Effect of MDR1 gene polymorphism on progression of end-stage renal disease1
Introduction
The MDR1 (multidrug resistance gene encoding for P-gp) gene product P-glycoprotein (ABCB1) is a membrane protein, which functions as an ATP-dependent exporter of xenobiotics from cells. P-glycoprotein is expressed in normal tissues with excretory function such as the intestine, liver and kidneys, in capillary endothelial cells of brain, placenta, and testis and in peripheral blood cells[1]. In kidneys, P-glycoprotein is expressed in the brush border membrane of proximal tubular cells[1,2]. It mediates active secretion of its substrates into urine. Renal P-glycoprotein is likely to function as a protective mechanism against toxic substances in the glomerular filtrate. Both clinical and experimental studies have reported the renoprotective effects of removing uremic toxins by peritoneal dialysis and oral charcoal adsorbent in delaying the progression of chronic renal disease[3–7]. Thus, individuals with a low renal P-glycoprotein expression would potentially be exposed to higher concentrations of toxic agents and should be more susceptible to their damaging effects.
Multiple mutations were found in the human MDR1 gene[8,9]. We selected two single nucleotide polymorphisms (SNP) that had been previously reported to be associated with the expression or activity of MDR1. People with mutation C3435T were associated with a lower P-glycoprotein expression in the kidneys, compared with subjects homozygous for the wild-type allele[10,11]. Another SNP G1199A has also been reported to increase the intracellular accumulation of rhodamine-123 in vitro[12].
As uremic toxins have been suggested to promote the progression of chronic renal failure by damaging tubular cells, based on these observations, we hypothesized that genetically predisposed subjects carrying T mutation allele at C3435T or the A mutation allele in G1199A might be at high risk for developing end-stage renal disease (ESRD). Therefore, we decided to examine whether MDR1 is a susceptible gene for renal disease in patients.
Materials and methods
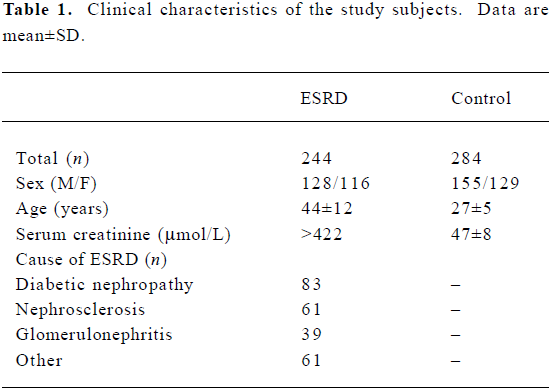
Subjects We studied 244 ESRD patients from the Division of Nephrology (Ruijin Hospital, Shanghai Jiaotong University, Shanghai, China), and 284 healthy controls. Clinical information and biochemical parameters were retrieved retrospectively from hospital records. The subject characteristics are presented in Table 1. According to the “Practice of Internal Medicine”[13], patients with serum creatinine >442 µmol/L were allocated to the ESRD group. Two hundred and eighty-four healthy patients were randomly selected and used for comparison with the ESRD patients. The healthy patients were determined by their medical history, physical examination, routine blood tests, and electrocardiography, and had no history of hypertension, diabetes, renal failure, vascular disease, stroke and cardiomyopathy. This research was approved by the Ethics Committee of Ruijin Hospital. Informed consent was obtained from the patients and controls participating in the study, and the hospital ethical committee approved the study.
