Preparation of ion-activated in situ gel systems of scopolamine hydrobromide and evaluation of its antimotion sickness efficacy1
Introduction
Scopolamine (SCOP), a naturally occurring antimuscarinic agent, has been used for the prevention and treatment of nausea and vomiting associated with motion sickness for almost 200 years. However, this drug has a low and variable oral bioavailability (10.7%–48.2%) because of extensive hepatic first-pass metabolism[1]. The variability in absorption and poor bioavailability of oral SCOP indicate that this route is neither reliable nor effective for this drug. In addition, the SCOP transdermal patch has been developed. Pharmacokinetic studies showed that peak plasma concentrations (Cmax) were reached about 12–16 h after dosing[2,3]. Moreover, SCOP concentrations in plasma also declined more slowly after the patches were removed than after an iv dose. The reported delay in the drug reaching the circulation after patch application, and the potential for prolonged unwanted side effects such as dry mouth, dizziness, or blurred vision, led us to look for an alternative route of administration to oral or transdermal delivery.
About 50 years ago, the nasal absorption of SCOP in solution was reported by Hyde et al[4]. They found that SCOP could produce faster responses and greater therapeutic activity than an equivalent oral dose. Recently, Scopolamine Hydrobromide 0.2% Nasal Spray was on the market. However, problems still exist, such as a short duration of therapeutic effect due to the rapid elimination of the instilled drug from the nasal cavity by mucociliary beating (a clearance half-life of 15 min[5,6]), and consequently, a frequent dosing regimen was needed.
Several approaches have been used in designing nasal dosage forms with high absorption and lasting drug effects. For example, hydrogels made of carbopol, methylcellulose, and hydroxypropyl methylcellulose can increase the drug contact time with the mucosa, therefore reducing its rapid clearance and resulting in increased absorption. However, viscous gels have the disadvantage of being difficult to administer.
These problems have been overcome by the use of in situ gels. In situ gels are instilled as low viscosity solutions into the nasal cavity, and upon contact with the nasal mucosa, the polymer changes conformation producing a gel. This type of gel combines the advantages of a solution, administration convenience, and exact dosing, with the favorable residence time of a gel. The phase transition can be induced by a shift in pH, as for cellulose acetate phthalate[7], a shift in temperature as for the thermo gelling Poloxamer 407[8,9] or by the presence of cations as for gellan gum[10].
Taken the information into account, in the present study a nasal in situ gel system for SCOP was developed. Since nasal mucosa is covered with approximately 0.1 mL mucus in humans, which consists of sodium, potassium, and calcium ions, a cation-responsive polymer gellan gum was chosen. Gellan gum is an anionic deacetylated, exocellular polysaccharide secreted by Pseudomonas elodea with a tetrasaccharide repeating unit of 1β-L-rhamnose, 1β-D-glucuronic, acid and 2β-D-glucose. The mechanism of gelation involves the formation of double-helical junction zones followed by aggregation of the double-helical segments to form a 3-D network by complexation with cations and hydrogen bonding with water[11–13]. Gellan gum has been widely used in ophthalmic drug delivery[10,14]. Nevertheless, there is little literature on its potential as a vehicle for nasal application.
The present work aimed to formulate an ion-activated in situ gel for SCOP with gellan gum and to investigate its viscosity, in vitro release, nasal ciliotoxicity, the nasal mucosal residence time, and antimotion sickness capacity.
Materials and methods
Materials SCOP was gifted by the Department of Pharmaceutics, School of Pharmacy, Fudan University (Shanghai, China). Gellan gum was purchased from ZhongWei Biochemical Ltd (Shanghai, China). The SCOP injection (0.3 g/L) was obtained from Shanghai Harvest (Shanghai, China). Technetium-99m-diethylentriamine pentaacetic acid (99mTc-DTPA) was prepared in the Department of Nuclear Pharmacy at Fudan University (China).
The ion compositions of artificial nasal fluid included 150±32 mmol/L Na+, 41±18 mmol/L K+, and 4±2 mmol/L Ca2+, prepared according to Lorin et al[15]. All other reagents were of commercially analytical grade.
