Protective effect of raloxifene on lipopolysaccharide and acid- induced acute lung injury in rats
Introduction
Almost 40 years have passed since the clinical symptoms of acute lung injury (ALI) and its more severe form, acute respiratory distress syndrome (ARDS), were first described by Ashbaugh et al in 1967[1]. Despite the strategies for treating ALI/ARDS being extensively investigated, the overall mortality of ALI/ARDS still remains high at approximately 30%–70%[2]. Treatment options are limited and most of them are supportive; the only intervention that can reduce the mortality rate is the use of low tidal volume mechanical ventilation[3]. While drug therapy for ARDS/ALI is disappointing, to date, no pharmacological intervention has been proven to be effective to the survival rate of ARDS/ALI patients clinically[4]. Extensive neutrophil influx into the lungs, the production of pro-inflammatory mediators, and damage to lung epithelial and endothelial surfaces are the main characteristics of ALI[5,6].
Clinically, the development of ALI/ARDS is complex and it is rare for only 1 single instigating factor to cause ALI/ARDS[7,8]. Many researchers suggest that 2-hit models are more appropriate to reflect the real situation present in ALI patients[9–12]. Our previous results have shown that rats with lipopolysaccharide (LPS) intraperitoneal (ip) injection and HCl aspiration had significant lung injury when compared to the saline solution control group (only LPS injection group, or only HCl instillation group).
Raloxifene, a selective estrogen receptor modulator (SERM), has now been widely used in the prevention and treatment of osteoporosis in postmenopausal women[13]. Recently, raloxifene has been reported to inhibit interleukin-6 (IL)-6 production as well as having an anti-inflammatory role in a murine model induced by LPS[14,15]. It was also found to have protective effects on the development of carrageenan-induced edema and pleurisy[16]. Although early studies have shown that estrogens have some salutary effects on ALI, and these effects may be mediated via ER-β[17,18], we did not come across raloxifene usage or experimental studies on ALI/ARDS. In the present study, we used the 2-hit model to investigate whether raloxifene had some protective effects on ALI.
Materials and methods
Animals Male Sprague-Dawley rats (Grade II), each weighing between 180 and 210 g, were purchased from the Animal Center of Zhejiang University school of Medicine (Hangzhou, China). All the animals were housed in air-filtered, temperature-controlled units with access to food and water ad libitum. All experimental protocols were approved by the animal care committee and all experiments were done in conformity with the Guiding Principles for Research Involving Animals of Zhejiang University School of Medicine.
Experimental protocol The rats were divided into 3 groups: the raloxifene-LPS-HCl group (n=10), LPS-raloxifene-HCl group (n=10), and the placebo group (n=10). All the rats were challenged with ip administration of LPS (Escherichia coli, serotype 0111, B4, Sigma, St Louis, MO, USA) at the dose of 5 mg/kg. Raloxifene (Evista, Lilly, SA, Spain) was solubilized in normal saline solution and was orally administered 1 h before and 14 h after the LPS ip injection into the raloxifene–LPS–HCl and LPS–raloxifene–HCl groups at the dose of 30 mg/kg, respectively. Administration was done by gentle gavage with a ball-tipped (18 gauge) needle, while the placebo group received nothing. Sixteen hours after the LPS ip injection, all the animals were anesthetized with an ip injection of sodium pentobarbital (40 mg/kg) and placed in a 60° inclined position. The femoral artery was cannulated and connected to a pressure transducer to record the mean arterial pressure (MAP) on a polygraph recorder (Mindary Company, Shenzhen, China). The trachea was surgically exposed and all the animals received a direct intratracheal (IT) injection of HCl (pH 1.2; 0.5 mL/kg). Blood gas samples (0.3 mL each) were obtained before HCl instillation and at 30, 90, 240 min after the instillation and replaced by the same volume of saline solution. The samples were analyzed using a blood gas analyzer (OMNI C, Roche, Roswell, GA, USA).