Full table
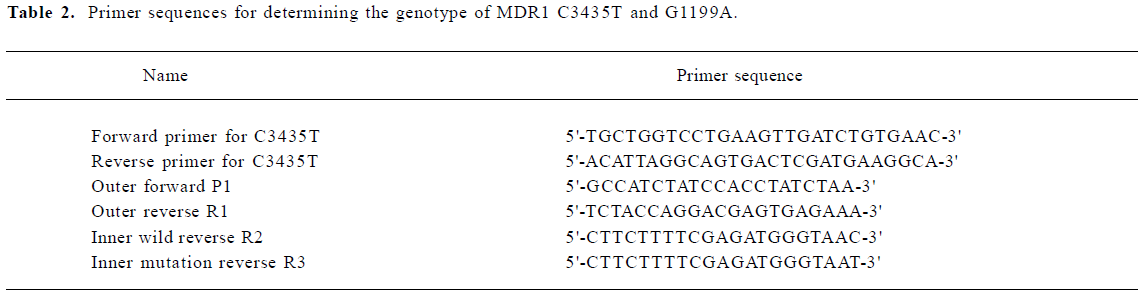
DNA isolation and genotyping analysis Genomic DNA was obtained from peripheral blood by proteinase K digestion and phenol-chloroform extraction and ethanol precipitation. Genotyping of the C3435T polymorphism was carried out by polymerase chain reaction-restriction fragment length polymorphism assay according to the method of Hoffmeyer et al with minor modifications[9]. Allele specific-polymerase chain reaction (AS-PCR) was used to deter-mine the genotype of G1199A. The AS-PCR consisted of 2 rounds of PCR. In the first round, 1 µL genomic DNA sample was added to 25 µL of reaction volume composed of PCR buffer, 1.5 mmol/L MgCl2, 0.5 unit of Taq polymerase, 0.2 mmol/L dNTP, 6.25 pmol of forward primer, and 6.25 pmol of reverse primer; 35 cycles were carried out in a GeneAmp PCR system 2700 (Biocompare, CA, USA). Each cycle consisted of 30 s at 94 °C for denaturation, 30 s at 54 °C for annealing, and 30 s at 72 °C for elongation. The second round was carried out in 2 reaction tubes. Each tube contained 25 µL of reaction volume composed of PCR buffer, 1.5 mmol/L of MgCl2, 0.5 unit of Taq polymorphism, 0.2 mmol/L dNTP, and 6.25 pmol of allele-specific primer as the reverse primer, and the same forward primer as that used in the first round of PCR. First-round products (20 times diluted) 1 µL was used as template for the second round. The second round consisted of 20 cycles performed in the same GeneAmp PCR system (30 s at 94 °C for denaturing; 30 s at 64 °C for annealing, and 30 s at 72 °C for elongation). Products (from each second-round reaction tube) in the volume of 10 µL were then analyzed directly on 1.5% agarose gel with 0.5 mg/mL of ethidium bromide. The sequence of the oligonucleotide primers employed in the AS-PCR assays are depicted in Table 2.
Full table
Statistical analyses We used the Hardy-Weinberg equilibrium for frequency deviation. The comparison of the allele and genotype frequencies between the different groups was evaluated by Chi-square test. ANOVA tests were used to compare genotype groups in terms of clinical and laboratory characteristics. The SPSS software package version 11.0 (SPSS Inc, Chicago, IL, USA) was used to perform these statistical analyses with P<0.05 as the minimal level of statistical significance.
