Expression and regulation of versican in neural precursor cells and their lineages1
Introduction
In recent years, significant progress has been made towards the understanding of molecular mechanisms that control the growth of neuronal processes during the development and maturation of the central nervous system (CNS). Special attention has been paid to the extracellular matrix molecules (ECM), which not only form as a physical framework in the CNS, but also exert profound effects on cell shape and behavior, including cell adhesion, spreading, migration, proliferation, and differentiation[1]. In the brain, chondroitin sulfate proteoglycans (CSPG) are prominent components of the ECM and are assumed to play a particularly important role in controlling neuronal differentiation and development[2,3]. The mammalian brain contains many different types of CSPG core proteins, and during development, these proteoglycans exhibit a complex pattern of expression. As the CNS develops from early gestation into late postnatal ages, the amount of CSPG steadily increases and reaches adult levels several weeks after birth[4].
Versican is one of the major extracellular proteoglycans in the developing and adult brains. It was originally isolated from human fibroblasts and developing chicken limb buds[5,6] and was also detected in normal human CNS and brain tumors[7]. In the rat CNS, it was highly expressed in white matter tracts and closely linked to myelination[8]. The highly interactive nature of versican provides a basis for its importance as a structural molecule to create loose and hydrated matrices during the key events in development and disease. By interacting either directly with cells or indirectly with molecules that associate with cells, it regulates cell survival, proliferation, adhesion, migration, and differentiation, as well as ECM assembly and cell phenotype[9–13].
Structurally, versican is made up of an N-terminal G1 domain, a chondroitin sulfate (CS) attachment region, and a C-terminal G3 domain. The latter contains 2 epidermal growth factor (EGF)-like repeats, a lectin-like motif, and a complement binding protein-like motif[14]. The CS sequence can be divided into 2 alternatively spliced domains, termed CSα and CSβ. The alternative splicing of the versican gene generates 4 isoforms that are expressed in distinct spatiotemporal patterns[6,10], namely V0, V1, V2, and V3[14,15]. V0 contains both CSα and CSβ; V1 and V2 possess only CSβ and CSα, respectively; and V3 contains neither CSα nor CSβ. Versican is known to associate with a number of other molecules in the ECM, including hyaluronan, tenascin, fibulin-1, fibrillin, fibronectin, CD44, selectins, and the link protein[16–21]. Studies of brain development and maturation have shown that versican V1/V0 and V2 exhibit complementary expression patterns in the CNS[22]. Although versican V1/V0 isoforms are mainly expressed in the late stages of embryonic development, they frequently associate with little adhesive, fast-proliferating tissues that display a high ECM turnover rate[23]. Versican V2, on the other hand, is mainly expressed in adults and becomes a major CSPG in the mature brain[24]. The different expression patterns of versican isoforms suggest that they may play distinct roles in neuronal differentiation and neurite outgrowth. Versican V2 inhibits neural differentiation and neurite growth[25], whereas versican V1 induces neural differentiation and promotes neurite outgrowth[26]. These distinct functions of versican isoforms may be a result of the different CS domains they possess.
Remodeling of the ECM occurs continuously as a part of physiological processes, such as development, growth, and aging. It also occurs in pathological processes, such as traumatic and inflammatory lesions. The variation in the relative composition of different proteoglycans thus influences the organization and thereby the properties of the matrix. Factors involved in the regulation of ECM remodeling include cytokines, such as transforming growth factor-β (TGF-β), which are known to be potent regulators in the inflammatory process. Other cytokines, such as pro-inflammatory cytokines interleukin-1β (IL-1β) and TNF-α may also play important roles. They can alter the synthesis of ECM molecules and have varying effects depending on the type of cells involved. It has been well known that after injury to the adult CNS, numerous cytokines and growth factors are released that contribute to reactive gliosis and ECM production and therefore limit the capacity for axon regeneration. Previous reports also revealed that versican was upregulated in the injured CNS and presented in the environment in which axon regeneration failed[27]. However, little is known concerning the effect of injury or pro-inflammatory cytokines on the expression of versican isoforms.
In the present study, we examined in vitro the temporal expressional profiles of versican in neural precursor cells (NPC) and their linage cells, especially the oligodendrocyte (OL) lineage cells. We compared the expressional patterns of versican isoforms V1/V0 and V2 at different developmental stages from NPC to mature oligodendrocytes. We examined the effects of 2 pro-inflammatory cytokines, TNF-α and IFN-γ, on the expression of versican isoforms in NPC in vitro. The objective of this study was to determine the expression and regulation of versican isoforms in oligodendrogliogenesis and the pathological environment.
Materials and methods
Cell culture The isolation and cultivation of NPC derived from embryonic rat neural precursor cells were prepared according to the method described by Hu et al[28]. Briefly, NPC were isolated from the embryonic spinal cords (SC) of E16 Wistar rats. After removing the amnion and dura, the SC were dissected in sterile dishes containing ice-cold Leibovitz’s L-15 medium (Invitrogen, Grand Island, NY, USA), dissociated by gentle mechanical pipetting through fire-polished Pasteur pipettes to achieve single-cell suspen-sions, then filtered through a 70 μm nylon mesh. The dissociated viable cells were then seeded into a T25 Corning tissue culture flask (Corning, Corning, NY, USA) containing growth medium, at a density of 1×105 cells/mL, then incubated in a humidified atmosphere containing 5% CO2 at 37 °C. The culture medium for NPC was composed of 1×Dulbecco’s modified Eagle’s medium/F12 (Invitrogen, USA), 1×N2 (Invitrogen, USA), 1% B27 (Invitrogen, USA), 0.06% glucose, 2 mmol/L glutamine (Invitrogen, USA), 1.34 mmol/L sodium bicarbonate, 0.5 mmol/L HEPES (N-2-hydroxyethylpipera-zine-N''-2-ethane sulfonic acid), 2 μg/mL heparin, freshly added 20 ng/mL EGF (all from Sigma, St Louis, MO, USA), and 20 ng/mL Basic fibroblast growth factor (bFGF, Invitrogen, USA). After incubation for 3 or 4 d, the cells developed into visible neurospheres of 50–200 cells/sphere and then were mechanically pipetted into single cells for passage. The NPC of passage 2 were collected for RNA extraction or immunocytochemistry (ICC).
For cytokine stimulation, the dissociated NPC of passage 2 were incubated with different concentrations of recombinant rat IFN-γ or TNF-α or a combination (both from PeproTech EC, London, UK) in NPC culture medium, 8 h after seeding. The control groups were grown under identical conditions except for the cytokines. Forty eight hours later, the cells of both groups were harvested for RNA extraction.
