Purification and partial characterizations of coagulant protein FIa from Daboia russelli siamensis (Myanmar) venom1
Introduction
Snake venoms are complex mixtures containing many different biologically active proteins and peptides. A number of these proteins affect the mammalian coagulation system by cleaving limited bonds in the blood coagulation factors. Factor V, factor X, prothrombin activators, and thrombin-like enzymes have been separated from different snake venoms[1–3]. Among those, snake venoms of the Viperinae family have been reported to contain strong procoagulants. Because of their biological activities, some of these venom proteins are useful for basic studies of hemostasis and thrombosis and for pharmacological and clinical applications[16]. Daboia russelli siamensis is widely found over southern China, central and southern Myanmar, and central Thailand. Although we have known that the venom of Daboia russelli siamensis also contains some hemostatic fractions, studies about the hemostatic effect and procoagulant mechanism of Daboia russelli siamensis (Myanmar) venom are minimal.
In the present investigation, we purified the hemostatic fraction FIa from Daboia russelli siamensis (Myanmar) venom, determined the hemostatic activities of FIa and investigated the mechanism of hemostasia and the potential application of FIa.
Materials and methods
Snake venom Daboia russelli siamensis (Myanmar) venom was purchased from Guangzhou Medical College (Guangzhou, China) and lyophilized and stored in a desiccator.
Reagents CM-Sephadex C-50, Sephadex G-75, and Superdex 75 were purchased from Pharmacia (Uppasala, Sweden). Human fibrinogen and thrombin were purchased from Sigma (St Louis, MO, USA). Factor X, factor Xa, prothrombin, and the reagent packs for the activity assays of factor Xa, prothrombin, and thrombin were purchased from Merck (Darmstadt, Germany). All other reagents were of analytical grade and obtained from commercial sources (Guangzhou, China).
Purification of FIa from Daboia russelli siamensis (Myanmar) venom
CM-Sephadex C-50 ion exchange chromatography Daboia russelli siamensis (Myanmar) venom (1.0 g) was dissolved in 0.5 mol/L ammonium acetate (pH 5.8), and the supernatant was applied to a column (2.0 cm×80 cm) of CM-Sephadex C-50. The fractions were eluted with a linear gradient consisting of 0.5 mol/L ammonium acetate (pH 5.8) as the starting buffer and 1 mol/L ammonium acetate (pH 8.0) as the limit buffer. The absorbance of the eluates was measured and then the hemostatic fraction was dialyzed and lyophilized.
Sephadex G-75 gel filtration Gel filtration was performed on a Sephadex G-75 column (2.0 cm×100 cm) equilibrated with 0.02 mol/L sodium phosphate buffer (pH 7.4). The first fraction (FI, 100 mg) from CM-Sphadex C-50 chromatography was eluted with the same buffer.
Superdex 75 gel filtration Gel filtration was performed on a Superdex 75 column (1.0 cm×80 cm) equilibrated with 0.02 mol/L sodium phosphate buffer (pH 7.4). The first fraction (FIa, 30 mg) from Sephadex G-75 gel filtration by gel filtration was eluted with the same buffer.
Molecular weight and isoelectric point determination SDS-PAGE was performed according to the method of Laemmli[17]. The molecular weight standards were MBP-G-galactosidase (175 000), MBP-paramyosin (83 000), glutamic dehydrogenase (62 000), aldolase (47 500), triosephosphate isomerase (32 500), G-lactoglobulin A (25 000), lysozyme (16 500), and aprotinin (6 500). Isoelectrofocusing–PAGE was carried out by the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China), with a pH gradient of 3–10 generated by ampholine (pH 3–10; Amersham Biosciences, Uppsala, Sweden).
