Clonidine, moxonidine, folic acid, and mecobalamin improve baroreflex function in stroke-prone, spontaneously hypertensive rats
Introduction
Arterial baroreflex (ABR) is one of the most important regulatory mechanisms in the cardiovascular system. Baroreflex function, expressed as baroreflex sensitivity (BRS), is an important determinant for many cardiovascular diseases. Clinically, it has been proven that ABR dysfunction is associated with sudden death in patients with acute myocardial infarction and mortality in patients with congestive heart failure[1-4]. Our previous works demonstrated that ABR function was closely related to atheroscleosis and end-organ damage in hypertension[5,6]. Furthermore, ABR function also plays an important role in the onset and prognosis of stroke. There is established evidence of abnormal ABR function in both animal models of stroke[7,8] and patients with acute stroke[9-11]. Recently, a study within our department indicated that ABR function affected the survival time of stroke-prone spontaneously-hypertensive rats (SHR-SP). The study also found that restoring impaired ABR function could prevent stroke in hypertension, suggesting that BRS is a new and important predictor for stroke incidence and that the restoration of ABR function is a new target for the prevention of stroke[12]. Since ABR function is associated closely with stroke, it is of great clinical importance to discover drugs with the ability to improve impaired ABR function.
Clonidine and moxonidine, representing the first and second generation of antihypertensive drugs, act on the central nervous system. It was demonstrated that clonidine improved ABR function in conscious mice through parasympathetic activation[13]. However, little is known about the effect of moxonidine. Both folic acid (vitamin B9) and mecobalamin (methyl-vitamin B12) belong to the vitamin B group. During the past decade, interest in the health benefits of these has increased considerably. Recent interest of B vitamin focused on their beneficial effects on preventing cardiovascular diseases. Clinical studies indicated that folic acid improved ABR function in hypertensive patients[14]. In many cases, folic acid involved biochemical processes that required the participation of vitamin B12[15]. Therefore, we postulated that mecobalamin might improve ABR function in conscious SHR-SP.
This study was designed to examine the effects of clonidine, moxonidine, folic acid, and mecobalamin on impaired ABR function in SHR-SP and the possible mechanisms involved.
Materials and methods
Animals and drugs Female SHR-SP rats, aged 20−24 weeks, were provided by the Animal Center of the Second Military Medical University (Shanghai, China). The rats were housed under controlled temperatures (23–25 °C) and lighting (8:00–20:00 light, 20:00–8:00 dark) with free access to food and tap water. All the animals used in the experiment received humane care in compliance with institutional guidelines for health and care of experimental animals.
Clonidine (Sigma Chemical Co, St Louis, MO, USA), moxonidine (Yahu Pharmaceutical Co, Shanghai, China), folic acid (Fushu Pharmaceutical Co, Shanghai, China), mecobalamin (Shenyang Wanlong Pharmaceutical Co, Shenyang, China). All were dissolved with sterile saline and stored at 0–4 °C.
Blood pressure and heart period measurement Systolic blood pressure (SBP) and diastolic blood pressure (DBP), and heart period (HP) were continuously recorded using a previously described technique[16,17]. Briefly, the rats were anesthetized with a combination of ketamine (50 mg/kg, ip) and diazepam (5 mg/kg, ip). A floating polyethylene catheter was inserted into the lower abdominal aorta via the left femoral artery for blood pressure (BP) measurement. Another catheter was placed into the left femoral vein for BRS measurement. A stomach catheter was inserted when intragastric (ig) administration was needed or a stainless steel cannula (0.7 mm OD) was stereotaxically (1.0 mm posterior to bregma, 1.6 mm lateral from the midline, 4.0 mm below the surface of the skull) implanted into the lateral cerebral ventricle for intracerebroventricular (icv) injection. All the catheters were exteriorized through the interscapular skin. After a 2 d recovery period, the animals were placed in individual cylindrical cages containing food and water for BP recording. The aortic catheter was connected to a BP transducer via a rotating swivel that allowed the animals to move freely in the cage. After about 4 h habituation, the BP signal was digitized by a microcomputer and beat-to-beat SBP, DBP, and HP values were determined online. The mean values during a period of 30 min were calculated and served as the SBP, DBP, and HP.
