Arsenic trioxide: safety issues and their management1
Introduction
Arsenic is infamous as a poison, but has recently gained fame as a remedy. It has featured in traditional Chinese pharmacopoeia for millennia, according to the traditional Chinese dictum of using poison against poisonous diseases[1]. In Western medicine, arsenic became popular as a drug after Dr Thomas Fowler in Edinburgh prepared a potassium bicarbonate based solution of arsenic, which was to bear his name. Arsenic also continued to be used as a poison, Napoleon Bonaparte being allegedly its victim[2].
Around the end of the nineteenth century and the turn of the twentieth century, arsenic was a standard medication for chronic myeloid leukaemia, there being no other more effective treatment. However, with the advent of modern pharmacology and chemotherapeutic agents in the latter half of the twentieth century, the use of arsenic declined, and description of its efficacy in chronic myeloid leukaemia disappeared from standard haematology textbooks after the 1950’s[3]. Although rarely now used as a poison, in the Indian sub-continent alone, chronic arsenic poisoning due to drinking water contamination has been estimated to affect over 120 million people[4,5].
There has been a rekindling in the interest of the therapeutic use of arsenic, due predominantly to the observation that arsenic trioxide (As2O3) induced a high rate of remission in patients with relapsed acute promyelocytic leukemia (APL)[6,7]. As2O3 induces partial differentiation and apoptosis in the APL cells through a variety of molecular mechanisms. Amongst these molecular actions, the targeting of the leukaemogenic fusion protein PML-RARA to proteasomal degradation is an important reason for the specificity of As2O3 for APL. As2O3 is now a standard drug in the treatment of newly diagnosed or relapsed APL. It is also now tested clinically in the treatment of other malignancies, notably multiple myeloma.
Owing to its notoriety as a poison, treatment with As2O3 is alarming to patients and physicians alike. Therefore, a thorough understanding of the safety and potential side effects of As2O3 as a therapeutic agent is necessary, in order to minimize its toxic complications.
Acute toxic effects of arsenic poisoning
Toxicities of short and long term arsenic exposure have been documented from case reports, epidemiological studies and animal experiments[8].Up to 200 human enzymes are inactivated by arsenic. The severity of the toxicity depends on the arsenic compound, and the route, pace and duration of absorption. The acute lethal dose varied from 100 to 300 mg of elemental arsenic[9]. Oral arsenic is methylated into active metabolites in the liver. Animal studies have shown that arsenic is concentrated in the liver, urinary bladder, and lungs. Clearance from the liver and bladder is rapid, but clearance of methylated arsenic metabolites from the kidney, heart and lungs takes a longer duration[10]. Since arsenic is mainly renal excreted, haemodialysis is the most effective and rapid way of detoxification. The use of chelating agents may also help[11].
Toxicities of acute arsenic poisoning include oesophagitis, abdominal colic, diarrhoea, arrhythmia, and mental confusion[8]. Chronic arsenic toxicity, observed mainly from studies of environmental low dose arsenic exposure, include skin pigmentation, neuropathy, skin cancers, liver cirrhosis and hepatocellular carcinoma (HCC) [8]. Environmental arsenic poisoning may account for many of the cases of idiopathic Indian childhood cirrhosis[12,13]. When arsenic is prescribed therapeutically in a controlled manner, none of these toxic side effects have been observed.
Use of arsenic as a therapeutic agent
The current therapeutic use of As2O3 is limited to the treatment of malignancies. Chinese investigators in Harbin and later Shanghai have shown that intravenous (iv) As2O3 at 0.07–0.17 mg·kg-1·d-1 is highly effective in relapsed APL, resulting in a complete remission (CR) rate of over 95%[14]. The effect is specific, with remissions not achieved in other types of leukaemia[7]. These results have been confirmed subsequently worldwide[15,16]. As2O3 therapy is largely safe and few patients require cessation of treatment due to side effects[17]. Persistent and durable molecular remission is achieved occasionally with As2O3 treatment alone[18]. Moreover, As2O3 is increasingly used in combination with all trans retinoic acid (ATRA) to exploit their synergistic interactions, in the first-line treatment and maintenance of APL[1,19]. In these studies, As2O3 is administered as a daily iv infusion. The duration of iv-As2O3 leading to remission ranged from 10–60 (median: 23) days[1,19].
To obviate the problems associated with iv administration, an oral formulation of As2O3 has also been prepared. It has comparable efficacy with the iv formulation, and poses no severe first-pass toxic side effects to the liver[20]. Oral-As2O3 has important advantages in cost savings and patient convenience, as it can be administered in the outpatients[21]. Diarsenic tetrasulphide has also been formulated orally for APL treatment, although its low solubility means that a much larger oral dose is required[22]. Data on the use of diarsenic tetrasulphide, particularly on its safety and pharmacokinetics, are limited.
As2O3 exerts differentiation and pro-apoptotic actions on APL leukaemic cells[6]. In vitro studies with cell lines and primary tumor cell cultures have also shown that other leukaemias and cancers are potentially sensitive to As2O3. These include multiple myeloma[23,24], myeloid leukaemias, lymphomas, squamous cell carcinomas and neuroblastomas[25]. Based on these results, clinical trials have been initiated for As2O3 treatment in these malignancies.