To induce the differentiation of NPC, the dissociated cells in the single-cell suspension were seeded onto poly-L-lysine (200 μg/mL, Sigma, USA) coated coverslips in 35 mm dishes at a density of 5×104 cells/coverslip. Then the growth factors were removed and 1% fetal bovine serum (FBS, Invitrogen, USA) was added. The cultures were allowed to differentiate for 5–7 d in vitro before being fixed for immunostaining.
All embryonic rats were obtained from female pregnant Wistar rats bred in the Animal Care Facility at Shanghai Jiaotong University School of Medicine (Shanghai, China). All animal care was performed in accordance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals.
Induction and passage of oligodendrocyte precursor cells The induction of oligodendrocyte precursor cells (OPC) from NPC was performed as previously described[29,30] with modifications. Briefly, freshly dissociated NPC from SC were seeded at 1×105 cells/mL in NPC medium supplemented with 10 ng/mL bFGF and EGF for 1 or 2 d to develop small neurospheres and then gradually replaced with fresh OPC medium every other day 3 times. The OPC medium comprised of NPC medium (except bFGF/EGF) with the addition of 0.1% bovine serum albumin (Amresco, Solon, OH, USA), 10 ng/mL biotin (Sigma, USA), 10 ng/mL recombinant rat platelet-derived growth factor (PDGF-AA, R&D, Minnea-polis, MN, USA), and 15 ng/mL bFGF (Invitrogen, USA). During the induction process, the cells bearing the characteristic morphology of OPC migrated from the neurospheres and attached to the bottom of the flask. After removal of the necrotic spheres and fragmented cells, the OPC were cultured for an additional 5–7 d until visible oligospheres of 50–200 cells/sphere were formed.
For OPC passage, cultures were incubated with 1.2 mL accutase solution (Innovative Cell Technologies, San Diego, CA, USA) for 12 min at 37 °C. After the OPC/oligospheres detached entirely from the bottom of the flask, the cell suspension was harvested, gently triturated using a fire-polished Pasteur pipette to dissociate spheres, and then centrifuged at 134×g for 8 min at 20 °C. The dissociated cells were resuspended and reseeded into a T25 flask containing fresh OPC medium at a density of 1×105 cells/mL. After 7–10 d, new oligospheres formed, which were passaged again. The OPC of passage 2 were harvested for later experi-ments, such as the identification of OPC purity, RNA extract, ICC, or induction of OPC differentiation. To examine the purity of OPC, the cells were plated onto poly-L-lysine (200 μg/mL, Sigma, USA) coated coverslips in 35 mm dishes and cultured in fresh OPC medium for 3 d, then fixed for immunostaining.
Differentiation of OPC To induce OPC differentiation in vitro, the dissociated OPC of passage 2 were seeded into a poly-L-lysine (100 μg/mL, Sigma, USA) coated T25 flask or coverslips at a density of 2×105 cells/mL or 5×104 cells/coverslip, respectively. The growth medium consisted of OPC medium without PDGF-AA/bFGF, but with 1% FBS (Invitrogen, USA) and 30 μmol/L thyroid hormone (tri-iodothyronine [T3], Sigma, USA). The OPC were allowed to differentiate for 4 d (4DIV) or 14 d (14DIV) in vitro and then collected for RNA extraction or ICC analyses. Antibodies against A2B5, O4, O1, and myelin basic protein (MBP), respectively, were used to identify OPC and oligodendrocyte lineage cells at different developmental stages.
ICC Immunofluorescence double-labeling was used for the colocalization of versican and cell-specific markers. For NPC, floating spheres were fixed in 4% paraformaldehyde (PFA) overnight, washed in 0.01 mol/L phosphate-buffered saline (PBS; pH 7.4), cryoprotected in PBS containing 30% sucrose, embedded in Optimal Cutting Temperature (O.C.T) Compound (Sakura FineTec, Torrance, CA, USA), and sectioned with a cryostat. The cell cultures on poly-L-lysine coated coverslips were fixed with 4% PFA for 10 min at room temperature (RT), washed, and stored in 0.01 mol/L PBS (pH 7.4). The sections of neurospheres or cell cultures were blocked in 10% goat serum in PBS (for cell surface staining) or 0.3% Triton X-100-containing 10% goat serum in PBS (for intracellular staining) for 1 h at RT and incubated with the following primary antibodies overnight at 4 °C: rabbit antiversican antibody (1:400, a kind gift from Prof Yi-ping ZHANG, University of California, Irvine, CA, USA); the monoclonal mouse antibodies against nestin (1:800, BD Pharmingen, San Jose, CA, USA) for NPC, βIII-tubulin (1:800, Sigma, USA) for the neurons, glial fibrillary acidic protein (GFAP, 1:200, Sigma, USA) for the astrocytes, or Rip (1:25, a gift from Dr Scott R WHITTEMORE, University of Louisville, Louisville, KY, USA) for the oligodendrocytes; the monoclonal mouse antibodies Immunoglobulin M (IgM) against A2B5 (1:200), O4 (1:800), O1 (1:800, all from R&D, USA), and the monoclonal mouse antibodies against MBP (1:40, Oncogene Corp, Seattle, WS, USA) for identifying OPC and/or OL. After washing with PBS, the sections and cell cultures were incubated for 60 min at 37 °C with the appropriate secondary antibodies: fluoresceinisothiocyanate (FITC)-conjugated goat antirabbit Immunoglobulin G (IgG, 1:80, Sigma, USA) for versican, rhodamine-conjugated goat antimouse IgM (1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for A2B5, O4 and O1, and rhodamine-conjugated goat antimouse IgG (1:100, Sigma, USA) for the others. The slides or coverslips were rinsed and mounted with Gel/Mount aqueous mounting media containing Hoechst 33342 (1 μg/mL, Sigma, USA), a fluorescent nuclear dye. The images were acquired using an Olympus BX60 microscope (Olympus, Tokyo, Japan) equipped with a digital camera and SPOT 4.0.1(G) software (Diagnostic instruments, Sterling Heights, MI, USA). In all the experiments, primary antibody omission controls were used to confirm the specificity of ICC. The double-immunostaining of CD4 (the specific marker for Th lymphocyte) and versican for the sections of lymphnodes were also applied to confirm the specificity of the antiversican antibody. For NPC and OPC counting, at least 5 randomly-selected fields with more than 200 cells were counted, and more than 300 cells for each individual was counted for the others.