Baroreflex sensitivity measurement BRS was determined using a previously described method[18]. The principle of this method was to measure the prolongation of HP in response to an elevation of BP. Briefly, a bolus injection of phenylephrine (2–5 mg/kg) was used to induce an elevation of SBP between 20–40 mmHg. HP was plotted against SBP for a linear regression analysis and the slope of SBP–HP was expressed as BRS (ms/mmHg). The mean value of 2 measurements with the proper dose served as the final result.
Experimental protocol Eighty one SHR-SP were randomly divided into 7 groups. Four groups were designated for the ig administration of clonidine (1.0 and 10.0 µg/kg), moxonidine (0.1 and 1.0 mg/kg), folic acid (1.0 mg/kg), and mecobalamin (1.0 mg/kg). Three groups were used for the icv injection of clonidine (4 µg/4 µL), moxonidine (5 µg/4 µL), and mecobalamin (20 µg/4 µL). After 4 h habitua-tion, basal (predrug) BP was recorded continuously for 30 min and BRS was determined. Thereafter, a single dose of drug was given via an ig catheter or stainless steel cannula as designated. One hour (ig moxonidine) or 30 min (the other drugs) later, the BP was recorded for another 30 min and BRS was determined again. The mean values of SBP, DBP, HP and BRS served as post-drug values. For the ig administration of clonidine or moxonidine, 2 doses were conducted in 1 rat, that is, the lower dose was administrated and post-drug values were calculated; 30 min later, the higher dose was administered and the same protocol was performed.
Statistical analysis All data are expressed as mean±SEM. Statistical analysis was performed with Student’s paired t-test. P<0.05 was considered statistically significant.