Preparation of nasal formulations A certain amount of gellan gum was added to deionized water and dissolved by heating to 100 °C with moderate stirring. After cooling to below 40 °C, SCOP (0.4%, w/v), mannitol (5%, w/v), and chlorhexidine acetate (0.01%, w/v) were added and mixed well. Three kinds of SCOP in situ gels were prepared at the concentrations of gellan gum which were 0.2%, 0.5%, and 1.0% (w/v), respectively. The pH of all the formulations was between 4.0 and 6.0.
Viscosity measurement of in situ gel formulations The viscosity of the gellan gum formulations, either in solution or in gel made with artificial nasal fluid instead of 5% mannitol, were determined with a rotational viscometer (NDJ-5S, Shanghai, China) using a 20 mL aliquot of the sample. Measurements were performed using suitable spindle number at 6, 12, 30, 60 r/min, and the temperature was maintained at 37 °C. The viscosity was read directly from the viscometer display. All measurements were made in triplicate.
In vitro release of SCOP from gels The in vitro release of SCOP from the gels was measured through a cellulose acetate dialysis membrane employing Valia-Chien diffusion cells (2TYTP3A, GongYi Ltd, Shanghai, China) with a diffusional area of 1.75 cm2 and a receptor compartment volume of 16 mL. The receptor compartment, containing artificial nasal fluid to allow the establishment of the “sink condition” and to simulate the physiological condition, was stirred and thermostated at 37±0.5 °C in an incubator during the experiment. 2 mL gellan gum preparation loaded with 0.4% of the drug (n=6) was placed in the donor compartment. At predetermined time intervals, samples (1 mL) of receiving solution were withdrawn and replaced with the same volume of fresh release medium. The amount of SCOP in each sample was determined by HPLC (LC-10A, Shimadzu Co Ltd, Kyoto, Japan).
Chromatographic separation was achieved using a Dikma DiamonsilTM C18 column (Dikma Co Ltd, Beijing, China, 5 μm, 200 mm×4.6 mm) and a precolumn (Nova-Pak, 10 μm, C18 15, 220, Waters) at 40 °C. The mobile phase was a mixture of 0.008 mol/L sodium dodecyl sulphate containing 0.001 mol/L hydrochloric acid (pH 3.0) and acetonitrile (50:50 v/v) at a flow rate of 1 mL/min. The UV absorbance of the effluent was monitored (SPD-10A, Shimadzu Co Ltd, Kyoto, Japan) at a wavelength of 210 nm.
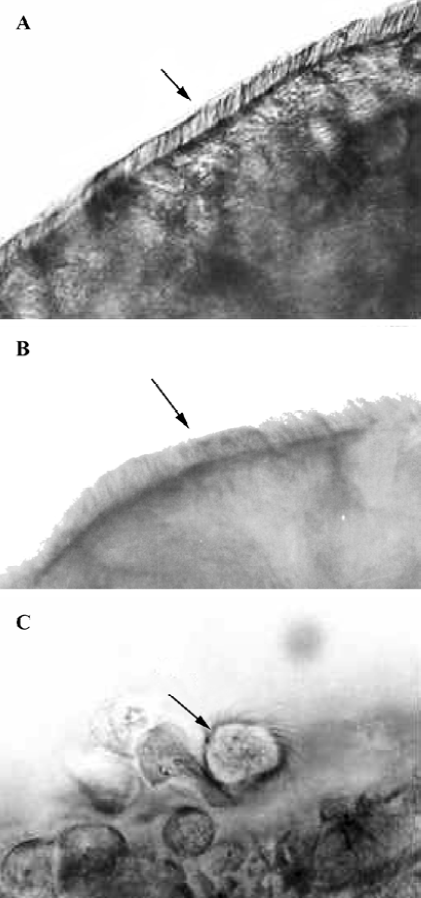
Nasal ciliotoxicity Nasal ciliotoxicity studies were carried out using an in situ toad palate model[16]. In brief, the upper palate of the toads (30–40 g, male and female, Experimental Animal Center of Fudan University, China, n=6) was exposed and treated with about 0.5 mL in situ gel (0.5% gellan gum) for 4 h. Then the test formulation was removed by washing the palate with saline, about 5×3 mm of the palate was dissected and the mucocilia was examined with a electron microscope (Nikon Fx-35A, Tokyo, Japan) at enlargements of 400×. Saline and sodium deoxycholate (one of the agents with serious nasal ciliotoxicity, 1% [w/v] solution) were used as the negative and positive controls, respectively.