In the last decade, thousands of patients have been treated with As2O3. Increasing numbers of clinical trials in other types of malignancies have suggested that As2O3 might also be therapeutically useful. Therefore, it will be opportune to review its side effects and toxicity profile.
Arsenic pharmacokinetics
After an iv infusion of As2O3, the plasma arsenic level reaches its peak in the first hour. At a dose of 10 mg, the median peak plasma arsenic level as measured by gas phase chromatography was 6.8 (5.54–7.30) μmol/L in a study involving 15 patients[14]. However, with the more specific and accurate methods of atomic absorption spectrometry or inductively coupled plasma mass spectrometry, the peak arsenic level after a one-hour iv infusion of As2O3 has been found to range from 0.5–2 μmol/L[20]. Repeated administration of As2O3 has little effect on the pharmacokinetic profile of iv-As2O3. Slightly less than 10% of the total dose of As2O3 is renal excreted. Tissue accumulation of arsenic occurs during As2O3 treatment. After completion of As2O3 therapy, urinary arsenic excretion continues for some time. At about four weeks after cessation of As2O3, plasma arsenic level declines to baseline levels, and arsenic urinary excretion stops.
The pharmacokinetics of oral-As2O3 follows a similar pattern. The peak plasma arsenic level achieved with the same dosage of oral-As2O3 (10 mg) is lower at 0.2–0.6 μmol/L. However, owing to gradual intestinal absorption, the area-under-the-curve (AUC) absorption of oral-As2O3 is comparable with that of iv-As2O3, implying that the bioavailability of oral-As2O3 is comparable with iv-As2O3[20]. The much lower peak arsenic plasma level after oral-As2O3 administration is an important reason for the improved safety as compared with iv-As2O3. Oral-As2O3 is also predominantly renal excreted.
Hepatic toxicity
Liver function tests (LFT) derangement is one of the commonest side effects. Typically, there is a hepatitis with increases in alanine and aspartate aminotransferases, starting about five to ten days after drug administration. The peak transaminase levels rarely exceed five times the upper reference value[21]. Increases in bilirubin and ductal enzymes including alkaline phosphatase and γ-glutamyl transpeptidase are uncommon, and if present should prompt investigations for other causes of cholestasis. A few cases of fulminant hepatic failure had been reported when As2O3 was used in patients with newly diagnosed APL[14]. However, this phenomenon has not been confirmed subsequently, suggesting that the observation is fortuitous only. As2O3 can be considered to be safe in all stages of APL.
When the transaminase elevations are less than three times normal, our experience shows that As2O3 therapy can be continued at half the original dose. The liver function usually normalizes within a week, and resumption of full-dose treatment or at the reduced dose is then well tolerated[14]. This transient hepatitis may or may not recur during subsequent As2O3 treatment. However, when the transaminases exceed three times normal, temporary cessation of As2O3 treatment may be needed. The hepatitis usually resolves within a week, and treatment at half the original dose, with gradual escalation to full dose, can be reinstated.
Different from iv-As2O3, the full dose of oral-As2O3 passes first through the portal circulation and therefore the liver. Despite this first-pass effect, oral-As2O3 does not cause more liver toxicity, so that the frequency and severity of LFT derangement are comparable with iv-As2O3.
Data from chronic arsenic poisoning suggest that liver fibrosis, cirrhosis and hepatocellular carcinoma may occur[5,26,27]. Therefore, the toxicity of prolonged therapeutic use of As2O3 may require close monitoring. So far, cirrhosis and hepatocellular carcinoma in after treatment with therapeutic doses of As2O3 have not been reported. In chronic carriers of the hepatitis B virus (HBV), lamivudine prophylaxis to prevent viral reactivation has been adovcated[28], although such a strategy has not been validated in control trials. Since both HBV and As2O3 predispose to cirrhosis and hepatocellular carcinoma[29], it may be prudent to prescribe prophylactic anti-viral treatment to avoid potential synergistic As2O3 and HBV hepatic damage.
Finally, other hepatotoxic drugs used in the clinical course of leukaemia, including antibiotics and the azole anti-fungal drugs, should also be used with caution during As2O3 therapy.
Dermatologic toxicity
Chronic arsenic exposure results in various skin manifestations, including hyperpigmentation, keratosis, bowenoid lesions and squamous cell carcinoma. The therapeutic use of As2O3 results in cumulative doses well below that reported for environmental or occupational arsenic exposure that leads to these skin manifestations[5]. The commonest dermatologic problem during As2O3 treatment is increased skin pigmentation[27]. So far, squamous cell carcinoma has not been reported. Abnormal pigmentation is reversible after cessation of As2O3 treatment. If severe or persistent pigmentation occurs, other causes potentially related to the underlying leukaemia, including porphyria and hemosiderosis, will have to be excluded[30].