Results
Effects of clonidine on BP, HP, and BRS in SHR-SP Figure 1 illustrates the effects of clonidine (ig) on BP, HP, and BRS in SHR-SP. SBP and DBP were significantly decreased (–16±3 mmHg and –12±3 mmHg) and HP was prolonged dramatically by 10.0 µg/kg clonidine. DBP was significantly decreased after 1.0 µg/kg clonidine while SBP and HP were not obviously affected. BRS was enhanced markedly from 0.30±0.03 ms/mmHg to 0.43±0.04 ms/mmHg by 1.0 µg/kg clonidine and from 0.30±0.03 ms/mmHg to 0.53±0.09 ms/mmHg by 10.0 µg/kg clonidine.
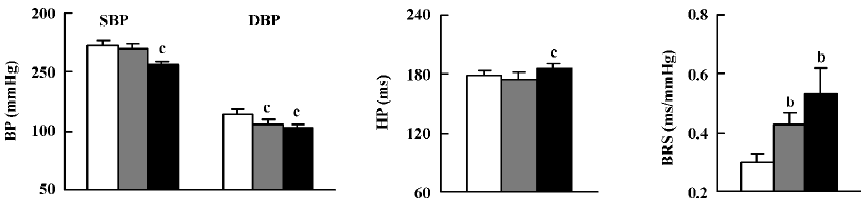
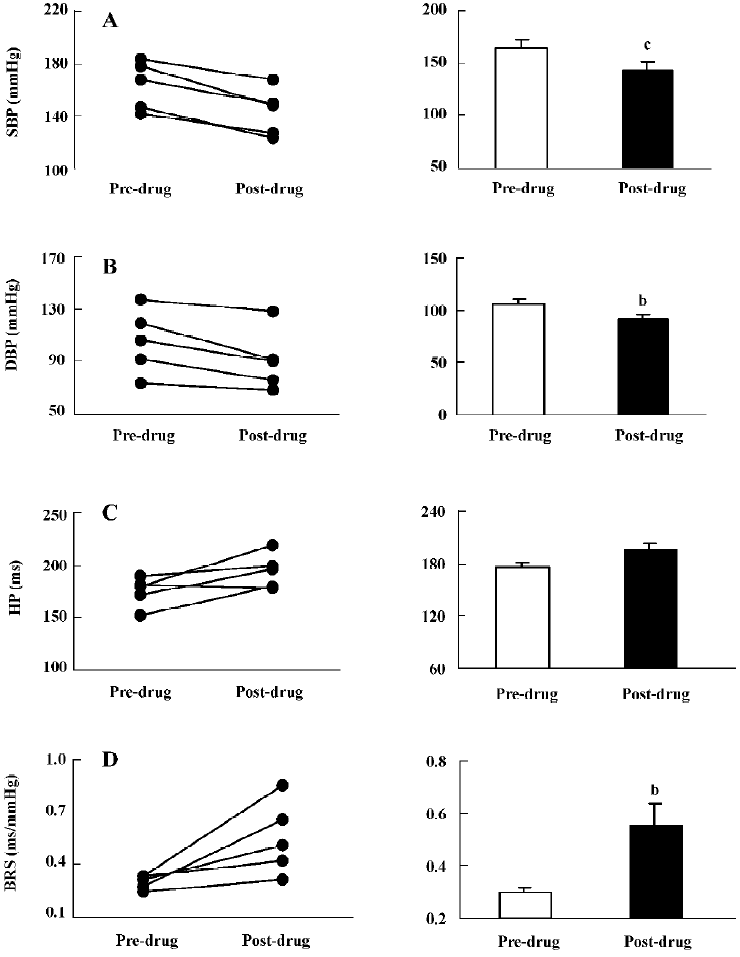
Clonidine (4 µg, icv; Figure 2) significantly decreased SBP and DBP (–21±3 mmHg and –15±4 mmHg), but HP was not significantly prolonged. BRS markedly increased from 0.30±0.02 ms/mmHg to 0.55±0.09 ms/mmHg.
Effects of moxonidine on BP, HP and BRS in SHR-SP As shown in Figure 3, both SBP and DBP were significantly decreased by 2 doses of moxonidine (ig). The reduction in SBP was 10±2 mmHg by 0.1 mg/kg and 17±3 mmHg by 1.0 mg/kg moxonidine. HP was significantly prolonged by 1.0 mg/kg moxonidine. BRS was significantly improved (0.27±0.03 vs 0.43±0.03 ms/mmHg and 0.27±0.03 vs 0.54±0.09 ms/mmHg) by 0.1 and 1.0 mg/kg moxonidine (ig).
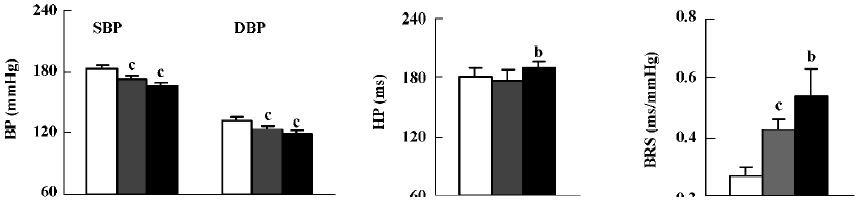
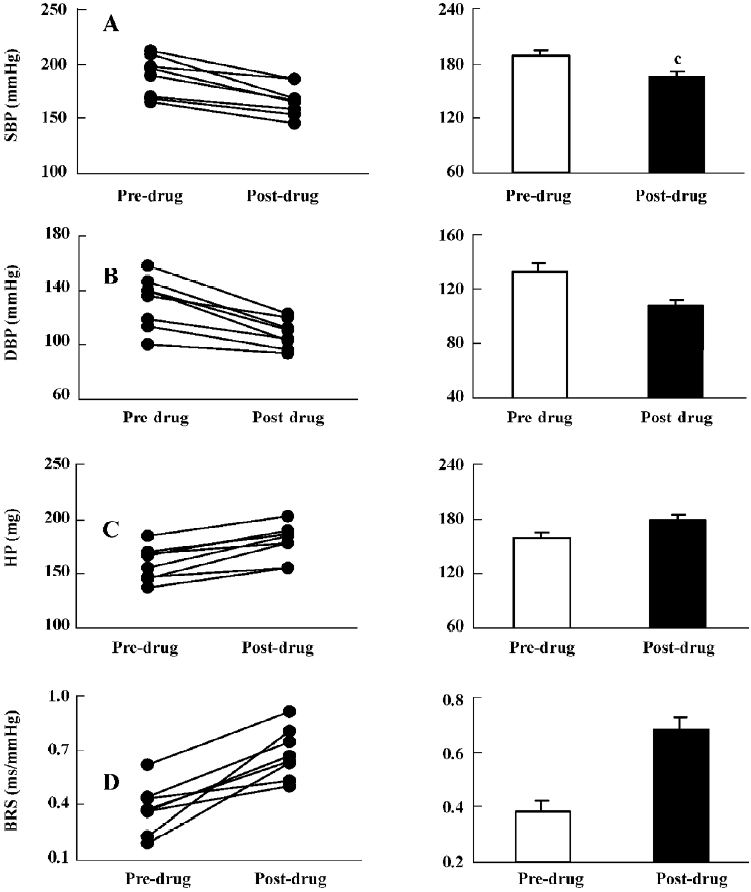
The effects of moxonidine (icv) are shown in Figure 4. Moxonidine (5 µg) significantly decreased SBP (189±7 vs 166±5 mmHg) and DBP (132±7 vs 108±4 mmHg). HP was markedly prolonged. BRS was significantly enhanced from 0.38±0.04 ms/mmHg to 0.68±0.05 mmHg. These results indicated that clonidine and moxonidine improved ABR function through central mechanisms.