Results
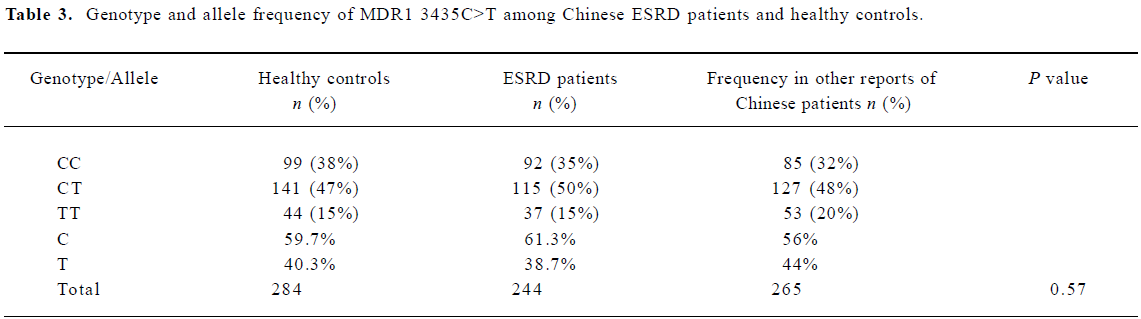
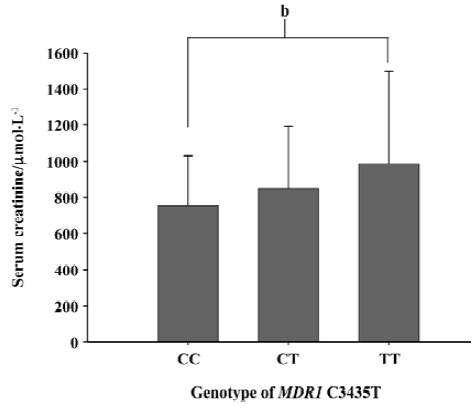
The different genotypic and allele frequency distributions for MDR1 C3435T in ESRD patients and controls are shown in Table 3. The genotype distribution was consistent with the Hardy-Weinberg equilibrium. No significant difference was observed in genotype frequencies between the ESRD patients and the control through the Chi-square test (P=0.573). However, as shown in Figure 1, the level of serum creatinine was significantly different between carriers with genotype CC and TT in ESRD patients, although the difference between the 3 genotypes, 3435CC, 3435CT, and 3435TT did not reach statistical significance. The value of serum creatinine for genotype 3435CC, 3435CT, and 3435TT were 753.8±276.0 µmol/L, and 849.6±342.2 µmol/L, 987.0±512.0 µmol/L, respectively. Of the 284 Chinese healthy subjects and 244 ESRD patients, no variant allele 1199G>A was found. All subjects were homozygous for 1199GG.
Full table
Discussion
To date, 48 SNP have been reported in the MDR1 gene[14]. The single nucleotide polymorphisms 1236C>T, 2677G>T/A, and 3435C>T are the most common variants in the coding region of ABCB1[10]. 1236C>T and 3435C>T are synonymous SNP, while the nonsynonymous 2677G>T/A causes an amino acid substitution (899Ala>Ser/Thr). These 3 SNP are in strong linkage disequilibrium, accounting for 2 abundant haplotypes (ABCB1*1: 1236C-2677G-3435C; and ABCB1*13: 1236T-2677T-3435T)[14,16]. So we selected the polymorphism 3435C>T to represent the other 2 SNP, 1236C>T and 2677G>T/A. It was reported that individuals homozygous for 3435TT showed significantly lower P-gp expression in the intestines, liver and kidneys, with increased plasma levels of the P-gp substrate[10,16,17]. Similar to its protective role at many biological barriers, P-glycoprotein as a plasma membrane efflux pump may be involved in the clearance of toxic compounds via the brush border of the tubular lumen and is critical in the processes of re-absorption and secretion. So we postulated that polymorphism C3435T should be associated with the severity of ESRD, or the frequency of mutation allele 3435C>T should be higher in ESRD patients compared with the controls.
In our results, we did not find any difference for the frequencies of the C3435T genotype and allele between the ESRD patients and the controls. The frequencies of C3435T in our study were consistent with other reports, as is shown in Table 3. However, the level of serum creatinine is higher in homozygote 3435TT than heterozygote 3435CT and homozygote 3435CC in ESRD patients. The difference between 3435TT and 3435CC reached statistical significance. According to the clinical characteristics in our subjects, the most common causes for ESRD were diabetes mellitus (34%), hypertension (25%), chronic glomerulonephritis (16%) and other (25%).The low expression of P-glycoprotein was not the etiological factor for the kidney disease, but it can contribute to the progression of ESRD and affect the severity.