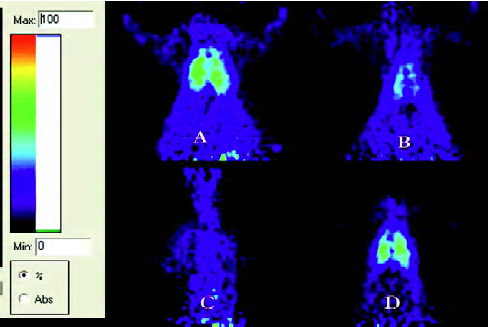
MicroPET examination Two hundred and ten minutes after HCl IT, 15 rats (5 in each group, respectively) were randomly selected and underwent microPET examination. PET was performed using a microPET R4 rodent model scanner (CTI Concorde Microsystems, Knoxville, TN, USA), which was equipped with a microPET manager for data acquisition in the list mode and Acquisition Sinogram and Image Processing (ASIPro) for preparing sinograms and image reconstruction. The scanner had a computer-controlled bed, a 10.8 cm transaxial, and a 7.8 cm axial field of view (FOV) with image resolution at <1.8 mm. Fluorodeoxyglucose (FDG) was prepared with a specific activity of 500 Ci/mmol at the Department of Nuclear Medicine, Zhejiang University School of Medicine. Before the examination, rats were re-anesthetized and injected with 10.56 MBq (0.3 mCi) FDG through the femoral artery cannulation, then 30 min later, the rats were placed at the center of the FOV of the microPET R4 scanner and underwent a 10 min static examination. The images were reconstructed by a maximum-a-posteriori probability (MAPP) algorithm. The ratio of the regions of interest (ROI) in the right lung to the muscle was calculated for each scan using ASIPro. These ROI were drawn and arranged by one of the authors who had extensive experience in manual ROI definition and was blinded to the results. The corrections for dead time, random scattering, and attenuation were performed for all the scans.
Wet/dry weight ratio At the end of the experiment, all the animals were killed by an injection of sodium pentobarbital. The right lung was excised and weighed, placed in an oven at 50 oC for 24 h, and then reweighed to determine the wet/dry (W/D) weight ratio. The left lung was used for the histopathological examination.
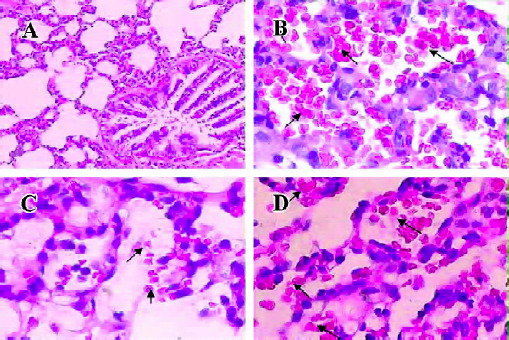
Histology The left lung was placed in 4% formalin, embedded in paraffin, and stained with hematoxylineosin (HE). According to an arbitrary 4-grade scale[19], all the sections were examined and graded by a pathologist who knew nothing about the experimental conditions of each individual animal. Briefly, the sections were assessed by the airway epithelial necrosis, intra-alveolar edema, hyaline membranes, hemorrhage, and the recruitment of inflammatory cells to the air space. Each characteristic was scored 0 to 3 (0=absent; 1=mild; 2=moderate; and 3=prominent).
Statistical analysis Data were expressed as mean±SD and analyzed by SPSS 13.0 statistical software (SPSS, Chicago, IL, USA). ANOVA with repeated measurements analysis was used to compare samples obtained at several time-points from the same animals. Independent samples t-test was used to determine which group was significantly different. The correlation between 2 variables was measured by Bivariate test. A P-value less than 0.05 was considered statistically significant.
Results
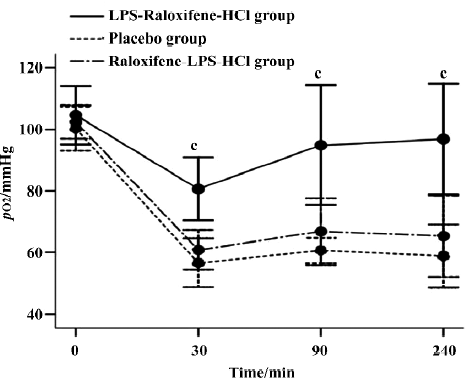
Blood gas analysis of pO2 The pO2 remained normal and there were no differences between the 3 groups immediately prior to tracheal acid instillation. As expected, the pO2 showed an initial decline in all animals 30 min after acid instillation, but the pO2 in the placebo group decreased more markedly and there were significant differences compared with that in the LPS-raloxifene-HCl group (P<0.01). As time passed, the pO2 in the LPS-raloxifene-HCl group slowly returned to normal ranges[20] (pO2: 80–120 mmHg; pCO2: 35–45 mmHg; pH: 7.40–7.50; and MAP: 70–110 mmHg), while that of the placebo group at the end of the experiment was 58.81±10.27 mmHg (Figure 1).