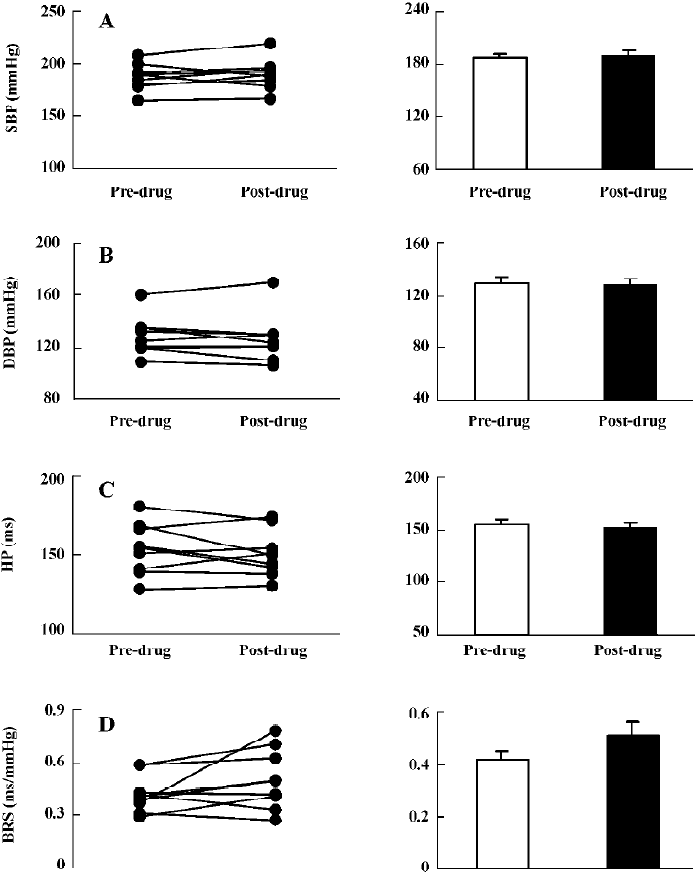
Effects of mecobalamin on BP, HP, and BRS in SHR-SP Figure 5 shows that 1.0 mg/kg mecobalamin (ig) did not cause changes in SBP, DBP, and HP. BRS was increased significantly (0.33±0.02 vs 0.44±0.04 ms/mmHg). Similar to the ig administration, the icv administration of 20 µg mecobalamin (Figure 6) did not affect SBP, DBP, and HP. However, BRS was not changed by icv injection. These results revealed that mecobalamin increased BRS through peripheral mechanisms.