The cause of ESRD and interindividual differences in susceptibility remain elusive. Many studies have recently focused on this aspect. Kim et al found that SNP and haplotypes of the SLC12A3 [solute carrier family12 member (sodium/chloride) 3] gene, especially Arg913Gln, are significantly associated with ESRD caused by diabetic nephropathy in the Korean population[18]. It was reported that the polymorphism of promoter -511, exon -5+3953 in IL-β and a variable number of tandem repeats in the interleukin-1 receptor antagonist gene affects the risk of development of ESRD[19]. Meanwhile, Lamnissou et al[20] reported that patients with autosomal dominant polycystic kidney disease, who carried allele A in the nitric oxide synthase (NOS3-4) gene, progressed to ESRD more quickly. The result from Koupepidou et al[21] support the hypothesis that Caucasian patients with essential hypertension, homozygous for 677TT or doubly heterozygous for 677CT/1298AC genotypes in the methylenetetra-hydrofolate reductase gene, are predisposed to develop hypertensive nephrosclerosis and chronic renal failure (CRF). Since more than 1 single gene was associated with the progression of ESRD, a study with the multiple linear regression method is needed in the future.
The substitution of G to A in the position 1199 of MDR1 results in a serine-to-asparagine substitution at amino acid 400 in a cytoplasmic domain of P-gp. Alteration in the efflux transport of P-gp owing to the G1199A transition has been observed in a recombinant expression system. Mean intracellular R123 fluorescence for MDR1wt and MDR1G1199A cells were 3.91±0.11 and 18.56±0.46 (P<0.001), respectively, an approximate 4.75-fold higher accumulation of R123 in MDR1G1199A cells[12], which meant that G1199A mutation resulted in the decrease of the transport function of P-gp. Accordingly, the decrease efflux function of P-gp made the toxins more easily accumulated in the body, so the individual with variant 1199G>A was more likely to suffer from ESRD. It is expected that the frequency of variant allele will be higher in ESRD patients than in healthy controls. However, no variant allele was found in our study, neither in healthy controls nor in ESRD patients. Comparatively, the frequency of genotype 1199GG, 1199GA, and 1199AA is 88.9%, 11.1%, and 0 in 461 German volunteers, respectively[22]. Hoffmeyer et al reported that the frequency of 1199GA was 12.9% and 1199AA was zero in Caucasians[9], so there is no evidence to associate 1199G>A with the progression or severity of ESRD. As the human body is a complex organism, data obtained from in vitro experiments sometimes cannot be applied to the human body directly.
In conclusion, we found that SNP of the MDR1 gene, especially C3435T, were significantly associated with the severity of ESRD in the Chinese population. Chinese people do not carry the 1199G>A variant allele. More study is needed to clarify the cause and interindividual differences in the susceptibility for the risk of ESRD.
References
- Thiebaut F, Tsuruo T, Hamada H, Gottesman M, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA 1987;84:7735-8.
- Cordon-Cardo C, O’Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, et al. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci USA 1989;86:695-8.
- Motojima M, Nishijima F, Ikoma M, Kawamura T, Yoshioka T, Fogo AB, et al. Role for ‘uremic toxin’ in the progressive loss of intact nephrons in chronic renal failure. Kidney Int 1991;40:461-9.
- Lysaght MJ, Vonesh EF, Gotch F, Ibels L, Keen M, Lindholm B, et al. The influence of dialysis treatment modality on the decline of remaining renal function. ASAIO Trans 1991;37:598-604.
- Rottembourg J. Residual renal function and recovery of renal function in patients treated by CAPD. Kidney Int 1993;43:S106-10.
- Sanaka T, Sugino N, Teraoka S, Ota K. Therapeutic effects of oral sorbent in undialyzed uremia. Am J Kidney Dis 1988;12:97-103.