Rashes are the next commonest problem. A late-onset painful, erythematous rash can be seen after prolonged arsenic treatment, which may be related partly to the vasoconstrictive effects of arsenic[31]. The concomitant use of ATRA may also worsen the rashes. In severe cases, temporary dose reduction or even cessation of As2O3 may be required. An allergic type of morbilliform to pruritic rash has been observed[32]. Rashes respond well to corticosteroid treatment, and As2O3 treatment can be continued without interruption. Swelling of hands, legs and face has also been found[33], which may be related to fluid retention as part of the APL differentiation syndrome.
Another intriguing side effect of As2O3 treatment is reactivation of latent herpes virus infection[34]. Both herpes simplex and herpes zoster reactivation may occur. In fact, herpetic reactivation had been found to complicate arsenic poisoning since the late nineteenth century. During the British beer arsenic-poisoning episode of 1900, herpetic skin eruptions increased to epidemic proportions. Dr E.S. Reynolds, who investigated these cases of “alcoholic neuritis”, was prompted by the frequent shingles (herpes zoster) in the victims to conclude that “there must be arsenic in the beer the people are drinking … because, of all known drugs arsenic is the only drug which causes shingles.” [35]. During As2O3 treatment, herpes zoster reactivation occurs in up to 25% of patients within the first year of treatment[36]. Recognition of the association is important, because timely treatment of herpes zoster may shorten the duration of the attack and decrease post-herpetic complications.
Hematologic toxicity
Because of a partial differentiation effect of As2O3 on the leukemic clone, leucocytosis occurs commonly. On continuation of As2O3 therapy, suppression of the leukaemic clone may lead to leucopenia. With haematologic remission and cessation of As2O3 treatment, leucopenia recovers quickly. In As2O3 maintenance treatment during remission, which lasts two weeks only, leucopenia rarely if ever develops[17].
In patients with other malignancies involving the marrow, including acute leukaemia, myelodysplasia, myeloma and lymphoma[37,38], continuous daily treatment with As2O3 (10 mg daily) may cause mild[24] to severe pancytopenia[39]. Indeed, myelosuppression is the main dose-limiting side effect in patients treated with As2O3 for leukaemias other than APL[37,38]. Concomitant administration of other myelosuppressive drugs may further aggravate the myelotoxicity. Therefore, As2O3 dosage may have to be reduced when concurrent chemotherapy or radiotherapy is used. In severely leucopenic cases, treatment with haematopoietic growth factors such as granulocyte colony stimulating factor rapidly restores normal leucocyte counts.
Cardiac toxicity
At therapeutic doses, As2O3 treatment results in prolongation of the QT interval[15,32,40]. Electrocardiographic (ECG) studies in patients receiving iv-As2O3 have shown significant QT interval prolongation in 35% of cases, with symptomatic torsades de pointes in 1–3% of cases[15,41]. Continuous ambulatory ECG monitoring detects various cardiac dysrrhythmias in higher frequencies[32]. The majority of these ECG abnormalities are asymptomatic. There are only few reports of patients with suspected cardiac death during As2O3 treatment. Even in these cases, arrhythmia attributable entirely to arsenic has not been unequivocally documented[42,43].
These cardiac toxicities have been investigated in vitro. Guinea pig papillary muscles showed delayed cardiac repolarization during As2O3 administration at 10–50 mg/kg[44]. Rabbit heart, however, did not show any detectable conduction abnormalities with short-term perfusion of As2O3 to up to 30 μmol/L, and cardiac conduction and repolarization abnormalities only occurred with short-term infusion of 300 μmol/L of As2O3[45]. On chronic administration of As2O3 at 30 μmol/L, QT prolongation and polymorphic ventricular tachycardia might result[45]. These conduction abnormalities may be due to decrease in surface expression of the potassium channel IKr protein hERG. This is related to arsenic-induced interference of hERG trafficking, as a result of inhibition of hERG-chaperone complexes formation[46]. The As2O3 concentration required to reduce hERG-chaperone formation by 50% was 3 μmol/L. Further studies have shown that the IKr and IKs potassium channels were inhibited by As2O3. The IC50 for IKr was 0.14±0.01 μmol/L, and that for IKs 1.13±0.06 μmol/L. However, another potassium channel IK-ATP was activated by As2O3 at 1 μmol/L[47]. Hence, the net effects may depend on a balance of activation and blockade of multiple repolarization potassium channels. It must be noted that the crucial observation of all these studies is that cardiac conduction defects are dependent on As2O3 concentrations, with a much increased risk when it exceeds 1 μmol/L.
Pharmacokinetic studies have shown that oral-As2O3 results in a lower peak plasma arsenic level, typically below 1 μmol/L (usually ranging from 0.2–0.6 μmol/L). This concentration falls well below 1–30 μmol/L required to lead to cardiac conduction defects in vitro. Indeed, continuous ambulatory ECG monitoring in patients on oral-As2O3 has shown that although QTc is prolonged during As2O3 administration, QTc prolongation >30 milliseconds only occurs at one time-point (2 hours) after oral-As2O3, resulting in QTc >500 milliseconds in about 20% of patients, all within 4 hours of oral-As2O3 administration. No ventricular proarrhythmias are observed[48]. The more favorable cardiac safety profile of oral-As2O3 may be due to the much lower plasma arsenic levels reached during oral As2O3 administration.