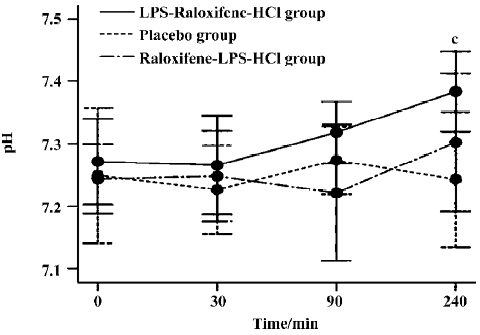
pH The pH values differed between the LPS-raloxifene-HCl group and the placebo group (P<0.05). In the placebo group, the pH value remained below the normal range, although it reached 7.273±0.054 at 90 min after instillation, while that of the LPS-raloxifene-HCl group gradually returned to the normal range after acid instillation and finally reached 7.380±0.064 (7.243±0.108 in the placebo group). However, there were no significant differences between the raloxifene-LPS-HCl group and the placebo group (Figure 2).
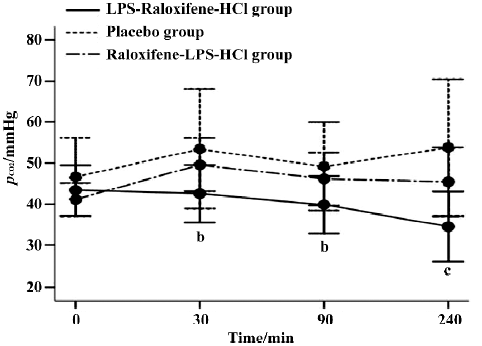
pCO2 In the LPS-raloxifene-HCl group, the average value of the pCO2 was 40.07±7.79 mmHg, while in the placebo group that value was 50.75±13.03 mmHg, much higher than that in LPS-raloxifene-HCl group (P<0.05; Figure 3). There were also no significant differences between the raloxifene-LPS-HCl group and the placebo group.
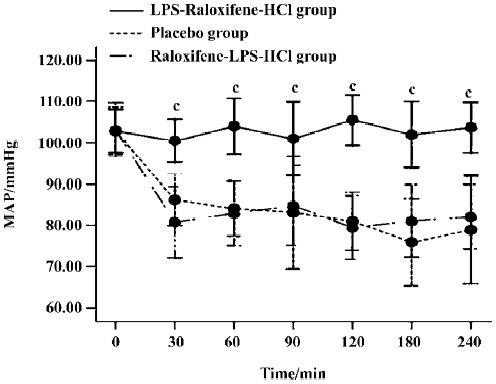
MAP There were no differences between the three groups in initial measurements of MAP. However, after HCl instillation, MAP in the LPS-raloxifene-HCl group remained stable, while MAP in placebo-treated group and raloxifene-LPS-HCl group showed a marked decrease. There were significant differences between the LPS-raloxi-fene-HCl group and the placebo group (P<0.01; Figure 4).
MicroPET The ratio of ROI in the right lung to the muscle was 9.01±1.58 in the placebo group, significantly higher than that (4.67±1.33) of the LPS-raloxifene-HCl group (P<0.01; Figure 5). There were no significant differences between the raloxifene-LPS-HCl group and the placebo group.
W/D weight ratio The W/D weight ratio was 5.886±0.257 in the placebo group, 5.335±0.198 in the LPS-raloxifene-HCl group, and 5.766±0.203 in the raloxifene-LPS-HCl group. There were significant differences between the placebo group and the LPS-raloxifene-HCl group (P<0.01).