Measurement of hemostatic activities Three different groups were established at various concentration intervals. The FIa groups were given 0.5, 0.25, 0.125, 0.625, and 0.3125 mg/100 µL and each concentration was tested 6 times. Thrombin was used as a positive control and 25, 12.5, 6.25, 3.125, 1.5625, 0.78125, and 0.390625 U/100 µL thrombin was given. Each concentration was also tested 6 times. Normal saline was used as a negative control.
The hemostatic activities of thrombin and FIa were measured according to the coagulation time determined by the method of Williams and Esnouf[4]. The citrated plasma (100 µL) was incubated with 100 µL of 0.01 mol/L Tris-HC1 buffer (pH 7.3) containing 0.15 mol/L NaCl at 37 °C for 3 min. 100 µL of 0.05 mol/L CaCl2 plus thrombin (100 µL) or FIa (100 µL) were added to the pre-incubation mixture respectively. The clotting time of the plasma was then recorded.
Effect of FIa on human blood factor X Using the method of Gowda et al[6], the activation of purified human factor X was followed by using the FXa differential chromogenic substance. Purified human blood factor X (100 U) was incubated at 37 °C in 50 mmol/L Tris-HCl (pH 7.4) containing 0.1 mol/L NaCl, 0.01 mol/L CaCl2, and 12 µg of FIa in a total reaction volume of 500 µL. Aliquots (50 µL) were removed at various times. 50 µL of FXa differential chromogenic substance solution [dissolved in 50 mmol/L Tris-HCl (pH 7.4) containing 0.1 mol/L NaCl (final concentration of 0.2 mmol/L) was added into the aliquots. The light absorbance was recorded at 405 nm. FXa was used as a positive control and normal saline was used as a negative control.
Effect of FIa on human prothrombin Using the method of Hofmann and Bon[7], the activation of the purified prothrombin was followed by using the thrombin differential chromogenic substance. Purified human prothrombin (6.67 µg/µL) was incubated at 37 °C in 50 mmol/L Tris-HCl (pH 7.4) containing 0.1 mmol/L NaCl and 10 mmol/L CaCl2 with FIa. Aliquots (50 µL) were removed at various times. 450 µL of thrombin differential chromogenic substance solution [dissolved in 50 mmol/L Tris-HCl (pH 7.4) containing 0.1 mol/L NaCl] was added into the aliquots. The light absorbance was recorded at 405 nm. Thrombin was used as a positive control and normal saline was used as a negative control.
Effect of FIa on fibrinogen Using the method of Gao et al[8], fibrinogen-clotting activity was determined by mixing 20 µL of FIa (concentrations were 0.5, 0.25, 0.125, 0.0625, and 0.02125 mg/100 µL, respectively) with 200 µL of human fibrinogen solution (3 mg/mL) in 50 mmol/L Tris-HCl buffer (pH 7.4) and 0.1 mol/L NaCl (containing 10 mmol/L CaCl2) and incubated at 37 °C. The clotting time was recorded. Thrombin was used as a positive control and normal saline was used as a negative control.