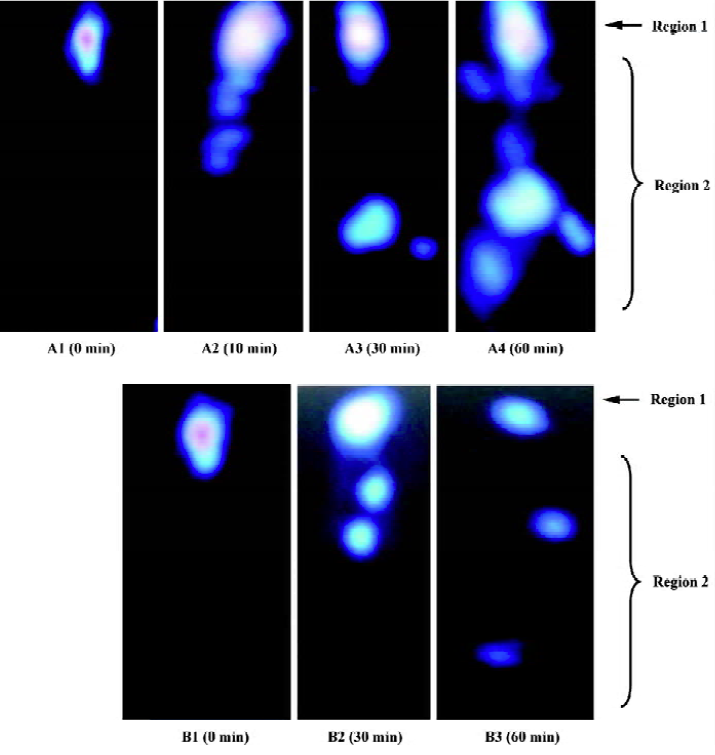
Scintigraphic studies The nasal mucosal residence time of the in situ gel was studied using single photon emission computing tomography (ZLC 3700, Münich, Germany) auto tuned to detect the 140 KeV radioactivity of 99mTc-DTPA. In situ gel incorporating 99mTc-DTPA at the gellan gum concentration of 0.5% was prepared as described earlier. The solution (99mTc-DTPA dissolved in phosphate buffer saline) was used as a control.
Eight male, New Zealand, white rabbits (2.0±0.2 kg, Experimental Animal Center of Fudan University, China) were divided into 2 groups. Each group received gel and solution formulations in a crossover design with at least a 3 d washout period. The rabbit was positioned 10 cm in front of the probe and 100 µL of the radio labeled gel or solution, which were stored in 20 °C for 30 min before use, were instilled into the nasal surface. Recording started 5 s after administration and continued for 60 min using a 128×128 pixel matrix. A total of 78 frames of dynamic images were recorded in a sequence of 36×20 s followed by 12×40 s then 30×80 s frames.
The images were analyzed by medical system ICONP workstation (Siemens, Münich, Germany). All of the graphs were divided into 2 regions of interest. A circular region was manually drawn around the nose area as region 1, and region 2 represented the rest of the body.
Antimotion sickness efficacy
Animals Adult, male Wistar rats weighing 200–250 g were used for the study. The animals were deprived of food 24 h prior to the experiment, but were allowed free access to water until 2 h before the experiment. The animal experiment was carried out in compliance with the protocol of Animal Use and Care by Medical Center of Fudan University (China).
Animal handling and drug administration In order to induce motion sickness in rats, a modified procedure[17] was used. In brief, the animals were placed individually in specifically-designed perspex restrainers (20 cm×5 cm) with a sliding door at one end to adjust the size for adequate ventilation. With the help of a hook, the restrainers were hanged to the shaft of the blade of the centrifuge. The hanging rope was only 20 cm long so as to minimize the centrifugal effect. The distance at the shaft of the blade was kept such that the diameter of rotation was fixed at 35 cm. The blade was run at a speed so as to obtain rotations of 300 rpm(35 g). Each rat was rotated for 15 min.