Histology An histological examination revealed lung injury of varying degrees in all animals in the 3 groups, but were more prominent in the placebo group. Briefly, the mean lung injury score in the placebo group was 12.6±0.97, much higher than that (8.20±1.23) of the LPS-raloxifene-HCl group (P<0.01). That score in the raloxifene-LPS-HCl group was 11.4±1.65, no significant difference when compared with the placebo group (Figure 6).
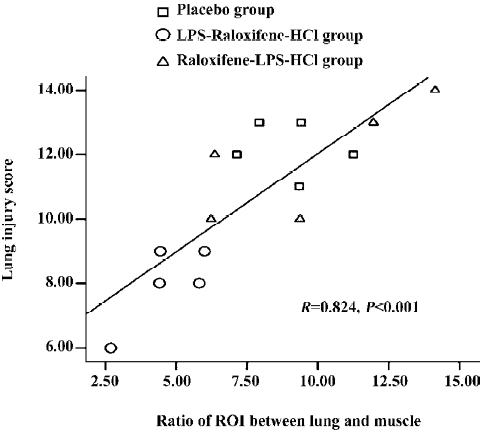
Correlation The ratio of the ROI between the right lung and the muscle correlated significantly with the histological lung injury score (r=0.824, P<0.001; Figure 7).
Discussion
In acute lung injury, the predominant inflammatory cells are neutrophils[21], which play an important role in the development of most cases of ALI[22] . When acute lung injury occurs, neutrophils adhere to the injured capillary endothelium, marginate into the air spaces, and are stimulated by cytokines, such as TNF-α. The activated neutrophils in return begin to release leukotrienes, oxidants, proteases, and other inflammatory mediators[21,23]. In short, it is the adhesion and activation of neutrophils that produce lung injury[24,25]. The activated neutrophils also take up [18F]FDG at an accelerated rate after [18F]FDG injection, thus generating a PET imaging signal[26]. As can be seen in the present study, an increasing number of reports are appearing in the field[27–30]. [18F]FDG PET/CT has become a useful tool for evaluating pulmonary lesions. Furthermore, the pulmonary transcapillary escape rate for 68 Ga-transferrin obtained by PET has been shown to be the only method which correlates with lung damage histopathologically[31,32], although PET is expensive and not readily available at all times or at all institutions.
One of the most commonly used procedures to quantify neutrophils during ALI is bronchoalveolar lavage (BAL), but BAL can only detect neutrophils which penetrate into the air spaces. Other activated neutrophils may be neglected, furthermore, part of the neutrophils in BAL might not be activated and are not involved in the inflammatory process. So when the number of neutrophils in BAL is used as the indicator of activated neutrophils, it is inaccurate and may underestimate the influx of neutrophils in the lungs, while [18F]FDG PET imaging is more accurate when at least using increased glucose uptake as the criterion for neutrophil activation[26]. The expression of CD11b, which is a marker of neutrophil activation in vivo during ALI[33], by flow cytometry examination again is less accurate compared with [18F]FDG PET because it may also neglect the activated neutrophils which do not penetrate into the air spaces. Furthermore, BAL is invasive, while [18F]FDG PET is non-invasive and can be easily accepted clinically.
Raloxifene, a benzothiophene derivative which has anti-estrogenic properties in breast tissues, a neutral effect on the endometrium, and potentially beneficial estrogen-like effects in non-reproductive tissues, such as bone[34], was also shown to have an anti-inflammatory role in acute inflammation. Esposito et al[16] demonstrated that raloxifene plays a protective role in the development of carrageenan-induced edema and pleurisy. They speculated that this effect may be caused by reducing inducible pro-inflammatory enzymes (COX-2 and iNOS) and by decreasing the infiltration of neutrophils into the inflamed paw and pleural cavity. Suuronen et al[15] showed that SERM, raloxifene, and tamoxifen, but not 17β-estradiol, suppressed the secretion of IL-6 and nitric oxide and induced a significant, concentration-dependent anti-inflammatory response both in rat primary microglial cells and in mouse N9 microglial cells which were induced by LPS.