Results
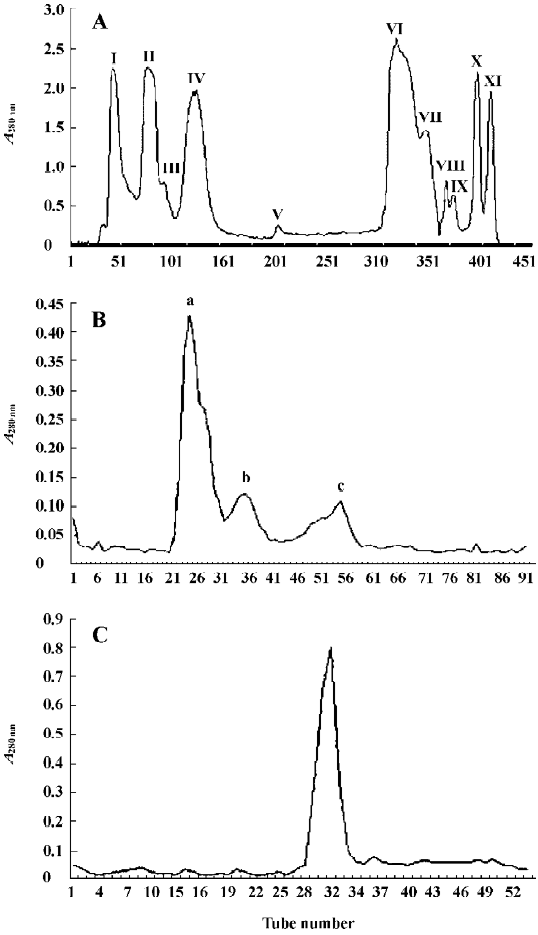
The isolation of FIa was achieved by using 3 steps of purification (Figure 1A–1C). Ion exchange chromatography of crude venom using a column of CM-Sephadex C-50 yielded 11 fractions (Figure 1A). The first fraction (FI) had the highest hemostatic activity. Its hemostatic activity, expressed by the thrombin coagulation time, was 430±5 s/31.25 µg (n=6). Further chromatography of FI using a column of Sephadex G-75 resulted in 3 fractions: Fa, Fb, and Fc (Figure 1B). The hemostatic activity of Fa was 350±5 s/31.25 µg (n=6), which was higher than that of Fb and Fc. The collection of the sharp peak of Fa was then applied to a Superdex 75 column. The single peak obtained was named FIa (Figure 1C). The hemostatic activity of FIa was 290±5 s/31.25 µg (n=6). From 1 g of crude venom, 10.75 mg of FIa was obtained.
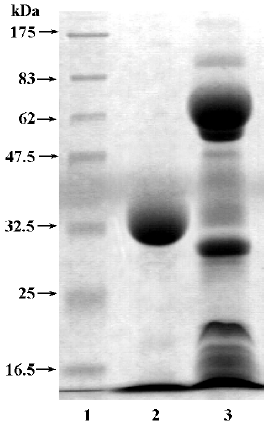
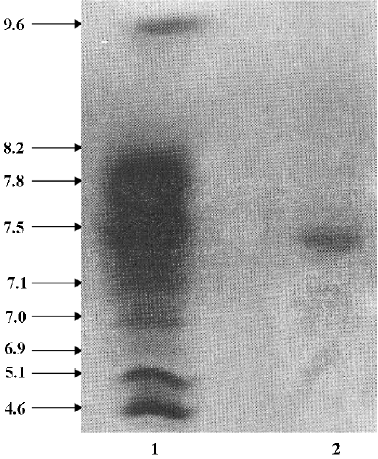
FIa was found to be electrophoretically homogeneous by SDS-PAGE (Figure 2) and isoelectric focusing (Figure 3). SDS-PAGE analysis gave a calculated molecular weight of 34 479. Isoelctric focusing studies indicated that FIa was a neutral protein with a pI of 7.2.
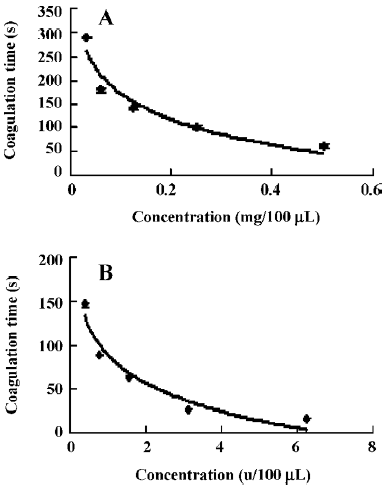
The hemostatic effect of purified enzyme FIa and thrombin was determined by coagulation time. The clotting time of normal human plasma was 960±5 s (n=6). FIa (100 µL) at several different dosages, 0.5, 0.25, 0.125, 0.0625, and 0.03125 mg, was applied. The clotting times of FIa were 60±5 s, 100±5 s, 142±3 s, 180±4 s, and 290±2 s, respectively (n=6). It was shown the clotting times increased with the decreased dosages (Figure 4A). The hemostatic effect of thrombin (100 µL) at different dosages (5, 12.5, 6.25, 3.125, 1.5625, 0.78125, and 0.390625 u, was also determined. The clotting times of thrombin were 6.88±0.1 s, 12.13±0.15 s, 16.52±0.08 s, 26.56±0.21 s, 64±1.2 s, 89±1.4 s, and 146±3 s, respectively (n=6). It was shown that the clotting times also increased with the decreased dosages of thrombin (Figure 4B).