In most of the cases of symptomatic cardiac arrhythmias reported previously, co-existing risk factors existed, including electrolyte abnormalities such as hypokalaemia and hypomagnesaemia, impaired cardiac function due to underlying heart diseases, and old age. Previous anthracycline exposure, however, did not appear to be important[49]. Although the risk of cardiac arrhythmias is minimal for patients without underlying heart diseases, certain precautions are nevertheless prudent. Firstly, As2O3 dosage should be reduced to the minimal effective amount, especially in elderly patients with impaired renal function. Secondly, concurrent drugs known to prolong the QT interval, including type I anti-arrhythmic agents, anti-histamines and tricyclic antidepressants, should be avoided[50]. Thirdly, electrolyte levels, especially potassium and magnesium, should be regularly tested and maintained at normal levels. Finally, regular ECG monitoring during the initiation of As2O3 treatment is needed, until the risks of arsenic-induced arrhythmia are clarified. Patients should be fully informed of the risks of arrhythmias, and cardiac symptoms including palpitations should be prompted reported. The efficacy of prophylactic anti-arrhythmic agents in symptomatic cases is undefined[46]. Finally, oral-As2O3, with its much more favorable cardiac safety profile, may be the preferred formulation for long-term As2O3 therapy[48].
Leucocytosis and the APL differentiation syndrome
Leucocytosis and the APL differentiation syndrome are important complications during the induction treatment of APL with As2O3, occurring in 37–58% of cases[15,32,51]. The two conditions are closely related. Both complications occur only in APL, and have not been reported after As2O3 treatment in other leukaemias and malignancies. A rapid increase in leucocyte and promyelocyte counts is reported in up to 50% of APL patients on As2O3. This may be accompanied by fever, fluid retention, pulmonary infiltrates, elevated lactate dehydrogenase levels, and occasionally pleural and pericardial effusions[33]. The clinical and laboratory features may be indistinguishable from the ATRA-syndrome that occurs during ATRA treatment of APL, where similar problems develop as the leukaemic clone differentiates and proliferates. The APL differentiation syndrome usually occurs within the first two weeks of As2O3 treatment, during which regular monitoring of the leucocyte count is mandatory. However, unlike the ATRA syndrome, As2O3-induced APL differentiation syndrome is rarely if ever life-threatening.
The early recognition of the As2O3-induced APL differentiation syndrome is critical to its subsequent successful treatment. Dexamethasone leads to symptomatic improvement, and may be used to tide over the whole period of leucocytosis until the leucocyte count falls later due to arsenic-induced apoptosis of the APL cells. Practically, however, cyto-reduction with chemotherapy is more effective and safe. Drugs including hydroxyurea, daunorubicin, idarubicin and mitoxantrone have all been successfully used. The current recommendation is to start chemotherapy once the leucocyte count rises above 5×109–10×109/L. Since an anthracycline is used in almost all regimens for induction treatment of APL, it will be appropriate to start the drug early during the leucocytosis. Another reason for early treatment of leucocytosis is because high leucocyte counts have been associated with central nervous system (CNS) deposits and infarction[52], and possibly extramedullary relapses in the future. It will be prudent to withhold As2O3 therapy if clinical signs of the APL differentiation syndrome occur. Subsequent to successful treatment of the syndrome, the re-institution of As2O3 is not compromised.
Neurologic toxicity
Peripheral neuropathy is reported in up to 10% of As2O3 treated patients[18,32]. The incidence may be higher when other predisposing conditions are present, including old age, diabetes mellitus, multiple myeloma, and the concurrent administration of neurotoxic drugs. A glove and stocking sensory neuropathy is typical, with electrophysiological studies showing reduced sensory action potentials with delayed conduction. Muscle atrophy has been reported in occasional cases after prolonged exposure[33]. Gradual improvement occurs when As2O3 is reduced in dosage or stopped. Sural nerve biopsies in a few severe cases have not shown specific histopathological features. Severe functional deficits are unusual, and the presence of serious neuropathies during As2O3 treatment should prompt investigations for other causes.
The blood brain barrier prevents heavy metals, including arsenic, from penetrating the CNS. Therefore, CNS side effects and encephalopathies during As2O3 therapy have not been reported. Hence, mental confusion in a patient on As2O3 should lead to investigations for other causes, such as CNS leukaemia, viral encephalitis, alcoholism or metabolic derangements[30]. For similar reasons, the CNS may be a sanctuary site for leukaemic cells, and isolated CNS relapse in patients who have remitted following As2O3 treatment has been described frequently[53]. Suspected Wernicke’s encephalopathy associated with As2O3 treatment has been reported[54]. However, abnormalities in thiamine pyrophosphate and erythrocyte transketolase levels in consecutive patients on prolonged As2O3 treatment have not been observed, so that routine vitamin supplements do not seem to be warranted. Long-term follow-up has not shown unusual CNS manifestations in patients after chronic treatment with As2O3, although behavioral abnormalities have been reported in animals with chronic arsenic exposure since birth[55].