RNA extraction and single-stranded cDNA synthesis Total cellular RNA was extracted using TRI reagent (Molecular Research Center, Cincinnati, OH, USA) according to the manufacturer’s instructions. Residual DNA contamination was eliminated by DNase I (Promega, Madison, WI, USA) treatment (5 U DNase I for 45 min at 37 °C). The integrity of the RNA samples was checked by the density ratio of 28S against 18S RNA in 1.0% agarose gel electro-phoresis. The concentration and purity of RNA was determined by repeated spectrophotometric measurements at 260 and 280 nm. 1 μg RNA was subjected to synthesize single-strand cDNA with the reverse transcription system from Promega (USA). The 20 μL reaction mixture contained 15 U AMV reverse transcriptase, 10 U recombinant RNasin® ribonuclease inhibitor, 5 mmol/L MgCl2, 1×reverse transcription buffer, 0.5 μg random primer, and 1.0 mmol/L dNTP mixture. After denaturation at 70 °C for 10 min, the reaction mixture was incubated at 42 °C for 50 min followed by heat inactivation of the enzyme at 95 °C for 5 min, then cooled on ice for 5 min and stored at -20°C.
Semiquantitative PCR Initial dilution and the cycle series experiments were carried out to determine the linear exponential amplification phase and select the appropriate quantity of input cDNA and cycle numbers in PCR. The housekeeping gene β-actin was used as the internal control. The primer sequences were as follow: V1/V0: forward 5'-GAT GTA ACA ACC ACT CCG TCA G-3', reverse 5'-CGC AAC ACT TTC ATA CAG GC-3'; V2: forward 5'-TCA AAG CCT CCT GTA ATG C-3', reverse 5'-CCG ACA AGG GTT AGA GTG A-3'; and β-actin: forward 5 -ATT GTA ACC AAC TGG GAC G-3', reverse 5'-TTG CCG ATA GTG ATG ACC T-3'.
The 25 μL PCR reaction contained 1.5 U Taq DNA polymerase (Promega, USA), 1×PCR buffer, 1.0 μL cDNA, 1.5 mmol/L MgCl2, 0.2 mmol/L dNTP mix, 0.4 μmol/L primers for V1/V0 and V2, and 0.06 μmol/L primers for β-actin. The reactions were incubated for 5 min at 94 °C, followed by 25 cycles of denaturation (30 s at 94 °C), annealing (30 s at 57 °C), elongation (30 s at 72 °C), and final extension (10 min at 72 °C). Then 10 μL of PCR products was separated on 1.5% agarose gels with 0.1% ethidium bromide and photographed under UV illumination. The ratio value of the signal intensity of the tested genes versus β-actin was quantified with the National Institute of Health Image.
Real-time PCR Real-time PCR was carried out on an ABI7900 PCR detection system (Applied Biosystems, Foster City, CA, USA) using the SYBR Green PCR Master Mix (Applied Biosystems, USA). To normalize the gene expres-sion, an endogenous reference HPRT (hypoxanthine guanine phosphoribosyl transferase, housekeeping gene) was performed for parallel amplification. The primer sequences were: V1/V0: The same as those in semiquantitative RT-PCR above; V2: forward 5'-TCA AAG CCT CCT GTA ATG C-3', reverse 5'-ATA GCA GGT GCC TCC AT-3'; and HPRT: forward 5'-CTC ATG GAC TGA TTA TGG ACA GGA C-3', reverse 5'-GCA GGT CAG CAA AGA ACTT ATA GCC-3'.
Each PCR reaction (total volume of 10 μL) contained 1.0 μL cDNA, 5.0 μL of 2×SYBR Green PCR Master Mix, and 0.4 μmol/L of each primer. The reaction solutions were incubated at 50 °C for 2 min, then at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s, and annealing at 60 °C for 1 min. The PCR cycle number at which the fluorescent crosses a threshold (CT value) could be related to the amount of initial templates. The relative expression level of target mRNA was calculated by the normalized expression of the target gene with respect to the HPRT gene. The difference of target gene expression between the treatment group and the control group was computed with the equation: Folds=2-ΔΔCT, where ΔΔCT is the difference between the ΔCT of the treatment group and the ΔCT of the control, ΔCT is the difference between the CT of the target gene and the CT of the HPRT within the same sample.
Statistical analysis Data were presented as mean±SEM values. Statistical analysis was performed by SPSS 10.0 (SPSS, Chicago, IL, USA), and one-way ANOVA with post-hoc Tukey LSD (Least Significant Difference) test was used to determine statistical significance. A P-value of less than 0.05 was considered statistically significant.
Results
Expression of versican in NPC and their lineages After the dissociated NPC were plated in N2/B27 growth medium supplemented with EGF and bFGF, most of them proliferated rapidly to form floating neurospheres. Immunostaining of the sectioned neurospheres indicated that nearly all the cells within the spheres expressed the intermediate filament protein (nestin), a marker for NPC (Figure1B). After withdrawal of EGF and bFGF and the addition of 1% FBS, the NPC began to differentiate into a mixture of GFAP+ astrocytes (Figure1E), βIII-tubulin+ neurons (Figure1H), and Rip+ oligodendrocytes (Figure1K).
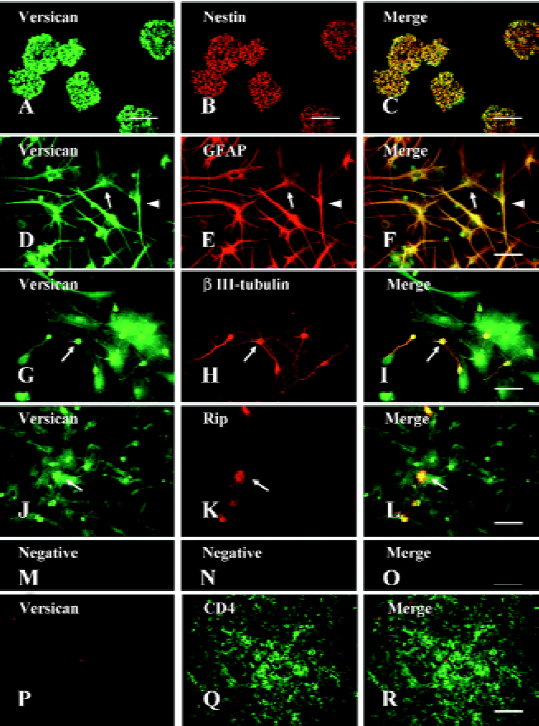
The double-immunofluorescence staining showed that versican was expressed in nestin+ NPC (Figure1C), GFAP+ astrocytes (Figure1F), βIII-tubulin+ neurons (Figure1I), and Rip+ oligodendrocytes (Figure1L). As the cells tested were fixed and permeabilized before the ICC assay, the staining should be the result of intracellular immunoreactivity. In the differentiated cells, the versican staining was observed in both the cell bodies and their processes. However, we could not observe the staining of versican in CD4+ lymphocytes in the lymph node, which had been reported to not express versican, and this negative staining result verified the specificity of the antiversican antibody used (Figure1P–1R). To exclude the possible influence on the expression of versican in NPC and their lineages due to differentiation in vitro, the neurons isolated from E18 rat embryonic cerebral cortex and astrocytes, mainly type-1 astrocytes obtained from newborn rats, were also tested for versican expression by ICC. The results showed that both cell types differentiated in vivo expressed versican (data not shown). We also observed the location of versican in the spinal cord in vivo by double-immunostaining (neural cell specific markers and versican) of the spinal cord sections and obtained the same results as that from the cell cultures in vitro (data not shown).