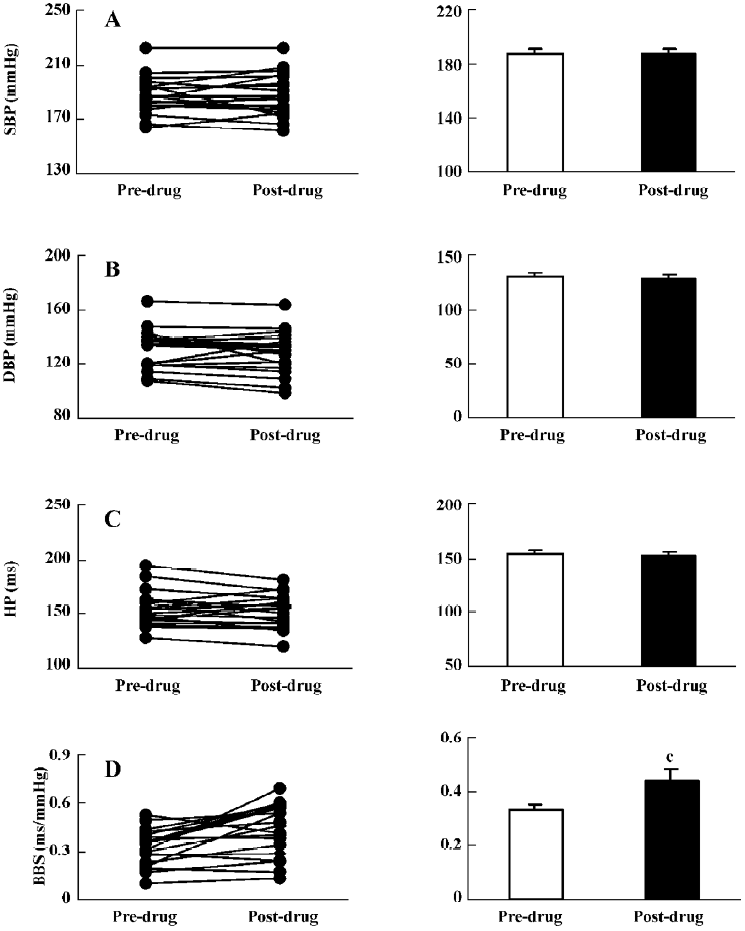
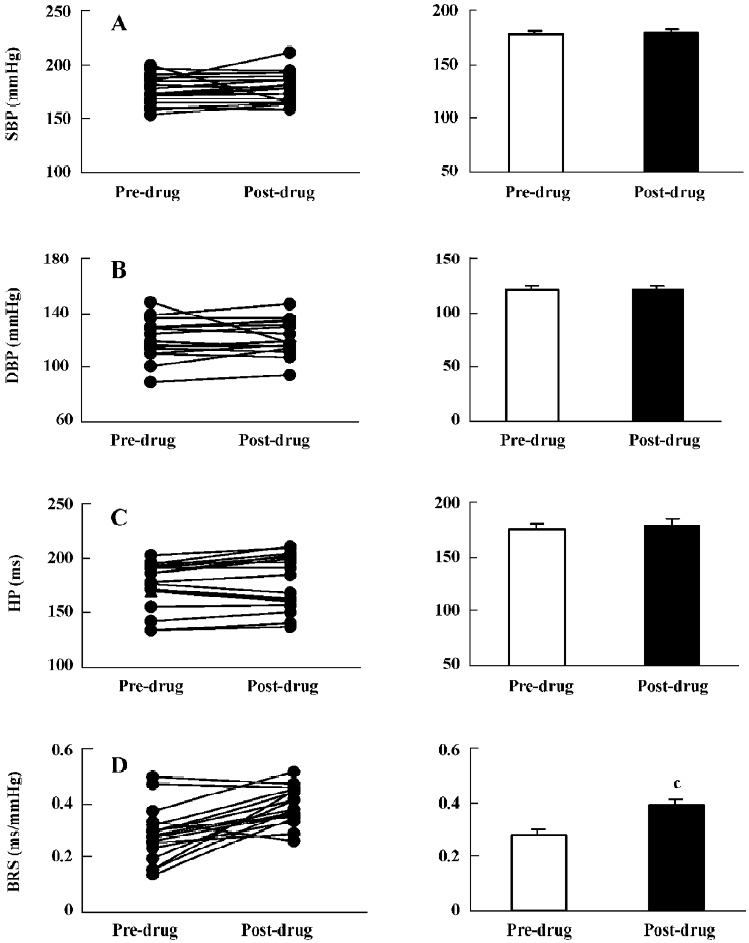
Effects of folic acid on BP, HP, and BRS in SHR-SP Similar to the ig administration of mecobalamin, 1.0 mg/kg of ig-administered folic acid (Figure 7) increased BRS without influence on the SBP, DBP, and HP levels. Icv injection was not conducted because folic acid could not reach the concentration needed in this study.