- Niwa T, Nomura T, Sugiyama S, Miyazaki T, Tsukushi S, Tsutsui S. The protein metabolite hypothesis, a model for the progression of renal failure: An oral adsorbent lowers indoxyl sulfate levels in undialyzed uremic patients. Kidney Int 1997;52:S23-8.
- Mickley LA, Lee JS, Weng Z, Zhan Z, Alvarez M, Wilson W, et al. Genetic polymorphism in MDR-1: a tool for examining allelic expression in normal cells, unselected and drug-selected cell lines, and human tumors. Blood 1998;91:1749-56.
- Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA 2000;97:3473-8.
- Siegsmund M, Brinkmann U, Schaffeler E, Weirich G, Schwab M, Eichelbaum M, et al. Association of the P-glycoprotein transporter MDR1(C3435T) polymorphism with the susceptibility to renal epithelial tumors. J Am Soc Nephrol 2002;13:1847-54.
- Roy JN, Barama A, Poirier C, Vinet B, Roger M. Cyp3A4, Cyp3A5, and MDR-1 genetic influences on tacrolimus pharmacokinetics in renal transplant recipients. Pharmacogenet Genomics 2006;16:659-65.
- Woodahl EL, Yang Z, Bui T, Shen DD, Ho RJ. Multidrug resistance gene G1199A polymorphism alters efflux transport activity of P-glycoprotein. J Pharmacol Exp Ther 2004;310:1199-207.
- Chen HZ. Practice of internal medicine. 12 ed. Beijing: People’s Medical Publishing House.
- Kroetz DL, Pauli-Magnus C, Hodges LM. Sequence diversity and haplotype structure in the human ABCB1(MDR1, multidrug resistance transporter) gene. Pharmacogenetics 2003;13:481-94.
- Zhang WX, Chen GL, Zhang W, Tan ZR, Liu J, Zhou G, et al. MDR1 genotype do not influence the absorption of a single oral dose of 100 mg talinolol in healthy Chinese males. Clin Chim Acta 2005;359:46-52.
- Song P, Lamba JK, Zhang L, Schuetz E, Shukla N, Meibohm B, et al. G2677T and C3435T genotype and haplotype are associated with hepatic ABCB1 (MDR1) expression. J Clin Pharmacol 2006;46:373-9.
- Nakamura T, Sakaeda T, Horinouchi M, Tamura T, Aoyama N, Shirakawa T, et al. Effect of the mutation (C3435T) at exon 26 of the MDR1 gene on expression level of MDR1 messenger ribonucleic acid in duodenal enterocytes of healthy Japanese subjects. Clin Pharmacol Ther 2002;71:297-303.
- Kim JH, Shin HD, Park BL, Moon MK, Cho YM, Hwang YH, et al. SLC12A3 (solute carrier family 12 member [sodium/chloride] 3) polymorphisms are associated with end-stage renal disease in diabetic nephropathy. Diabetes 2006;55:843-8.
- Manchanda PK, Kumar A, Bid HK, Mittal RD. Interleukin-1beta and receptor antagonist (IL-1Ra) gene polymorphisms and the prediction of the risk of end-stage renal disease. Biomarkers 2006;11:164-73.
- Lamnissou K, Zirogiannis P, Trygonis S, Demetriou K, Pierides A, Koptides M, et al. Evidence for association of endothelial cell nitric oxide synthase gene polymorphism with earlier progression to end-stage renal disease in a cohort of Hellens from Greece and Cyprus. Genet Test 2004;8:319-24.
- Koupepidou P, Deltas C, Christofides TC, Athanasiou Y, Zouvani I, Pierides A. The MTHFR 677TT and 677CT/1298AC genotypes in Cypriot patients may be predisposing to hypertensive nephrosclerosis and chronic renal failure. Int Angiol 2005;24:287-94.
- Cascorbi I, Gerloff T, Johne A, Meisel C, Hoffmeyer S, Schwab M, et al. Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin Pharmacol Ther 2001;69:169-74.