Expression of versican on oligodendrocyte lineage cells A previous report revealed that versican was mainly a product of oligodendrocyte lineage cells[27]. To study the expressional profile of versican at different developmental stages of oligodendrocytes, an in vitro model was established, which could mimic the environment of oligodendrogliogenesis in vivo and generate A2B5+ OPC, O4+ pre-oligo-dendrocytes, O1+ immature oligodendrocytes, and MBP+ mature oligodendrocytes over time.
The OPC generated from NPC in this study displayed bipolar and tripolar morphology (Figure 2A–2C) and most of them (97.33%±3.06%, n=3) were positive for A2B5 (Figure 2B), a marker of OPC, indicating that the NPC-induced OPC were highly purified. Immunofluorescent double-staining showed that versican was expressed on the surface of soma and processes of OPC (Figure 2A) colocalizated with A2B5 (Figure 2C).
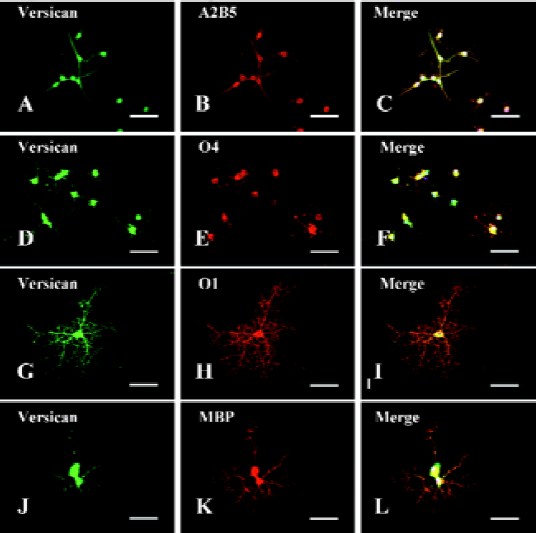
When OPC were cultured in differentiation medium with 1% FBS and T3, they progressively extended more processes and expressed more mature markers, such as O4, O1, and MBP, while A2B5 decreased quickly. Double-immunofluorescence staining showed that versican was expressed in O4+ pre-oligodendrocytes (Figure 2D–2F), O1+ immature oligodendrocytes (Figure 2G–2I), and MBP+ mature oligodendrocytes (Figure 2J–2L). Versican was expressed on both soma and processes of these cells. As the staining was carried out on the fixed and unpermeabilized cells, it indicated that the epitopes of versican were localized on the extracellular surface of the glial membrane.
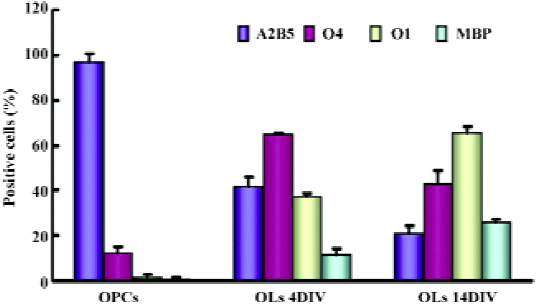
Expression patterns of versican isoforms on NPC and oligodendrocyte lineage cells As we were unable to obtain antibodies to distinguish V1 from V2, semiquanti-tative RT-PCR was performed to examine the transcription levels of versican V1/V0 and V2 in NPC and oligodendrocyte lineage cells, that is, OPC, OL (4DIV), and OL (14DIV). The characteristics of OL at these selected differentiated stages were identified by immunofluorescence based on their differentiation markers (Figure 3). The results showed that O4+ cells increased from 11.95%±2.88% at OPC to 43.34%±5.48% at OL (14DIV). O1+ and MBP+ cells could hardly be detected at OPC, but along the OPC maturation, O1+ and MBP+ cells increased from 37.19%±1.68% and 11.43%±2.70% at OL (4DIV) to 65.26%±3.25% and 25.76%±1.31% at OL (14DIV), respectively, while A2B5+ cells dropped greatly from 97.33%±3.06% at OPC, to 20.99%±3.50% at OL (14 DIV).
Consistent with the results of immunostaining for versican expression, the RT-PCR result revealed that the mRNA of V1/V0 and V2 were present in NPC as well as oligodendrocyte lineage cells, but the expression patterns of 2 isoforms at different development stages were different (Figure 4). The statistical analysis showed that the expression of V1/V0 in NPC was the lowest (P<0.01) among all analyzed cells. It increased dramatically in OPC (1.42-fold) and was maintained at high levels in OL at 4 and 14DIV (Figure 4C). However, the expression pattern of V2 was somewhat different from that of V1/V0. The mRNA level of V2 in NPC was very low, peaked in OPC (2.13 fold vs NPC, P<0.01), slightly decreased in OL at 4DIV, and markedly decreased in OL at 14DIV. The expression of V2 in OL at 14DIV was significantly lower than that in OPC (P<0.01) and OL at 4DIV (P<0.05), and showed no significant difference with that in NPC (Figure 4D).