Purified enzyme FIa from Daboia russelli siamensis (Myanmar) venom readily cleaves a number of commercially available chromogenic substrates that have been designed for human blood factor X. The parameters for chromogenic substrate conversion by the venom activator are higher than by normal saline (Figure 5). It was shown that FIa can active factor X to factor Xa. However, in the test of chromogenic substrates to prothrombin, the parameters of FIa had no change compared to the normal saline group (Figure 6). It showed that FIa had no catalytic efficiency on protrombin.
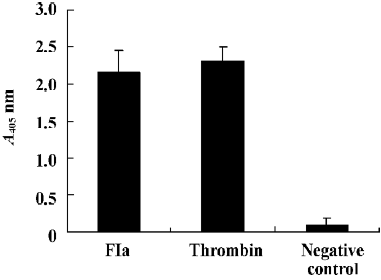
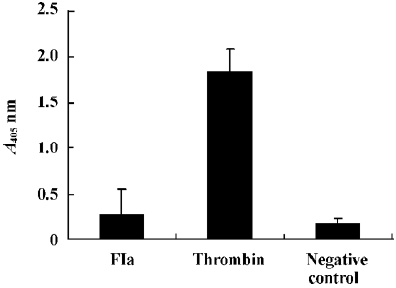
In the test of fibrinogen-clotting activity, we found that FIa at dosages of up to 0.5 mg/100 µL could not coagulate human fibrinogen, which was the same with the normal saline group. It showed that FIa had no effect on human fibrinogen.
Discussion
Studies on Russell’s viper venoms have been conducted for a long time. It is known that fractions of Russell’s viper venom exhibit a number of hemostatic activities such as the proteases from Russell’s viper venom that activate factor X, factor V, and prothrombin[9–12].
In this paper, we have described the purification and characterization of the factor X-activating fraction FIa from Daboia russelli siamensis (Myanmar) venom.
With a 3-step procedure, we obtained a purified factor X-activating fraction FIa. Its molecular weight was 34 479 and its pI was 7.2, showing a difference when compared with RVV-X obtained by Esnouf and Williams[4]. It is also different from the factor X-activating fraction obtained by Yang et al from Thailand Daboia russelli siamensis[5]. Judging from the above facts, FIa is a new factor X-activating protein found from the venom of Daboia russelli siamensis (Myanmar) venom, or these difference is a different derivation of viper russellii. We found that FIa might shorten clotting time, and the hemostatic activity of 0.5 mg FIa was equal to that of 1.5625 u of thrombin.
We know that the activation of the extrinsic coagulation pathway initiated by an expression of prothrombinase (prothrombin, FXa) plays an important role in hemostasis. Factor Xa, the physiological activator of prothrombin, converts prothrombin to active thrombin. Thrombin directly activates platelets and cleaves fibrinogen to fibrin monomers[13,14]. In order to understand the relationship between FIa and this process, we determined the activating activities of FIa on factor Xa prothrombin in vitro. In light of our data, we found that FIa could increase the activity of factor X, which has a concentration-time relationship. However, we found that FIa could not affect the activity of prothrombin directly.
Fibrinogen and fibrin play essential roles in blood clotting. During coagulation, the soluble fibrinogen is converted to insoluble fibrin, and this process is initiated by thrombin[15]. In this study, we found that FIa had no effect on human fibrinogen in vitro. The results showed that FIa could not cleave or clot fibrinogen directly.