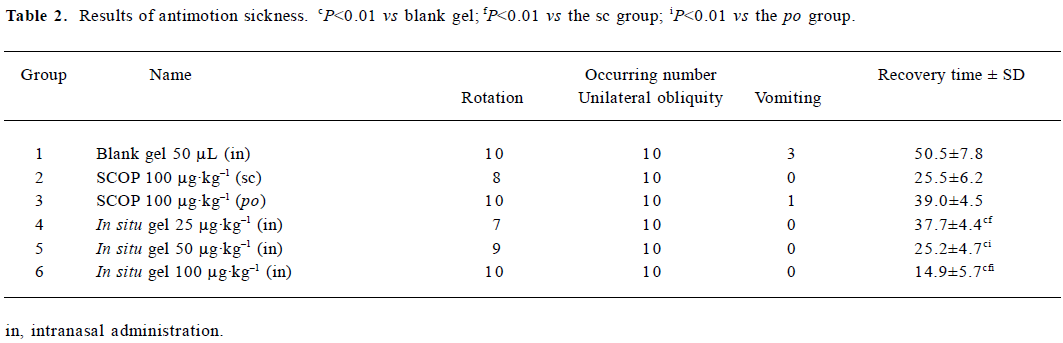
Sixty rats were used in this experiment and randomly divided into 6 groups: group 1 was administered intranasally with 50 µL blank gel (the excipient control); group 2 and group 3 with 100 µg/kg SCOP aqueous solution administered subcutaneously and orally, respectively; and groups 4, 5, and 6 were administered SCOP in situ gel (0.5% gellan gum) at the dose of 25, 50, and 100 µg/kg, respectively. Fifteen minutes after administration, all the groups were tested employing the above treatment, except the oral group which needed 30 min.
After the treatment, the rats were placed on the floor and their behavior, such as continuous rotation, unilateral obliquity, and vomiting, were observed for 1 h, the recovery time was recorded.
Statistical analysis The results were expressed as mean± SEM. ANOVA was used to test the differences between the calculated parameters using the SPSS Statistical Package (Version 10, SPSS Inc, Chicago, IL, USA). Differences were considered statistically significant when P<0.05.
Results
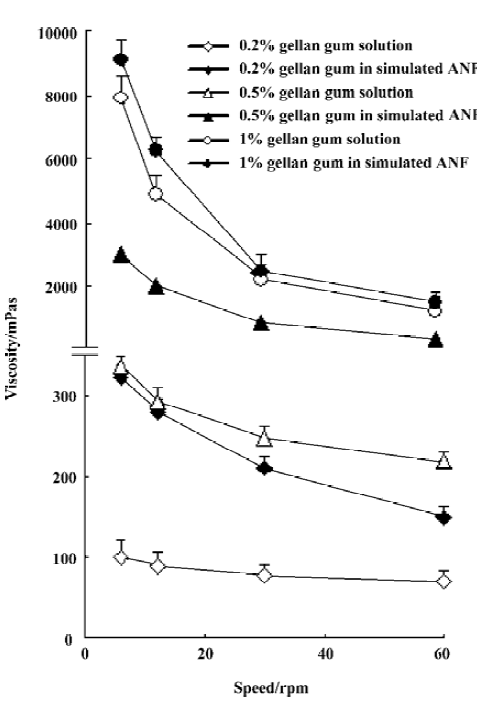
Viscosity of in situ gel formulations All gellan gum formulations, either in solution or in gel, showed pseudo plastic behavior (Figure 1). The viscosity of the test gels increased with increasing concentrations of gellan gum, and a large viscosity change was found when gellan gum underwent sol-gel transition at lower concentrations (0.2% and 0.5%). Due to a very viscous solution obtained with 1% gellan gum, a slight viscosity increase was observed after gel formation.
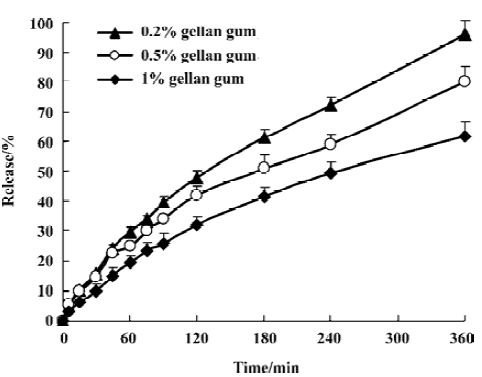
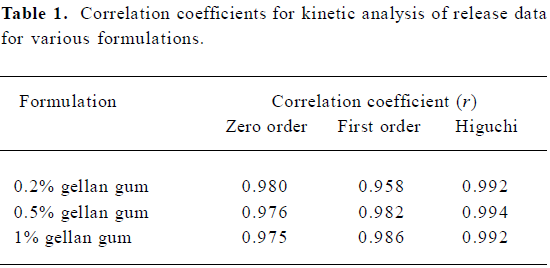
In vitro release experiments SCOP release from nasal preparations was moderate under sink condition (Figure 2). After incubated for 6 h, about 96.1±4.5%, 80.0±5.4%, and 61.9±4.9% SCOP release from 3 gels was found, respectively. At fixed drug concentrations, the release rate depended on gellan gum concentration: the higher the gellan gum con-cen-tration, the lower the rate of drug release. The in vitro release data were kinetically analyzed according to zero-order, first-order, and the diffusion-controlled release mechanism. The relative high correlation coefficient values obtained from the analysis of the amount of the drug released versus the square root of time indicated the release followed the Higu-chi[18] kinetic model, as shown in Table 1.