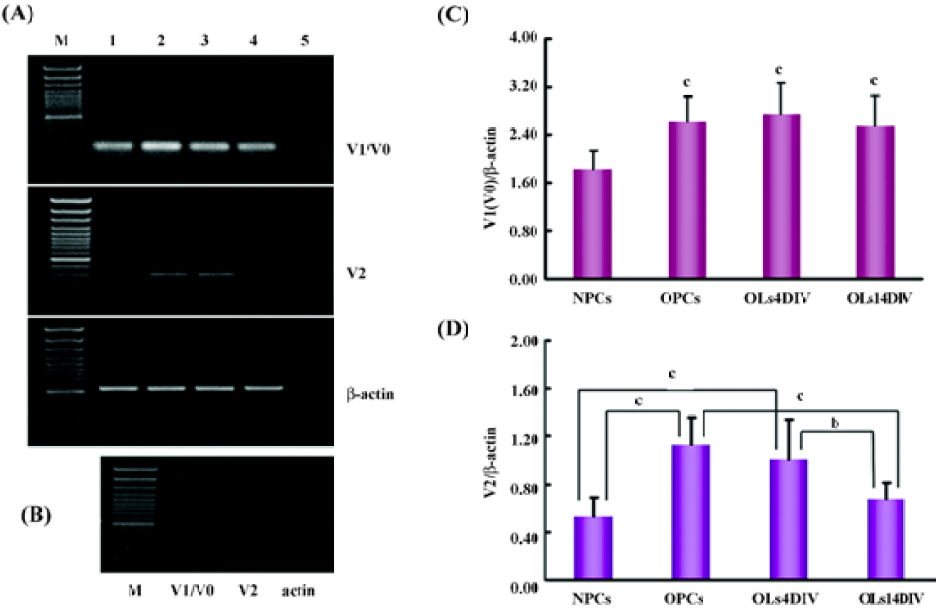
Regulation of versican expression in NPC by pro-inflammatory cytokines A previous report demonstrated that TNF receptor I and IFN-γ receptor were expressed on the NPC[31] making it possible to study the effect of TNF-α and IFN-γ on NPC. To determine whether the expression patterns of versican isoforms could be regulated by these 2 pro-inflammatory cytokines, NPC were treated for 48 h with different doses of TNF-α, IFN-γ, or both, and collected for relative quantitative real-time PCR. The melting curve analysis (Figure 5A) and the agarose gel electrophoresis analysis (Figure 5B) for V1, V2, and HPRT confirmed the specificity of these amplified products.
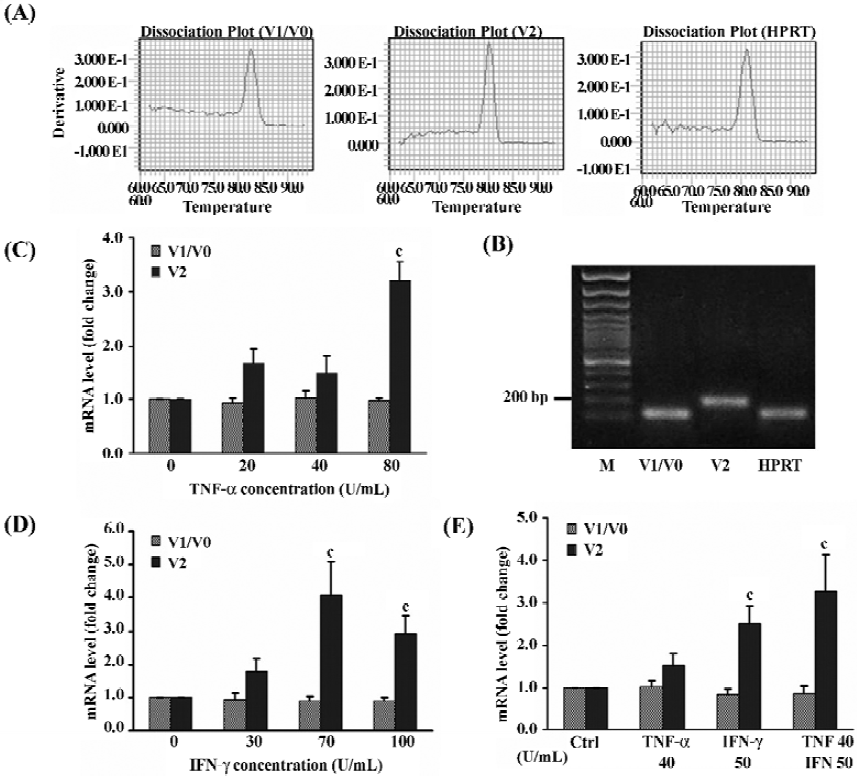
The results revealed that the 2 pro-inflammatory cytokines, used separately or in combination, did not affect the expression of V1/V0, but obviously affected the V2 mRNA expression in NPC in a dose-dependent manner (Figure 5C–5E). The 80 U/mL TNF-α could significantly increased the level of V2 mRNA by 3.2-fold over the untreated control (P<0.01), but low-dose TNF-α had no obvious effects (Figure 5C). Similarly, treatment with 30, 70, and 100 U/mL IFN-γ, could upregulate V2 mRNA levels by 1.8- (P>0.05), 4.1- (P<0.01), and 2.9-fold (P<0.01), respectively (Figure 5D). A combination of the 2 cytokines exhibited an additive effect and the level of V2 mRNA was increased by 3.3-fold (P<0.01) compared to 1.5-fold (P>0.05) with TNF-α or 2.5-fold (P<0.01) with IFN-γ alone (Figure 5E).
Discussion
Versican expression in NPC and their lineages Versican is a large chondroitin sulfate proteoglycan identified as one of the major extracellular molecules in the brain and belongs to the family of lecticans[32,33]. Previous reports showed that versican was selectively expressed in embryonic tissues that act as barriers to neural crest cell migration and axon outgrowth[23,34]. In this study, we showed that versican was expressed in NPC isolated from the embryonic rat spinal cord, detected by both immunofluorescence double-staining and RT-PCR. The expression of versican in NPC may be related to the highly precise and coordinated migration of neural crest cells during the early phase of embryonic development.
Our ICC results also showed that versican was widely located in cells differentiated from NPC, including neurons, astrocytes, and oligodendrocytes. To strengthen these results, we employed RT-PCR to verify the expression of versican in neurons isolated from the E18 rat embryonic cerebral cortex and cultured in vitro for 7 d. The results of the RT-PCR completely coincided with the immunostaining results showing that both V1/V0 and V2 mRNA were expressed in neurons.