In conclusion, FIa is a factor X-activating enzyme, which could activate factor X to factor Xa, but has no effects on prothrombin and fibrinogen.
These activities indicate the potential therapeutic application of FIa for hemostasis. Therefore, the clinical application of FIa remains to be explored and evaluated. Moreover, because habitat, climate, age, and environment influence the venom ingredient and toxicity at different levels, the stability of natural venom is not ideal. It is more interesting to produce medical venom products by molecular cloning and expression. The N-terminus amino acid sequences of FIa have been determined, but the data are not shown in the present paper, which would be very useful in following studies.
Acknowledgement
We gratefully acknowledge the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences for technical assistance.
References
- Iwanaga S, Suzuki T. Enzymes in snake venom. In: Lee CY, editor. Handbook of experimental pharmacology. New York: Springer-Verlag; 1979. p 61–158.
- Morita T, Iwanaga S. Prothrombin activator from Echis carinatus venom. Methods Enzymol 1981;80:303-11.
- Kisiel W, Canfield WM. Snake venom proteases that activate blood-coagulation factor V. Methods Enzymol 1981;80:275-85.
- Williams WJ, Esnouf MP. The fractionation of Russell’s-viper (Vipera russellii) venom with special reference to the coagulant protein. Biochem J 1962;84:52-62.
- Yang LJ, Liu GF, Wang QC. Purificaiton and partial properties of blood coagulation factor X activator from the venom of Vipera russelli siamensis. J Fujian Med Univ 2002;36:242-6.
- Gowda DC, Jackson CM, Hensley P, Davidson EA. Factor X-activating glycoprotein of Russell’s viper venom. Polypeptide composition and characterization of the carbohydrate moieties. J Biol Chem 1994; 269: 10 644–50.
- Hofmann H, Bon C. Blood coagulation induced by the venom of Bothrops atrox. 1.Identification, purification, and properties of a prothrombin activator. Biochemistry 1987;26:772-80.
- Gao R, Manjunatha Kini R, Gopalakrishnakone P. A novel prothrombin activator from the venom of Micropechis ikaheka: isolation and characterization. Arch Biochem Biophys 2002;408:87-92.
- Lee CY. Toxicological studies on the venom of Vipera russelli formosensis, Maki. Part I. Toxicity and pharmacological pro-perties. J Formosan Med Assoc 1948;47:65-84.
- Schiffman S, Theodor I, Rapaport SI. Separation from Russell’s viper venom of one fraction reacting with factor X and another reacting with factor V. Biochemistry 1969;8:1397-405.
- Tans G, Govers-Riemslag JW, van Rijn JL, Rosing J. Purification and properties of a prothrombin activator from the venom of Notechis scutatus scutatus. J Biol Chem 1985;260:9366-72.
- Speijer H, Govers-Riemslag JW, Zwaal RF, Rosing J. Prothrombin activation by an activator from the venom of Oxyuranus scutellatus (Taipan snake). J Biol Chem 1986;261:13258-67.
- Rosing J, Tans G, Govers-Riemslag JW, Zwaal RF, Hemker HC. The role of phospholipids and factor Va in the prothrombinase complex. J Biol Chem 1980;255:274-83.
- Morita T, Iwanaga S, Suzuki T. The mechanism of activation of bovine prothrombin by an activator isolated from Echis carinatus venom and characterization of the new active intermediates. J Biochem (Tokyo) 1976;79:1089-108.
- Weisel JW, Stauffacher CV, Bullitt E, Cohen C. A model for fibrinogen: domains and sequence. Science 1985;230:1388-91.
- Fujimura Y, Kawasaki T, Titani K. Snake venom metallo-endopeptidases: reprolysis. Methods Enzymol 1996;24:345-68.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-5.