Full table
Nasal ciliotoxicity A micrograph showed that there was a great number of cilia at a fast-beating rate on the edge of the mucosa treated with in situ gel for 4 h (Figure 3), and the beating lasted for about 12 h after the palate was dissected. The cilia density was judged normal compared with the saline, indicating that in situ gel had no obvious effect on cilia movement.
Scintigraphic studies The mucociliary clearance graphs of the 99mTc-labeled formulation are shown in Figure 4. Instilled solution exhibited a rapid initial drainage of radioactivity due to nasal turnover, followed by a slow decrease in remaining radioactivity. Ten minutes after administration of the solution, the radioactivity in region 2 was observed, 60 min later, the radioactivity was found around the whole body. However, a clear tendency that was less pronounced after administration of the gellan gum formulation was visualized. There was about 80% of the gel deposited in the rabbit nasal cavity 30 min after administration, 60 min later, more than 60% of the gel was in contact with the mucociliary surface as compared with only about 20% of the applied reference solution. These results indicated that 0.5% gellan gum could increase the residence time of the formulation, which was further demonstrated by the pharmacodynamic.
Antimotion sickness efficacy The present study was conducted to test the efficacy of in situ gel for motion sickness in an experimental rat model. In the rats that were rotated for a period of 15 min after administration of the blank gel, there were almost complete symptoms of motion sickness (Table 2). The phenomenon could last 7–8 min, during which some rats vomited slightly. Fifteen minutes later, the rats were static and convoluting with the symptom of nystagmus to alleviate the suffering, which could last about 45–60 min. The rats treated with SCOP aqueous solution (100 µg/kg sc and 100 µg/kg, po) prior to the test also showed the symptoms of motion sickness after rotation, which could last 5 min. Twenty minutes later, the rats were static and convoluting without nystagmus, and these symptoms disappeared 30 min post rotation. Intranasal SCOP in situ gel at a dose of 100 µg/kg (group 6) decreased the degree of motion sickness significantly in the rats compared with the oral and subcutaneous group (P<0.01). In addition, although the dose of subcutaneous injection was 2 times that of the nasal dose, the recovery time was approximately equal for the rats in groups 2 and 5, suggesting that a reduced nasal dose might be expected.
Full table
Discussion
Motion sickness, also known as travel, car, sea, air, rail, or space sickness, is induced through whole body vibrations by stimulation of the vestibular organ. Motion sickness is a very common disease characterized by various sym-ptoms, such as pallor, cold sweating, nausea, vomiting, fatigue, self-rotation, unilateral obliquity, or the slowing of brain waves[19–20]. SCOP has been used successfully for motion sickness for almost a century. At present, SCOP in motion sickness is administered by transdermal patches. However, the patch must be attached at least 4–6 h before the onset of action and is therefore unsuitable for acute therapy. Recently, Scopolamine Hydrobromide 0.2% Nasal Spray was on the market, which claimed to have a fast onset of action within 30 min after administration[20]. However, due to the mucociliary clearance mechanism, solution formulations, that are not mucoadhesive, are generally rapidly cleared from the nasal cavity and result in a short duration. Therefore, to overcome these barriers, a novel in situ gel system for the nasal delivery of SCOP was developed in the present study. The results of the animal experiments revealed that 15 min after intranasal administration, in situ gel produced obvious antimotion sickness effects on rats at all the tested doses. Moreover, in situ gel at a dose of 100 µg/kg showed a better therapeutic efficacy when compared to oral and subcutaneous administration. These results suggested that SCOP nasal in situ gel is a promising therapeutic alternative to existing medications for motion sickness. Further pharmacokinetic investigation about the nasal absorption of SCOP from gellan gum preparation is in progress in our laboratory.