It has long been believed that versican is a glial product based on its distribution in the normal and injured CNS. High levels of versican were found in white matter tracts and it was also readily detectable in the spinal cord gray matter, but very little in the cerebral cortex[8]. Our finding that versican was also expressed in neurons has added a new dimension to previous findings and raised an intriguing question as to what possible physiological role versican may play in neurons during development or after injury. Further, we also questioned whether the expression of versican in neurons contributes to the formation of peri-neuronal nets (PNN). PNN, a specialized form of extracellular matrix around the cell body and dendrites of many classes of neurons in the adult CNS, are composed of several molecules including CSPG, hyaluronan, tenascin-R, and link protein[35]. Some CSPG, such as aggrecan and versican, members of the lectican family, are associated with PNN. These molecules play a role in restricting the plastic capabilities of adult neurons[36] and act as a protection for neurons against oxidative stress. However, until now, the cellular sources of PNN components remain unclear. In this work, we described the expression of versican in neurons and glial cells by ICC and/or RT-PCR, and the results suggested that both neurons and glial cells might participate in synthesizing PNN components. The neuronal expression of versican may indicate that this molecule may play a role in maintaining synaptic stability and preventing plasticity in the mature CNS. On the other hand, the wide distribution of versican in neurons and glial cells suggests that versican may be critical in the interaction between matrices and these cells.
Versican expression in oligodendrocyte lineage cells Previous in vitro studies revealed that versican was mainly a product of oligodendrocyte lineage cells[27]. In this study, based on high purity OPC generated from rat embryonic NPC, an in vitro model which could mimic the environment of oligodendrogliogenesis in vivo was established. Therefore, we could conveniently obtain oligodendrocyte lineage cells at different developmental stages in vitro to elucidate the expression pattern of versican isoforms during oligodendrogliogenesis. The ICC results showed that versican was not only expressed in bipolar or tripolar A2B5+ OPC, but also on the soma and processes of multipolar O4+ pre-oligodendrocytes, more branched O1+-immature oligodendrocytes, and even more differentiated MBP+-myelin-forming oligodendrocytes. These results were mostly in accordance with previous reports[27] except the expression of versican on MBP+ oligodendrocytes. However, other investigators had detected the expression of versican in MBP+ oligodendrocytes by in situ hybridization in vivo[25]. We supposed that the difference in the methods used for OPC induction might have brought up the discrepancy with the reports.
Previous studies showed that different isoforms of versican exhibited distinct and complementary expression patterns in the developing CNS[22]. Versican V2 was present at relatively low levels during the late embryonic stage and further decreased by approximately 50% between 1 and 2 weeks’ postnatal, then increased steadily to reach a maximum at 100 d, 7-fold more that at 10 d postnatal[37], whereas versican V1/V0 was mainly expressed in the late stages of embryonic development[23], and its level doubled between E14 and birth, after which V1/V0 decreased by 90% to reach a low “mature” level that remained stable throughout adulthood[37]. These observations and clear-cut changes suggest that versican V1/V0 and V2 play different roles during neurogenesis and homeostasis of the mature brain.
In this study, we examined the expression patterns of different versican isoforms in the different developmental stages of oligodendrocyte lineage cells. Our RT-PCR results showed that embryonic spinal cord-derived NPC expressed both versican V1/V0 and V2, but at relatively low levels. Once they differentiated into oligodendrocyte lineage cells, the expression of both isoforms increased significantly. However, the expression patterns of V1/V0 and V2 were different during the course of OL differentiation. From OPC to early (4DIV) and late OL (14DIV), the expression of versican V1/V0 did not change significantly, whereas that of versican V2 decreased markedly in OL at 14DIV compared with OPC or OL at 4DIV. Notably, these expression patterns of versican isoforms in oligodendrogliogenesis in vitro are different from those in the developing brain as mentioned above[37]. As the in vivo study reflected, there was a complete change of versican expressional profiles in the extracellular matrix and in all cell types, including neurons, OL lineage cells, astrocytes, as well as non-neural cells, such as meningeal cells[27]. This suggests that the expression patterns of versican isoforms in different cells during CNS development may be different. Further study of the expression patterns of these isoforms in different cell types may be beneficial for the understanding of the function of versican isoforms in the developing CNS.
It has been recognized that different versican isoforms may have distinct biological functions. For example, the expression of versican V2 in the white matter and CNS myelin suggests a role of this molecule in restricting structural plasticity and regeneration of CNS fiber tracts[25]. However, coculture experiments showed that versican V1/V0 had no inhibitory effect on axon extension[38] and instead induced neuronal differentiation and promoted neurite outgrowth in vitro[26]. Thus, the complementary effects of V1/V0 and V2 on neurite outgrowth imply that the dynamically balanced expression patterns of these isoforms may provide a suitable extracellular environment for neurite development and homeostasis of the mature brain.
In the development of CNS, the distribution of OPC is consistent with their function in axonal guidance, perhaps channeling axons and preventing them from straying into inappropriate areas. Furthermore, OPC were present at greater numbers during the last 2–3 d of gestation and the first week of rat postnatal life[39], which is in accordance with their function in preventing errors and axonal straying. Versican V2, as well as other CSPG produced by OPC, such as NG2, may also play a critical role during the development of axonal pathways. From this point on, it was not surprising that putatively inhibitory isoform versican V2 was expressed at the highest level at the OPC stage. The results presented here suggest that the surface of OPC might be non-permissive for axon growth and repel growing axons.
However, in another observation made at our laboratory, OPC/oligodendrocytes cocultured with dorsal root ganglion (DRG) explants showed that OPC were less inhibitory to neurite outgrowth of DRG neurons than mature oligodendrocytes (Zheng-wen MA, personal communication). It implied that the high-level expression of versican V2 in OPC might not be parallel to their inhibitory function, and the inhibition effect of OL lineage cells may be determined by a combination of a series of inhibitory molecules, including Nogo, OMgp, and Myelin-associated glycoprotein (MAG), not only by versican V2.
Effect of pro-inflammatory cytokines on versican expression After injury to the adult CNS, one of the earliest responses to injury is the infiltration of macrophages and the activation of microglia, which starts a cytokine/growth factor cascade[40]. Numerous growth factors and cytokines are released that could potentially regulate the expression of CSPG. Recently, it has been revealed that the presence of TGF-β1 and EGF greatly increased the production of several CSPG by astrocytes[41]. Moreover, it was reported that TGF-β and IL-1β could bring about an increase in the amount of versican in OPC and their differentiating cells, respectively[27]. It was also found that versican V2 expression was upregulated in response to brain injury and was suggested to be induced, directly or indirectly, by cytokines and growth factors released after injury[27]. In contrast, versican V1 and V0 were not detected in the damaged tissue or in the surrounding region after CNS injury[38]. These results suggest that the expression of versican isoforms, especially V2, could be regulated by cytokines and other molecules presented in injured CNS environments.