Entry of arsenic into the CNS, however, may occur when the blood brain barrier is breached. In a case of meningeal relapse of APL treated with oral-As2O3, penetration of arsenic into the cerebrospinal fluid to therapeutically meaningful levels has been observed[56]. Therefore, in patients in whom the blood brain barrier is compromised, As2O3 will have to be administered with caution[57].
A prominent but innocuous side effect is severe headache when As2O3 is administered together with ATRA[58]. Computerized tomographic scan and fundoscopic examination have occasionally shown signs of pseudotumor cerebri[59]. Although this side effect is distressful and alarming, the headache responds swiftly to analgesic treatment and dose splitting of ATRA or As2O3, and no long-term sequelae have been reported.
Miscellaneous toxicities
Gastrointestinal upset is frequently reported even with iv-As2O3[32]. For patients on oral-As2O3, mild nausea and dyspepsia are frequent[21,22]. Most patients respond to symptomatic treatment and cessation or dose reduction of As2O3 is unnecessary. Carcinogenicity and mutagenicity are common concerns for anti-neoplastic agents. In populations exposed to chronic environmental arsenic poisoning, a higher incidence of skin and liver cancer is observed, together with chromosomal instability[60]. An increased incidence of cancer of the skin, lung and liver has also been reported after industrial and agricultural arsenic exposure[61,62]. The risk of secondary cancers after As2O3 treatment is undefined. Solid tumors might be a chance occurrence in As2O3-treated patients[63]. Arsenic is a known mutagen in mouse embryos, especially with concomitant folate deficiency[64]. There is no experience of the use of As2O3 in pregnant woman, so that the fetal side effects of therapeutic As2O3 are unknown. Arsenic is excreted in the milk and breast-feeding should be avoided during As2O3 treatment.
Dose reduction of As2O3
The side effects of As2O3 are dose-related. The predominant renal excretion of arsenic means that in patients with impaired kidney function, As2O3 dosage should be reduced. With appropriate dose adjustment and monitoring of arsenic level, a patient on continuous ambulatory peritoneal dialysis with relapsed APL had been successfully treated with oral- As2O3 [65]. Due to the relatively fewer side effects as compared with chemotherapy, As2O3 is the drug of choice for treating APL in elderly patients[66]. However, the volume of distribution is lower in elderly patients, so that the arsenic concentration may be higher for the same dosage of As2O3. It may be prudent to reduce to half the dose of As2O3 for patients above the age of 70 years. When prolonged administration of As2O3 is planned, especially for myeloma, myelodysplasia or low-grade lymphoma, the cumulative dosage and tissue concentration of arsenic becomes an important issue. In this connection, it is interesting to note that a lower As2O3 dosage of 0.8 mg·kg-1·d-1 has been reported to be equally effective for APL[67].
Conclusions
Arsenic has a remarkable position in medicine. It is both poisonous and therapeutically useful. Its efficacy in differentiating APL cells makes it the treatment of choice for relapsed cases, with the possibility of replacing chemotherapy in frontline and maintenance treatment. The toxicity profile of both iv- and oral-As2O3 is acceptable compared with most chemotherapy regimens[68]. The frequencies of the various side effects in studies involving APL and patients with other malignancies are summarized in Table 1. Safety may be enhanced if dosing precautions are rigorously adhered to. Although cardiac toxicity is a major concern, the frequency of life-threatening arrhythmia is low, becoming insignificant with oral-As2O3. Most of the safety data are derived from APL treatment with As2O3. Whether the risk-benefit profile is applicable to other diseases remains to be clarified.
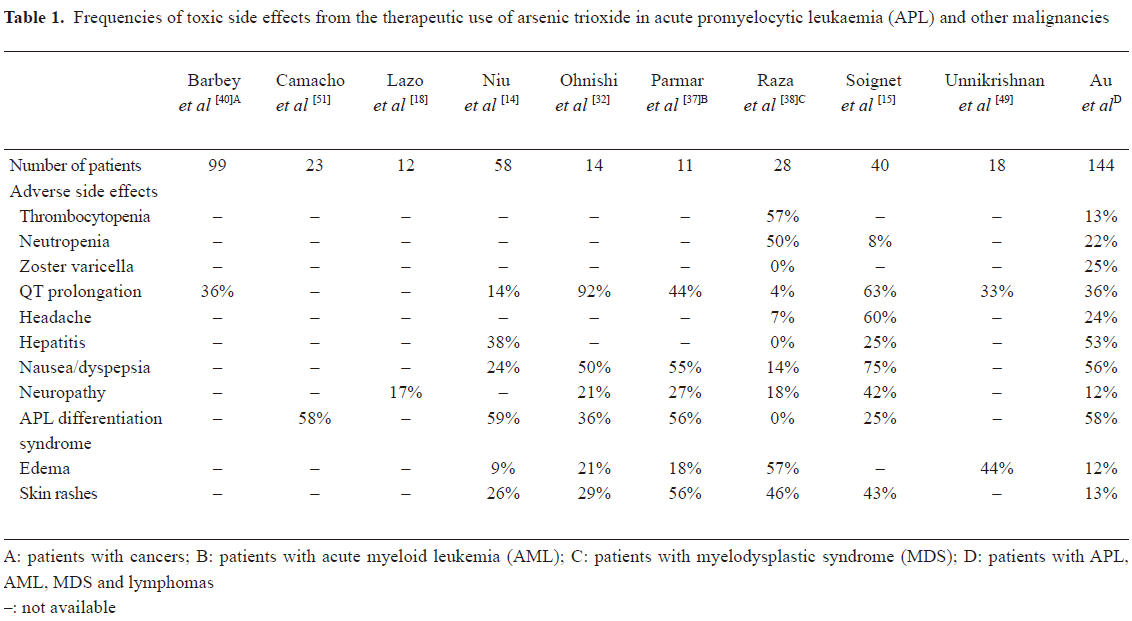
Full table
Acknowledgement
The authors thank the the SK YEE Medical Foundation generously supported the production of oral arsenic trioxide.