In the present study, we found that raloxifene also had an anti-inflammatory role during ALI. The results of the [18F]FDG microPET showed that the rats in the placebo group had significant influx of activated neutrophils in the lungs, while the rats receiving raloxifene treatment 2 h before the instillation of HCl exhibited diminished influx. It is well known that leukocyte activation at the site of inflammation is fundamental in the inflammatory process and raloxifene exerted a marked inhibition on leukocyte infiltration and activation in this 2-hit acute lung injury model. Oral raloxifene can also maintain MAP stability and improve pulmonary gas exchange. The W/D weight ratio, indicating pulmonary edema, was much lower in the LPS-raloxifene-HCl group. These data indicate that the oral administration of raloxifene offers a statistically significant protective effect on ALI.
Findings in the histological examination also showed that raloxifene had significant anti-inflammatory effects in the 2-hit model when administered 2 h before HCl IT. Neutrophil infiltration, hemorrhage, hyaline membranes, alveolar edema, and airway epithelial necrosis were common and prominent in the placebo group, but were rare in the LPS-raloxifene-HCl group. Furthermore, our findings also showed that there was a significant correlation between [18F]FDG uptake and lung damage histopathologically. Therefore, we concluded that the degree of histological lung injury can be roughly assessed with [18F]FDG PET.
The half-decay period of raloxifene in human is 27.7 h, but it is much shorter in rats. Therefore, there were no best blood drug level in the rats of the raloxifene-LPS-HCl group and raloxifene showed no protective effect on ALI at 17 h after administration. The pharmacology of raloxifene is very complex. It has recently been reported that raloxifene could block NF-κB biological activity through the modulation of the ER-α association with p65 in multiple myeloma[35], and because ER-α are also highly expressed in lung tissues[36], we hypothesized that raloxifene can also suppress NF-κB activity during ALI through the modulation of ER-α and thus exert a protective effect on ALI. This hypothesis needs to be further studied and verified.
In conclusion, oral raloxifene 2 h before HCl instillation can decrease neutrophil influx, suppress neutrophil activa-tion, improve pulmonary gas exchange, and maintain MAP stability in the 2-hit ALI model, which may therefore have an important therapeutic role on ALI patients clinically. Furthermore, the oral administration was of interest since oral use by humans is desirable compared to other methods of drug administration.
References
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet 1967;2:319-23.
- Isik AF, Kati I, Bayram I, Ozbek H. A new agent for treatment of acute respiratory distress syndrome: thymoquinone. An experimental study in a rat model. Eur J Cardiothorac Surg 2005;28:301-5.
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8.
- Jain R, DalNogare A. Pharmacological therapy for acute respiratory distress syndrome. Mayo Clin Proc 2006;81:205-12.
- Jeyaseelan S, Chu HW, Young SK, Freeman MW, Worthen GS. Distinct roles of pattern recognition receptors CD14 and Toll-like receptor 4 in acute lung injury. Infect Immun 2005;73:1754-63.
- Piantadosi CA, Schwartz DA. The acute respiratory distress syndrome. Ann Intern Med 2004;141:460-70.
- Lang JD, Hickman-Davis JM. One-hit, two-hit….is there really any benefit? Clin Exp Immunol 2005;141:211-4.
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334-49.
- Welty-Wolf KE, Carraway MS, Miller DL, Ortel TL, Ezban M, Ghio AJ, et al. Coagulation blockade prevents sepsis-induced respiratory and renal failure in baboons. Am J Respir Crit Care Med 2001;164:1988-96.
- Fan J, Kapus A, Li YH, Rizoli S, Marshall JC, Rotstein OD. Priming for enhanced alveolar fibrin deposition after hemorrhagic shock: role of tumor necrosis factor. Am J Respir Cell Mol Biol 2000;22:412-21.
- Fan J, Kapus A, Marsden PA, Li YH, Oreopoulos G, Marshall JC, et al. Regulation of Toll-like receptor 4 expression in the lung following hemorrhagic shock and lipopolysaccharide. J Immunol 2002;168:5252-9.
- Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 2003;167:1027-35.
- Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999;282:637-45.
- Viereck V, Grundker C, Blaschke S, Niederkleine B, Siggelkow H, Frosch KH, et al. Raloxifene concurrently stimulates osteoprotegerin and inhibits interleukin-6 production by human trabecular osteoblasts. J Clin Endocrinol Metab 2003;88:4206-13.