Discussion
The present study clearly demonstrated that clonidine, moxonidine, folic acid, and mecobalamin all improved the damaged ABR function in SHR-SP, whether or not the BP levels were changed or unchanged. It is well known that ABR function, damaged in many cardiovascular diseases, including myocardial infarction, heart failure, athero-sclerosis, diabetes, and end-organ damage in hypertension, plays a key role in the regulation of cardiovascular acti-vities. Since the end of 1980s, the pathological importance of ABR function has attracted the attention of many investigators. Nowadays, more and more studies have demonstrated that ABR function is closely associated with stroke. Robinson et al reported a significant reduction in cardiac BRS after acute stroke in patients[10]. Thereafter, they reported that post-stroke patients with impaired BRS values (≤5.0 ms/mmHg) had a significantly poorer prognosis than patients without impaired BRS (>5.0 ms/mmHg). Based on these results, they suggested therapeutic strategies for stroke by increasing BRS activity with drugs[11].
Given the clinical importance of BRS in stroke therapy, we believe it is necessary to find drugs that are effective in restoring damaged ABR function in stroke patients. The application of these drugs for the prevention of stroke incidence may be a new strategy in stroke therapy. In this study, SHR-SP were used. It was reported that SHR-SP had damaged ABR function compared with normotensive Wistar–Kyoto rats[19]. At the same time, SHR-SP are a useful experimental model for examining the pathogenesis of stroke as well as their treatment because the cerebrovascular lesions in these animals are similar to that in humans[20].
Clonidine, a centrally–acting, antihypertensive drug, is an agonist for both I1-imidazoline receptors and α2-adreno-ceptors. Tank et al reported that clonidine improved spontaneous BRS in conscious mice[13]. In this study, we found that clonidine enhanced BRS significantly after ig administration in SHR-SP. To determine the possible mechanism involved in this effect, icv-administered clonidine was conducted. The results indicated that icv-administered clonidine also improved BRS significantly in SHR-SP. These results suggested that the BRS-improving effect induced by clonidine was mediated through the central mechanism. Furthermore, 1.0 µg/kg clonidine did not decrease SBP, but increased BRS significantly, indicating that this BRS-improving effect was not secondary to a decrease in BP.
Moxonidine, an agent of the second generation, antihypertensive drugs acting on the central nervous system, mediates hypotensive effects by activating central I1-imidazoline receptors and subsequently decreasing sympathetic nerve activity[21]. In the present study, the effect of moxonidine on ABR function in conscious SHR-SP was tested. Our study was the first to find that ig–administered moxonidine significantly increased BRS in SHR-SP. Furthermore, icv-administered moxonidine was performed and the results indicated that BRS was also enhanced significantly, demonstrating that the central mechanism was involved in the BRS-improving effect of moxonidine. However, both 0.1 and 1.0 mg/kg moxonidine decreased SBP and increased BRS significantly, so it is unclear whether this effect was dependent on the decrease of blood pressure or not. Further studies are needed to testify this mechanism.
Folic acid was considered to have potential protection against cardiovascular diseases due to its homocysteine-lowering effect[22]. It was also suggested that folate might have a direct antioxidant role in vivo, which was independent of any indirect effects through the lowering of homocysteine levels[23]. Abundant attention has been focused on folic acid with its role in the prevention of cardiovascular disease[24,25]. Bechir et al[14] proved that folic acid improved impaired BRS in hypertensive patients. They speculated that the positive effects of folic acid seemed to be mediated by a reduction of oxidative stress because oxidative stress directly interfered with nerve endings of baroneurons in the arterial wall to damage ABR function[26]. They also found that folic acid had antioxidative properties[27]. In the present study, we examined effect of folic acid on ABR function in SHR-SP. It was found that BRS was significantly enhanced after ig administration of folic acid under the condition that both BP and HP were unchanged. The BRS-improving effect of folic acid in SHR-SP might be conducted through its antioxidative properties. As well as this, folic acid was reported to enhance endothelial function and increase the production of nitric oxide (NO)[28,29]; NO played an important role in the regulation of ABR function[30]. This might be another contribution to BRS improvement induced by folic acid. In this study, an icv injection was not conducted because folic acid could not reach the concentration needed in this study. Therefore, it was unclear whether the central mechanism was involved in this BRS-improving effect and further studies are needed.