It was reported that NPC had a tendency to migrate towards damaged regions after CNS injury in vivo[42,43], which was the first critical step in NPC engagement during regeneration. In addition, evidence revealed that 2 other cytokines, TNF-α and IFN-γ, increased after traumatic brain injury[44] and spinal cord injury[45–47]. Here we report that both TNF-α and IFN-γ can upregulate the transcription of versican V2 in NPC in a dose-dependent manner within a certain dose range, yet had no effect on V1/V0 expression. This result was in agreement with the phenomenon that versican was upregulated in the injured CNS and provided some explanations that TNF-α and IFN-γ are partly responsible for the different expression changes of versican isoforms after CNS injury. Combined with other reports[27,41], it is reasonable to conclude that injury-induced changes in cytokines might alter the expression pattern of CSPG and several important inhibitory proteoglycans, such as versican V2 and NG2[48] being overexpressed, therefore, causing regenerative failure around the lesion sites. In this case, for NPC transplantation to repair the CNS injury, some measures need to be taken to modulate or reduce CSPG production in the injured CNS prior to the transplantation. Treatment with chondroitinase by degrading its glycosaminoglycan components may abolish the inhibitory effect of versican V2 and other inhibitory proteoglycans on neurite outgrowth and promote axonal regeneration[36,49,50].
In this study, we also found that after exposure to IFN-γ, the floating neurospheres tended to attach to the bottom of the flask. Some cells migrated from the spheres and showed a significant tendency of differentiation. As we revealed in this study, once NPC differentiate into oligodendrocyte lineages, versican V2 expression increases significantly, therefore, it is possible that the increase in versican V2 expression was an indirect consequence of the differentiation of NPC induced by pro-inflammatory cytokines, rather than a direct effect of cytokines on NPC. Certainly, the cases in vivo may be more complex and the expression changes of versican isoforms upon these cytokines on other cell types in the CNS remain to be determined.
It has been revealed that a high dose of pro-inflammatory cytokines could inhibit NPC proliferation and increase apoptosis[31], and the change of cell vitality caused by cytokines might have an effect on versican gene expression. We also noted that the mRNA expression of versican V2 changed in a dose-dependent manner; within a certain dose range, it raised steadily, but after it reached its peak, it quickly declined. Therefore, to avoid the inhibitory effect of a high dose of cytokines on the status or vitality of NPC, both cytokines used in this study were restricted in medium concentrations and within a narrow range.
In conclusion, the present study demonstrated that versican was constitutively expressed in neurons, astrocytes, and oligodendrocyte lineage cells differentiated from NPC in vitro. By means of simple and efficient methods of OPC induction and differentiation in vitro, we compared the expression patterns of versican isoforms V1/V0 and V2 at different developmental stages of oligodendrocyte development from NPC. The results showed that the 2 versican isoforms expressed throughout the oligodendrocyte development stages, but inhibitory versican V2 expression peaked at the OPC stage. We also found that pro-inflammatory cytokines TNF-α and IFN-γ could upregulate the transcription of inhibitory versican V2 in NPC in a dose-dependent manner within a certain dose range, but had no effect on V1/V0 expression, which might be partly related to the formation of non-permissive surroundings for axonal regeneration after CNS injuries in vivo.
Acknowledgements
The authors thank Prof Yi-ping ZHANG, Department of Neurology, University of California, for the generous gift of versican antibodies and valuable suggestions.
References
- Ingber DE, Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol 1989;109:317-30.
- Mongiat M, Fu J, Oldershaw R, Greenhalgh R, Gown AM, Iozzo RV. Perlecan protein core interacts with extracellular matrix protein 1 (ECM1), a glycoprotein involved in bone formation and angiogenesis. J Biol Chem 2003; 278: 17 491–9.
- Walker HA, Whitelock JM, Garl PJ, Nemenoff RA, Stenmark KR, Weiser-Evans MC. Perlecan up-regulation of FRNK suppresses smooth muscle cell proliferation via inhibition of FAK signaling. Mol Biol Cell 2003;14:1941-52.
- Oohira A, Matsui F, Matsuda M, Shoji R. Developmental change in the glycosaminoglycan composition of the rat brain. J Neurochem 1986;47:588-93.
- Zimmermann DR, Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J 1989;8:2975-81.
- Shinomura T, Nishida Y, Ito K, Kimata K. cDNA cloning of PG-M, a large chondroitin sulfate proteoglycan expressed during chondrogenesis in chick limb buds. Alternative spliced multiforms of PG-M and their relationships to versican. J Biol Chem 1993; 268: 14 461–9.
- Paulus W, Baur I, Dours-Zimmermann MT, Zimmermann DR. Differential expression of versican isoforms in brain tumors. J Neuropathol Exp Neurol 1996;55:528-33.
- Bignami A, Perides G, Rahemtulla F. Versican, a hyaluronate-binding proteoglycan of embryonal precartilaginous mesenchyma, is mainly expressed postnatally in rat brain. J Neurosci Res 1993;34:97-106.
- Ang LC, Zhang Y, Cao L, Yang BL, Young B, Kiani C, et al. Versican enhances locomotion of astrocytoma cells and reduces cell adhesion through its G1 domain. J Neuropathol Exp Neurol 1999;58:597-605.
- Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol 2002;14:617-23.
- Zhang Y, Cao L, Yang BL, Yang BB. The G3 domain of versican enhances cell proliferation via epidermal growth factor-like motifs. J Biol Chem 1998; 273: 21 342–51.
- Zhang Y, Cao L, Kiani CG, Yang BL, Yang BB. The G3 domain of versican inhibits mesenchymal chondrogenesis via the epidermal growth factor-like motifs. J Biol Chem 1998; 273: 33 054–63.
- Zhang Y, Cao L, Kiani C, Yang BL, Hu W, Yang BB. Promotion of chondrocyte proliferation by versican mediated by G1 domain and EGF-like motifs. J Cell Biochem 1999;73:445-57.
- Ito K, Shinomura T, Zako M, Ujita M, Kimata K. Multiple forms of mouse PG-M, a large chondroitin sulfate proteoglycan generated by alternative splicing. J Biol Chem 1995;270:958-65.
- Lemire JM, Braun KR, Maurel P, Kaplan ED, Schwartz SM, Wight TN. Versican/PG-M isoforms in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 1999;19:1630-9.
- Aspberg A, Binkert C, Ruoslahti E. The versican C-type lectin domain recognizes the adhesion protein tenascin-R. Proc Natl Acad Sci USA 1995; 92: 10 590–4.
- Isogai Z, Aspberg A, Keene DR, Ono RN, Reinhardt DP, Sakai LY. Versican interacts with fibrillin-1 and links extracellular microfibrils to other connective tissue networks. J Biol Chem 2002;277:4565-72.