- Suuronen T, Nuutinen T, Huuskonen J, Ojala J, Thornell A, Salminen A. Anti-inflammatory effect of selective estrogen receptor modulators (SERMs) in microglial cells. Inflamm Res 2005;54:194-203.
- Esposito E, Iacono A, Raso GM, Pacilio M, Coppola A, Di Carlo R, et al. Raloxifene, a selective estrogen receptor modulator, reduces carrageenan-induced acute inflammation in normal and ovariectomized rats. Endocrinology 2005;146:3301-8.
- Speyer CL, Rancilio NJ, McClintock SD, Crawford JD, Gao H, Sarma JV. al. Regulatory effects of estrogen on acute lung inflammation in mice. Am J Physiol Cell Physiol 2005;288:C881-90.
- Yu HP, Hsieh YC, Suzuki T, Shimizu T, Choudhry MA, Schwacha MG, et al. Salutary effects of estrogen receptor-β agonist on lung injury after trauma-hemorrhage. Am J Physiol Lung Cell Mol Physiol 2006;290:L1004-9.
- Lu KW, William Taeusch H, Robertson B, Goerke J, Clements JA. Polymer-surfactant treatment of meconium-induced acute lung injury. Am J Respir Crit Care Med 2000;162:623-8.
- Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke 2003;34:2964-9.
- Cepkova M, Matthay MA. Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. J Intensive Care Med 2006;21:119-43.
- Abraham E. Neutrophils and acute lung injury. Crit Care Med 2003;4 Suppl 31:S195-9.
- Weinacker AB, Vaszar LT. Acute respiratory distress syndrome: physiology and new management strategies. Annu Rev Med 2001;52:221-37.
- Nemzek JA, Call DR, Ebong SJ, Newcomb DE, Bolgos GL, Remick DG. Immunopathology of a two-hit murine model of acid aspiration lung injury. Am J Physiol Lung Cell Mol Physiol 2000;278:L512-20.
- Goldman G, Welbourn R, Klausner JM, Kobzik L, Valeri CR, Shepro D, et al. Leukocytes mediate acid aspiration-induced multiorgan edema. Surgery 1993;114:13-20.
- Chen DL, Schuster DP. Positron emission tomography with [18F]fluorodeoxyglucose to evaluate neutrophil kinetics during acute lung injury. Am J Physiol Lung Cell Mol Physiol 2004;286:L834-40.
- Love C, Tomas MB, Tronco GG, Palestro CJ. FDG PET of infec- tion and inflammation. Radiographics 2005;25:1357-68.
- Rini JN, Palestro CJ. Imaging of infection and inflammation with 18F-FDG-labeled leukocytes. Q J Nucl Med Mol Imaging 2006;50:143-6.
- Vos FJ, Bleeker-Rovers CP, Corstens FH, Kullberg BJ, Oyen WJ. FDG-PET for imaging of non-osseous infection and inflammation. Q J Nucl Med Mol Imaging 2006;50:121-30.
- Jacene HA, Cohade C, Wahl RL. F-18 FDG PET/CT in acute respiratory distress syndrome: a case report. Clin Nucl Med 2004;29:786-8.
- Velazquez M, Weibel ER, Kuhn CD, Schuster DP. PET evaluation of pulmonary vascular permeability: a structure-function correlation. J Appl Physiol 1991;70:2206-16.
- Schuster DP, Stark T, Stephenson J, Royal H. Detecting lung injury in patients with pulmonary edema. Intensive Care Med 2002;28:1246-53.
- Saxton JM, Pockley AG. Effect of ex vivo storage on human peripheral blood neutrophil expression of CD11b and the stabilizing effects of Cyto-Chex. J Immunol Methods 1998;214:11-7.
- Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science 2002;295:2465-8.
- Olivier S, Close P, Castermans E, de Leval L, Tabruyn S, Chariot A, et al. Raloxifene-induced myeloma cell apoptosis: a study of nuclear factor-kappaB inhibition and gene expression signature. Mol Pharmacol 2006;69:1615-23.
- Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, et al. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab 1997;82:4258-65.