In this study, in situ gels at 3 different gellan gum concentrations were prepared. The preparations behave like a fluid, but form a rigid gel when exposed to cations. The viscosity of the test gel increased with higher gellan gum concentrations. It was proposed that as the concentration of gellan gum increased, the polymer chains approached closer, and the number of interactions between the polymer chains increases which leads to a denser 3-D network structure[21]. When the concentration of gellan gum achieved 1%, high viscosity made administration difficult with a conventional nebulizer.
Because the release rate of a drug directly affected its absorption process in vivo, SCOP release through the different gellan gum formulations was examined using the Valia-Chien diffusion cell method. The results showed that the release of SCOP from nasal preparations was moderate without any burst effects. It was most likely that gellan gum underwent a rapid sol-gel transition when exposed to artificial nasal fluid as confirmed by the viscosity experiment. During the hydrogel formation, a portion of SCOP might be loaded into the hydrogel phase, and thus the drug release became slow. In addition, the release rate also depended on the gellan gum concentration. The release from various gellan gum formulations could be ranked as follows: 0.2%>0.5%>1%. These results indicated that the structure of the gel became more closely packed and functioned as an increasingly resistant barrier to drug release as the concentration of polymer increased. Considering the viscosity and sustained release capacity, the gel at 0.5% gellan gum concentration seems to be a preferable formulation for nasal delivery of SCOP. Therefore, the 0.5% formulation was chosen for further study.
Nasal mucociliary clearance plays a crucial role in protecting the respiratory system from damage by inhaled substances[22]. Therefore, it is vital to examine the influences of drugs and drug excipients on nasal mucociliary clearance before clinical application. In the present study, we chose the toad palate model for the study of ciliotoxicity because the toad palate is a robust tissue giving reproductive results and the experimental technique was easy[16]. It was found that animals treated with the in situ gel (0.5% gellan gum) showed a mild effect on ciliary beating, suggesting it was safe for nasal application.
Several methods, both in vitro and in vivo, have been used to evaluate mucociliary transport rates[23–26]. Advantages of the gamma scintigraphic technique lie in the ability to non-invasively monitor the deposition and clearance of drug formulations, allowing both quantitative and photographic illustrations of distribution and clearance of the radiolabeled formulation. Employing this technique to evaluate the nasal clearance of mucoadhesive preparations requires a radiotracer which is stable and non-diffusible to prevent absorption into the vascular compartment. 99mTc tracer is reported as technically easy to perform and more representative of ciliary function since it investigates a large surface of the mucosa as a whole and not the fastest flow rate[26]. Therefore, 99mTc-DTPA was used in this study. As expected, in situ gel had a longer residence in rabbit nasal cavity compared with the solution.
Three kinds of SCOP in situ gels were prepared at the concentrations of gellan gum, which were 0.2%, 0.5%, and 1.0% (w/v), respectively. Its viscosity depended on the concentration of gellan gum. In vitro release experiments showed that the release of SCOP from the test gels was moderate. In situ gel had a longer residence time in the rabbit nasal cavity compared with the solution, and no nasal ciliotoxicity. The antimotion sickness experiment confirmed that intranasal in situ gel produced pronounced antimotion sickness efficacy, especially at a dose of 100 µg/kg. In conclusion, SCOP nasal in situ gel is a well-tolerated and promising therapeutic alternative to existing medications for motion sickness.
Acknowledgments
We wish to express our thanks to professor Jian-hua ZHU (Department of Nuclear Pharmacy, School of Pharmacy, Fudan University, Shanghai, China) for his help in the scintigraphic studies.
References
- Putcha L, Cintron NM, Tsui J, Vanderploeg JM, Kramer WG. Pharmacokinetics and oral bioavailability of scopolamine in normal subjects. Pharm Res 1989;6:481-5.
- Shaw J, Urquhart J. Programmed systemic delivery by the transdermal route. Trends Pharmacol Sic 1980;2:208-11.
- Cintron NM, Chen Y. A sensitive radioreceptor assay for determining scopolamine concentrations in plasma and urine. J Pharm Sci 1987;76:328-32.