Footnote
Conflict of interest
The University of Hong Kong holds a temporary patent for the use of oral arsenic trioxide in the treatment of leukaemia. Prof Yok-Lam KWONG is an employee of the University of Hong Kong.
References
- Shen ZX, Shi ZZ, Fang J, Gu BW, Li JM, Zhu YM, et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci USA 2004;101:5328-35.
- Mari F, Bertol E, Fineschi V, Karch SB. Channelling the Emperor: what really killed Napoleon? J R Soc Med 2004;97:397-9.
- Kwong YL, Todd D. Delicious poison: arsenic trioxide for the treatment of leukemia. Blood 1997;89:3487-8.
- Chowdhury UK, Biswas BK, Chowdhury TR, Samanta G, Mandal BK, Basu GC, et al. Groundwater arsenic contamination in Bangladesh and West Bengal, India. Environ Health Perspect 2000;108:393-7.
- Mazumder DN, Das Gupta J, Santra A, Pal A, Ghose A, Sarkar S. Chronic arsenic toxicity in west Bengal—the worst calamity in the world. J Indian Med Assoc 1998;18:4-7.
- Douer D, Tallman MS. Arsenic trioxide: new clinical experience with an old medication in hematologic malignancies. J Clin Oncol 2005;23:2396-410.
- Wang ZY. Ham-Wasserman lecture: treatment of acute leukemia by inducing differentiation and apoptosis. Hematology (Am Soc Hematol Educ Program) 2003.1-13.
- Ratnaike RN. Acute and chronic arsenic toxicity. Postgrad Med J 2003;79:391-6.
- Schoolmeester WL, White DR. Arsenic poisoning. South Med J 1980;73:198-208.
- Lin CJ, Wu MH, Hsueh YM, Sun SS, Cheng AL. Tissue distribution of arsenic species in rabbits after single and multiple parenteral administration of arsenic trioxide: tissue accumulation and the reversibility after washout are tissue-selective. Cancer Chemother Pharmacol 2005;55:170-8.
- Annonymous. Toxic summary for arsenic. Risk Assessment Information System database 1992; Cited Nov 2007. Available from URL: .http://risk.lsd.ornl.gov/tox/profiles/arseni_c.shtml
- Santra A, Das Gupta J, De BK, Roy B, Guha Mazumder DN. Hepatic manifestations in chronic arsenic toxicity. Indian J Gastroenterol 1999;18:152-5.
- Datta DV, Narang AP, Arya P, Sahni MM, Banerjee CK, Walia BN, et al. Indian childhood cirrhosis. Lancet 1979;2:641.
- Niu C, Yan H, Yu T, Sun HP, Liu JX, Li XS, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood 1999;94:3315-24.
- Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol 2001;19:3852-60.
- Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med 1998;339:1341-8.
- Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood 1997;89:3354-60.
- Lazo G, Kantarjian H, Estey E, Thomas D, O’Brien S, Cortes J. Use of arsenic trioxide (As2O3) in the treatment of patients with acute promyelocytic leukemia: the M. D. Anderson experience. Cancer 2003;97:2218-24.
- Raffoux E, Rousselot P, Poupon J, Daniel MT, Cassinat B, Delarue R, et al. Combined treatment with arsenic trioxide and all-trans-retinoic acid in patients with relapsed acute promyelocytic leukemia. J Clin Oncol 2003;21:2326-34.
- Kumana CR, Au WY, Lee NS, Kou M, Mak RW, Lam CW, et al. Systemic availability of arsenic from oral arsenic-trioxide used to treat patients with hematological malignancies. Eur J Clin Pharmacol 2002;58:521-6.
- Au WY, Kumana CR, Kou M, Mak R, Chan GC, Lam CW, et al. Oral arsenic trioxide in the treatment of relapsed acute promyelocytic leukemia. Blood 2003;102:407-8.
- Lu DP, Qiu JY, Jiang B, Wang Q, Liu KY, Liu YR, et al. Tetra-arsenic tetra-sulfide for the treatment of acute promyelocytic leukemia: a pilot report. Blood 2002;99:3136-43.