- Kawashima H, Hirose M, Hirose J, Nagakubo D, Plaas AH, Miyasaka M. Binding of a large chondroitin sulfate/dermatan sulfate proteoglycan, versican, to L-selectin, P-selectin, and CD44. J Biol Chem 2000; 275: 35 448–56.
- Matsumoto K, Shionyu M, Go M, Shimizu K, Shinomura T, Kimata K, . Distinct interaction of versican/PG-M with hyaluronan and link protein. J Biol Chem 2003; 278: 41 205–12.
- LeBaron RG, Zimmermann DR, Ruoslahti E. Hyaluronate binding properties of versican. J Biol Chem 1992; 267: 10 003–10.
- Olin AI, Morgelin M, Sasaki T, Timpl R, Heinegard D, Aspberg A. The proteoglycans aggrecan and versican form networks with fibulin-2 through their lectin domain binding. J Biol Chem 2001;276:1253-61.
- Bandtlow CE, Zimmermann DR. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev 2000;80:1267-90.
- Landolt RM, Vaughan L, Winterhalter KH, Zimmermann DR. Versican is selectively expressed in embryonic tissues that act as barriers to neural crest cell migration and axon outgrowth. Development 1995;121:2303-12.
- Schmalfeldt M, Dours-Zimmermann MT, Winterhalter KH, Zimmermann DR. Versican V2 is a major extracellular matrix component of the mature bovine brain. J Biol Chem 1998; 273: 15 758–64.
- Schmalfeldt M, Bandtlow CE, Dours-Zimmermann MT, Winterhalter KH, Zimmermann DR. Brain derived versican V2 is a potent inhibitor of axonal growth. J Cell Sci 2000;113:807-16.
- Wu Y, Sheng W, Chen L, Dong H, Lee V, Lu F, et al. Versican V1 isoform induces neuronal differentiation and promotes neurite outgrowth. Mol Biol Cell 2004;15:2093-104.
- Asher RA, Morgenstern DA, Shearer MC, Adcock KH, Pesheva P, Fawcett JW. Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. J Neurosci 2002;22:2225-36.
- Hu JG, Fu SL, Zhang KH, Li Y, Yin L, Lu PH, et al. Differential gene expression in neural stem cells and oligodendrocyte precursor cells: a cDNA microarray analysis. J Neurosci Res 2004;78:637-46.
- Fu SL, Hu JG, Li Y, Yin L, Jin JQ, Xu XM, et al. Induction of rat neural stem cells into oligodendrocyte precursor cells. Acta Physiol Sin 2005;57:132-8.
- Zhang SC, Lundberg C, Lipsitz D, O’Connor LT, Duncan ID. Generation of ligodendroglial progenitors from neural stem cells. J Neurocytol 1998; 27: 475–89. 3 1 Ben-Hur T, Ben-Menachem O, Furer V, Einstein O, Mizrachi-Kol R, Grigoriadis N. Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol Cell Neurosci 2003;24:623-31.
- Aspberg A, Miura R, Bourdoulous S, Shimonaka M, Heinegard D, Schachner M, et al. The C-type lectin domains of lecticans, a family of aggregating chondroitin sulfate proteoglycans, bind tenascin-R by protein-protein interactions independent of carbohydrate moiety. Proc Natl Acad Sci USA 1997; 94: 10 116–21. 3 3 Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci 2000;57:276-89.
- Dutt S, Kleber M, Matasci M, Sommer L, Zimmermann DR. Versican V0 and V1 guide migratory neural crest cells. J Biol Chem 2006;281:123-31.
- Celio MR, Spreafico R, De BS, Vitellaro-Zuccarello L. Perineu-ronal nets: past and present. Trends Neurosci 1998;21:510-5.
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 2002;298:1248-51.
- Milev P, Maurel P, Chiba A, Mevissen M, Popp S, Yamaguchi Y, et al. Differential regulation of expression of hyaluronan-binding proteoglycans in developing brain: aggrecan, versican, neurocan, and brevican. Biochem Biophys Res Commun 1998;247:207-12.
- Fidler PS, Schuette K, Asher RA, Dobbertin A, Thornton SR, Calle-Patino Y, et al. Comparing astrocytic cell lines that are inhibitory or permissive for axon growth: the major axon-inhibitory proteoglycan is NG2. J Neurosci 1999; 19: 8778–88. 3 9 Levine JM, Stincone F, Lee YS. Development and differentiation of glial precursor cells in the rat cerebellum. Glia 1993;7:307-21.
- Giulian D, Chen J, Ingeman JE, George JK, Noponen M. The role of mononuclear phagocytes in wound healing after traumatic injury to adult mammalian brain. J Neurosci 1989;9:4416-29.
- Smith GM, Strunz C. Growth factor and cytokine regulation of chondroitin sulfate proteoglycans by astrocytes. Glia 2005;52:209-18.
- Boockvar JA, Schouten J, Royo N, Millard M, Spangler Z, Castelbuono D, et al. Experimental traumatic brain injury modulates the survival, migration, and terminal phenotype of transplanted epidermal growth factor receptor-activated neural stem cells. Neurosurgery 2005;56:163-71.
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, . Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA 2004; 101: 18 117–22.
- Fan L, Young PR, Barone FC, Feuerstein GZ, Smith DH, McIntosh TK. Experimental brain injury induces differential expression of tumor necrosis factor-alpha mRNA in the CNS. Brain Res Mol Brain Res 1996;36:287-91.
- Hayashi M, Ueyama T, Nemoto K, Tamaki T, Senba E. Sequential mRNA expression for immediate early genes, cytokines, and neurotrophins in spinal cord injury. J Neurotrauma 2000;17:203-18.
- Nakamura M, Houghtling RA, MacArthur L, Bayer BM, Bregman BS. Differences in cytokine gene expression profile between acute and secondary injury in adult rat spinal cord. Exp Neurol 2003;184:313-25.
- Yan P, Li Q, Kim GM, Xu J, Hsu CY, Xu XM. Cellular localization of tumor necrosis factor-alpha following acute spinal cord injury in adult rats. J Neurotrauma 2001;18:563-8.
- Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci 2002;22:2792-803.
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002;416:636-40.
- Chau CH, Shum DK, Li H, Pei J, Lui YY, Wirthlin L, et al. Chondroitinase ABC enhances axonal regrowth through Schwann cell-seeded guidance channels after spinal cord injury. FASEB J 2004;18:194-6.