- Hyde RW, Tonndorf J, Chinn HE. Absorption from the nasal mucous membrane. Ann Otol Rhinol Laryngol 1953;62:957-68.
- Illum L, Jorgensen H, Bisgaard H, Krogsgaard O, Rossing N. Bioadhesive microspheres as a potential nasal drug delivery system. Int J Pharm 1987;39:189-99.
- Gizurason S. The relevance of nasal physiology to the design of drug absorption studies. Adv Drug Del Rev 1993;11:329-47.
- Gurny R, Boye T, Ibrahim H. Ocular therapy with nanoparticulate systems for controlled drug delivery. J Control Release 1985;2:353-61.
- Miller SC, Donovan MD. Effect of Poloxamer 407 gel on the miotic activity of pilocarpine nitrate in rabbits. Int J Pharm 1982;12:147-52.
- Edsman K, Carlfors J, Petersson R. Rheological evaluation of poloxamer as an in situ gel for ophthalmic use. Eur J Pharm Sci 1998;6:105-12.
- Rozier A, Mazuel C, Grove J, Plazonnet B. Functionality testing of gellan gum, a polymeric excipient material for ophthalmic dosage forms. Int J Pharm 1997;153:191-8.
- Grasdalen H, Smidsroed O. Gelation of gellan gum. Carbohydr Polym 1987;7:371-93.
- Chanrasekaran R, Puigjaner LC, Joyce KL, Arnott S. Cation interaction in gellan: an X-ray study of the potassium salt. Carbohydr Res 1988;181:23-40.
- Chanrasekaran R, Thailambal VG. The influence of calcium ions, acetate and l-glycerate groups on the gellan double helix. Carbohydr Polym 1990;12:431-2.
- Sanzgiri YD, Maschi S, Crescenzi V, Callegaro L, Topp EM, Stella VJ. Gellan-based systems for ophthalmic sustained delivery of methylprednisolone. J Control Release 1993;26:195-201.
- Lorin MI, Gaerlan PF, Mandel ID. Quantitative composition of nasal secretions in normal subjects. J Lab Clin Med 1972;2:275-81.
- Jiang XG, Cui JB, Fang XL, Wei Y, Xi NZ. Toxicity of drugs on nasal mucocilia and the method of its evaluation. Acta Pharm Sin 1995;30:848-53.
- Gupta YK, Chaudhary G. Effect of antiemetic drugs on decrease in gastric emptying in experimental model of motion sickness in rats. Acta Pharmacol Sin 2003;24:296-300.
- Higuchi WI. The analysis of data on the medicament release from ointments. J Pharm Sci 1962;51:802-4.
- Money K. Motion sickness. Physiol Rev 1970;50:1-39.
- Klöcker N, Hanschke W, Toussaint S, Verse T. Scopolamine nasal spray in motion sickness: a randomized, controlled, and crossover study for the comparison of two scopolamine nasal sprays with oral dimenhydrinate and placebo. Eur J Pharm Sci 2001;13:227-32.
- Nickerson MT, Paulson AT. Rheological properties of gellan, k-carrageenan and alginate polysaccharides: effect of potassium and calcium ions on macrostructure assemblages. Carbohydr Polym 2004;58:15-24.
- Ugwoke MI, Agu RU, Jorissen M, Augustijns P, Sciot R, Verbeke N, et al. Nasal toxicological investigations of Carbopol 971P formulation of apomorphine: effects on ciliary beat frequency of human nasal primary cell culture and in vivo on rabbit nasal mucosa. Eur J Pharm Sci 2000;9:387-96.
- Marttin E, Schipper NGM, Verhoef JC, Merkus FWHM. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv Drug Deliv Rev 1998;29:13-38.
- Puchelle E, Aug F, Pham QT, Bertrand A. Comparison of three methods for measuring nasal mucociliary clearance in man. Acta Otolaryngol 1981;91:297-303.
- Schipper NGM, Verhoef JC, Merkus FWHM. The nasal mucociliary clearance: Relevance to nasal drug delivery. Pharm Res 1991;8:807-14.
- Ingels K, Van Hoom V, Obrie E, Osmanagaoglu K. A modified technetium-99m isotope test to measure nasal mucocillary transport: comparison with the saccharine-dye test. Eur Arch Otorhinolaryngol 1995;252:340-3.