Mecobalamin is one of the active analogs of vitamin B12. It is the essential cofactor for methionine synthase. Deficiency in folic acid and vitamin B12 leads to the elevation of the plasma homocysteine level, which is considered an independent risk factor in the pathogenesis of atherosclerosis[31], acute myocardial infarction[32], stroke[33], and hypertension[34]. All of these diseases were characterized by poor ABR function. We speculated that vitamin B12 might affect BRS. In the present study, the effect of vitamin B12 on ABR function was examined in SHR-SP. It was found that ig-administered mecobalamin increased BRS markedly, but icv-administered mecobalamin did not. These results suggested that the central mechanism was not involved in this BRS-improving effect. The exact mechanism of this effect by vitamin B12 is still unclear. Visontai et al reported a negative correlation between the homocysteine concentration and BRS in exfoliation syndrome or exfoliation glaucoma patients[35]. At the same time, folic acid and vitamin B12 decreased blood plasma homocysteine[36]. So we postulated that the reduction of the homocysteine concentration might contribute to the BRS-improving effect of folic acid and mecobalamin.
In conclusion, this study is the first to directly demonstrate that clonidine, moxonidine, folic acid, and meco-balamin all improve impaired BRS in SHR-SP. The central mechanism was involved in this effect of either clonidine or moxonidine and mecobalamin improved ABR function through the peripheral mechanism.
References
- La Rovere MT, Specchia G, Mortara A, Schwartz PJ. Baroreflex sensitivity, clinical correlates and cardiovascular mortality among patients with a first myocardial infarction: a prospective study. Circulation 1988;78:816-24.
- La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 1998;351:478-84.
- Mortara A, La Rovere MT, Pinna GD, Prpa A, Maestri R, Febo Q, et al. Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation 1997;96:3450-8.
- Fukuma Y, Munakata K, Fukuma N, Kishida H, Hayakawa H, Takano T. Correlation between atrial natriuretic peptide and baroreflex sensitivity in patients with congestive heart failure. Jpn Circ J 1999;63:893-9.
- Shan ZZ, Dai SM, Su DF. Relationship between baroreceptor reflex function and end-organ damage in spontaneously hypertensive rats. Am J Physiol 1999;277:H1200-6.
- Cai GJ, Miao CY, Xie HH, Lu LH, Su DF. Arterial baroreflex dysfunction promotes atherosclerosis in rats. Atherosclerosis 2005;183:41-7.
- Doba N, Reis D. Role of the cerebellum and the vestibular apparatus in regulation of orthostatic reflexes in the cat. Circ Res 1974;34:9-18.
- Cechetto D, Wilson J, Smith K, Wolski D, Silver MD, Hachinski VC. Autonomic and myocardial changes in middle cerebral artery occlusion: stroke models in the rat. Brain Res 1989;502:296-305.
- Eames PJ, Blake MJ, Dawson SL, Panerai RB, Potter JF. Dynamic cerebral autoregulation and beat to beat blood pressure control are impaired in acute ischaemic stroke. J Neurol Neurosury Psychiatry 2002;72:467-72.
- Robinson TG, James M, Youde J, Panerai R, Potter J. Cardiac baroreceptor sensitivity is impaired after acutestroke. Stroke 1997;28:1671-6.
- Robinson TG, Dawson SL, Eames PJ, Panerai RB, Potter JF. Cardiac baroreceptor sensitivity predicts long-term outcome after acute ischaemic stroke. Stroke 2003;34:705-12.
- Liu AJ, Ma XJ, Shen FM, Liu JG, Chen H, Su DF. Arterial baroreflex: a novel target for preventing stroke in rat hyperten-sion. Stroke 2007;38:1916-23.
- Tank J, Jordan J, Diedrich A, Obst M, Plehm R, Luft FC, et al. Clonidine improves spontaneous baroreflex sensitivity in conscious mice through parasympathetic activation. Hypertension 2004;43:1042-7.
- Bechir M, Enseleit F, Chenevard R, Muntwyler J, Luscher TF, Noll G. Folic acid improves baroreceptor sensitivity in hyperten-sion. J Cardiovasc Pharmacol 2005;45:44-8.
- Mangoni AA, Jackson SH. Homocysteine and cardiovascular disease: current evidence and future prospects. Am J Med 2002;112:556-65.
- Su DF, Cerutti C, Barres C, Vincent M, Sassard J. Blood pressure and baroreflex sensitivity in conscious hypertensive rats of Lyon strain. Am J Physiol 1986;251:H1111-7.
