. Wuhan: Tongji Medical College, Huazhong University of Science and Technology; 2006. Chinese.
- Gallico L, Borghi PJ, Dalla RC, Ceserani R, Tognella S. Moguisteine: a novel peripheral non-narcotic antitussive drug. Br J Pharmacol 1994;112:795-800.
- Morikawa T, Gallico L, Widdicombe JG. Actions of moguisteine on cough and pulmonary rapidly adapting receptor activity in the guinea pig. Pharmacol Res 1997;35:113-8.
- Morita K, Kamei J. Involvement of ATP-sensitive K+ channels in the antitussive effect of moguisteine. Eur J Pharmacol 2000;395:161-4.
- Gordon R, Gordon RJ, Sofia D. Antitussive activity of some naturally occurring cannabinoids in anesthetized cats. Eur J Pharmcol 1976;35:309-13.
- Baekey DM, Morris K, Gestreau C, Li Z, Lindsey B, Shannon R. Medullary respiratory neurones and control of laryngeal motoneu-rones during fictive eupnoe and cough in the cat. J Physiol 2001;534:565-81.