- Borad MJ, Swift R, Berenson JR. Efficacy of melphalan, arsenic trioxide, and ascorbic acid combination therapy (MAC) in relapsed and refractory multiple myeloma. Leukemia 2005;19:154-6.
- Bahlis NJ, McCafferty-Grad J, Jordan-McMurry I, Neil J, Reis I, Kharfan-Dabaja M, et al. Feasibility and correlates of arsenic trioxide combined with ascorbic acid-mediated depletion of intracellular glutathione for the treatment of relapsed/refractory multiple myeloma. Clin Cancer Res 2002;8:3658-68.
- Wang ZY. Arsenic compounds as anticancer agents. Cancer Chemother Pharmacol 2001;48:S72-6.
- Guha Mazumder DN. Arsenic and liver disease. J Indian Med Assoc 2001;99:311-20.
- Liu J, Zheng B, Aposhian HV, Zhou Y, Chen ML, Zhang A, et al. Chronic arsenic poisoning from burning high-arsenic-containing coal in Guizhou, China. Environ Health Perspect 2002;110:119-22.
- Lau GK, Yiu HH, Fong DY, Cheng HC, Au WY, Lai LS, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology 2003;125:1742-9.
- Lu JN, Chen CJ. Prevalence of hepatitis B surface antigen carrier status among residents in the endemic area of chronic arsenicism in Taiwan. Anticancer Res 1991;11:229-33.
- Hughes GS Jr, Davis L. Variegate porphyria and heavy metal poisoning from ingestion of “moonshine”. South Med J 1983;76:1027-9.
- Tsai SM, Wang TN, Ko YC. Mortality for certain diseases in areas with high levels of arsenic in drinking water. Arch Environ Health 1999;54:186-93.
- Ohnishi K, Yoshida H, Shigeno K, Nakamura S, Fujisawa S, Naito K, et al. Arsenic trioxide therapy for relapsed or refractory Japanese patients with acute promyelocytic leukemia: need for careful electrocardiogram monitoring. Leukemia 2002;16:617-22.
- Huang SY, Chang CS, Tang JL, Tien HF, Kuo TL, Huang SF, et al. Acute and chronic arsenic poisoning associated with treatment of acute promyelocytic leukaemia. Br J Haematol 1998;103:1092-5.
- Tanvetyanon T, Nand S. Herpes zoster during treatment with arsenic trioxide. Ann Hematol 2004;83:198-200.
- Satterlee HS. The arsenic-poisoning epidemic of 1900. Its relation to lung cancer in 1960 - an exercise in retrospective epidemiology. N Engl J Med 1960;263:676-84.
- Au WY, Kwong YL. Frequent varicella zoster reactivation associated with therapeutic use of arsenic trioxide: portents of an old scourge. J Am Acad Dermatol 2005;53:890-2.
- Parmar S, Rundhaugen LM, Boehlke L, Riley M, Nabhan C, Raji A, et al. Phase II trial of arsenic trioxide in relapsed and refractory acute myeloid leukemia, secondary leukemia and/or newly diagnosed patients at least 65 years old. Leuk Res 2004;28:909-19.
- Raza A, Buonamici S, Lisak L, Tahir S, Li D, Imran M, et al. Arsenic trioxide and thalidomide combination produces multi-lineage hematological responses in myelodysplastic syndromes patients, particularly in those with high pre-therapy EVI1 expression. Leuk Res 2004;28:791-803.
- Hermine O, Dombret H, Poupon J, Arnulf B, Lefrere F, Rousselot P, et al. Phase II trial of arsenic trioxide and alpha interferon in patients with relapsed/refractory adult T-cell leukemia/lymphoma. Hematol J 2004;5:130-4.
- Barbey JT, Soignet S. Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia. Ann Intern Med 2001;135:842-3.
- Barbey JT, Pezzullo JC, Soignet SL. Effect of arsenic trioxide on QT interval in patients with advanced malignancies. J Clin Oncol 2003;21:3609-15.
- Unnikrishnan D, Dutcher JP, Varshneya N, Lucariello R, Api M, Garl S, et al. Torsades de pointes in 3 patients with leukemia treated with arsenic trioxide. Blood 2001;97:1514-6.
- Westervelt P, Brown RA, Adkins DR, Khoury H, Curtin P, Hurd D, et al. Sudden death among patients with acute promyelocytic leukemia treated with arsenic trioxide. Blood 2001;98:266-71.
- Chiang CE, Luk HN, Wang TM, Ding PY. Prolongation of cardiac repolarization by arsenic trioxide. Blood 2002;100:2249-52.
- Wu MH, Lin CJ, Chen CL, Su MJ, Sun SS, Cheng AL. Direct cardiac effects of As2O3 in rabbits: e9idence of reversible chronic toxicity and tissue accumulation of arsenicals after parenteral administration. Toxicol Appl Pharmacol 2003;189:214-20.
- Ficker E, Kuryshev YA, Dennis AT, Obejero-Paz C, Wang L, Hawryluk P, et al. Mechanisms of arsenic-induced prolongation of cardiac repolarization. Mol Pharmacol 2004;66:33-44.