- Miao CY, Xie HH, Yu H, Chu ZX, Su DF. Ketanserin stabilizes blood pressure in conscious spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 2003;30:189-93.
- Su DF, Chen L, Kong XB, Cheng Y. Determination of arterial baroreflex blood pressure control in conscious rats. Acta Pharmacol Sin 2002;23:103-9.
- Head GA, Minami N. Importance of cardiac, but not vascular, hypertrophy in the cardiac baroreflex deficit in spontaneously hypertensive and stroke-prone rats. Am J Med 1992;92:S54-9.
- Yamori Y, Okamoto K. Spontaneous hypertension in the rat: A model for human “essential” hypertension. Verh Dtsch Ges Inn Med 1974;80:168-70.
- Armah BI, Hofferber E, Stenzel W. General pharmacology of the novel centrally acting antihypertensive agent moxonidine. Arzneimittel-Forsch 1988;38:1426-34.
- Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. New Engl J Med 1997;337:230-6.
- Nakano E, Higgins JA, Powers HJ. Folate protects against oxidative modification of human LDL. Br J Nutr 2001;86:637-9.
- Hernandez-Diaz S, Werler MM, Louik C, Mitchell AA. Risk of gestational hypertension in relation to folic acid supplementation during pregnancy. Am J Epidemiol 2002;156:806-12.
- Graham IM, O’Callaghan P. The role of folic acid in the prevention of cardiovascular disease. Curr Opin Lipidol 2000;6:577-87.
- Chapleau MW, Cunningham JT, Sullivan MJ, Wachtel RE, Abbound FM. Structural versus functional modulation of the arterial baroreflex. Hypertension 1995;26:341-7.
- Verhaar MC, Wever RM, Kastelein JJ, van Loon D, Milstien S, Koomans HA, et al. Effects of oral folic acid supplementation on endothelial function in familial hypercholesterolemia. A randomized placebo-controlled trial. Circulation 1999;100:335-8.
- Chambers JC, Ueland PM, Obeid OA, Wrigley J, Refsum H, Kooner JS. Improved vascular endothelial function after oral B vitamins: An effect mediated through reduced concentrations of free plasma homocysteine. Circulation 2000;102:2479-83.
- Doshi SN, McDowell IF, Moat SJ, Payne N, Durrant HJ, Lewis MJ, et al. Folate improves endothelial function in coronary artery disease: an effect mediated by reduction of intracellular superoxide? Arterioscler Thromb Vasc Biol 2001;21:1196-202.
- Nightingale AK, Blackman DJ, Field R, Glover NJ, Pegge N, Mumford C, et al. Role of nitric oxide and oxidative stress in baroreceptor dysfunction in patients with chronic heart failure. Clin Sci 2003;104:529-35.
- Kerkeni M, Addad F, Chauffert M, Chuniaud L, Miled A, Trivin F, et al. Hyperhomocysteinemia, paraoxonase activity and risk of coronary artery disease. Clin Biochem 2006;39:821-5.
- Banu S, Mollah FH, Alam MK, Rahman MA, Hamid MA, Wahab MA, et al. Serum homocysteine concentration in patients with acute MI and chronic IHD. Mymensingh Med J 2005;14:54-7.
- Torbus-Lisiecka B, Bukowska H, Jastrzebska M, Chelstowski K, Honczarenko K, Naruszewicz M. Lp(a), homocysteine and a family history of early ischemic cerebral stroke. Nutr Metab Cardiovasc Dis 2001;11:52-9.
- Kahleova R, Palyzova D, Zvara K, Zvarova J, Hrach K, Novakova I, et al. Essential hypertension in adolescents: Association with insulin resistance and with metabolism of homocysteine and vitamins. Am J Hypertens 2002;15:857-64.
- Visontai Z, Merisch B, Kollai M, Hollo G. Increase of carotid artery stiffness and decrease of baroreflex sensitivity in exfoliation syndrome and glaucoma. Br J Ophthalmol 2006;90:563-7.
- Tungkasereerak P, Ong-ajyooth L, Chaiyasoot W, Ong-ajyooth S, Leowattana W, Vasuvattakul S, et al. Effect of short-term folate and vitamin B supplementation on blood homocysteine level and carotid artery wall thickness in chronic hemodialysis patients. J Med Assoc Thai 2006;89:1187-93.