- Drolet B, Simard C, Roden DM. Unusual effects of a QT-prolonging drug, arsenic trioxide, on cardiac potassium currents. Circulation 2004;109:26-9.
- Siu CW, Au WY, Yung C, Kumana CR, Lau CP, Kwong YL, et al. Effects of oral arsenic trioxide therapy on QT intervals in patients with acute promyelocytic leukemia: implications on long-term cardiac safety. Blood 2006;108:103-6.
- Unnikrishnan D, Dutcher JP, Garl S, Varshneya N, Lucariello R, Wiernik PH. Cardiac monitoring of patients receiving arsenic trioxide therapy. Br J Haematol 2004;124:610-7.
- Ohnishi K, Yoshida H, Shigeno K, Nakamura S, Fujisawa S, Naito K, et al. Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia. Ann Intern Med 2000;133:881-5.
- Camacho LH, Soignet SL, Chanel S, Ho R, Heller G, Scheinberg DA, et al. Leukocytosis and the retinoic acid syndrome in patients with acute promyelocytic leukemia treated with arsenic trioxide. J Clin Oncol 2000;18:2620-5.
- Roberts TF, Sprague K, Schenkein D, Miller KB, Relias V. Hyperleukocytosis during induction therapy with arsenic trioxide for relapsed acute promyelocytic leukemia associated with central nervous system infarction. Blood 2000;96:4000-1.
- Au WY, Ma SK, Ooi C, Liang R, Kwong YL. Unusual manifestations of acute leukemia. Case 1. CNS extramedullary relapse of acute promyelocytic leukemia after arsenic trioxide-induced remission. J Clin Oncol 2000;18:3435-7.
- Yip SF, Yeung YM, Tsui EY. Severe neurotoxicity following arsenic therapy for acute promyelocytic leukemia: potentiation by thiamine deficiency. Blood 2002;99:3481-2.
- Rodriguez VM, Carrizales L, Mendoza MS, Fajardo OR, Giordano M. Effects of sodium arsenite exposure on development and behavior in the rat. Neurotoxicol Teratol 2002;24:743-50.
- Au WY, Tam S, Fong BM, Kwong YL. Elemental arsenic entered the cerebrospinal fluid during oral arsenic trioxide treatment of meningeal relapse of acute promyelocytic leukemia. Blood 2006;107:3012-3.
- Au WY, Tam S, Kwong YL. Entry of elemental arsenic into the central nervous system in patients with acute promyelocytic leukemia during arsenic trioxide treatment. Leuk Res 2007. [Epub ahead of print].
- Au WY, Chim CS, Lie AK, Liang R, Kwong YL. Combined arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia recurring from previous relapses successfully treated using arsenic trioxide. Br J Haematol 2002;117:130-2.
- Galm O, Fabry U, Osieka R. Pseudotumor cerebri after treatment of relapsed acute promyelocytic leukemia with arsenic trioxide. Leukemia 2000;14:343-4.
- Chou WC, Hawkins AL, Barrett JF, Griffin CA, Dang CV. Arsenic inhibition of telomerase transcription leads to genetic instability. J Clin Invest 2001;108:1541-7.
- Axelson O, Dahlgren E, Jansson CD, Rehnlund SO. Arsenic exposure and mortality: a case-referent study from a Swedish copper smelter. Br J Ind Med 1978;35:8-15.
- Luchtrath H. The consequences of chronic arsenic poisoning among Moselle wine growers. Pathoanatomical investigations of post-mortem examinations performed between 1960 and 1977. J Cancer Res Clin Oncol 1983;105:173-82.
- Au WY, Kumana CR, Lam CW, Cheng VC, Shek TW, Chan EY, et al. Solid tumors subsequent to arsenic trioxide treatment for acute promyelocytic leukemia. Leuk Res 2007;31:105-8.
- Fascineli ML, Hunter ES 3rd, De Grava Kempinas W. Fetotoxicity caused by the interaction between zinc and arsenic in mice. Teratog Carcinog Mutagen 2002;22:315-27.
- Au WY, Cheung GT, Yuen TW, Kumana CR, Kwong YL. Successful treatment of relapsed acute promyelocytic leukemia in a patient receiving continuous ambulatory peritoneal dialysis with oral arsenic trioxide. Arch Intern Med 2005;165:1067-8.
- Fenaux P, Chevret S, de Botton S. Treatment of older adults with acute promyelocytic leukaemia. Best Pract Res Clin Haematol 2003;16:495-501.
- Shen Y, Shen ZX, Yan H, Chen J, Zeng XY, Li JM, et al. Studies on the clinical efficacy and pharmacokinetics of low-dose arsenic trioxide in the treatment of relapsed acute promyelocytic leukemia: a comparison with conventional dosage. Leukemia 2001;15:735-41.
- Au WY, Lie AK, Chim CS, Liang R, Ma SK, Chan CH, et al. Arsenic trioxide in comparison with chemotherapy and bone marrow transplantation for the treatment of relapsed acute promyelocytic leukaemia. Ann Oncol 2003;14:752-7.
