Effect of baicalin and berberine on transport of nimodipine on primary-cultured, rat brain microvascular endothelial cells1
Introduction
Nimodipine (NMD) is a dihydropyridine calcium channel blocker and is currently used to prevent and treat the ischemic damage caused by cerebral arterial spasm in subarachnoid hemorrhage[1]. NMD has also been used in other cerebrovascular disorders, such as ischemic stroke[2] and multi-infarct dementia[3].
Huanglian (Rhizoma Coptidis) and Huangqin (Radix Scutellariae) are a familiar medicine couple in many traditional complex prescriptions. Baicalin and berberine are the phytochemical markers for the quality control of Radix Scutellariae and Rhizoma Coptidis in Chinese pharmacopoeia, respectively. Thus, we choose baicalin and berberine as the experimental samples.
Baicalin has multiple biological activities such as vasodilatory[4], antioxidant[5], and antitumor activities[6,7]. Recent studies have demonstrated that baicalin has a protective effect against brain edema and cerebral ischemic damage[8–10].
Berberine has varied pharmacological effects including anti-diarrheic[11], antimicrobial[12], and anti-inflammatory[13]; it also exhibits protective effects against ischemic damage after ischemia/reperfusion[14–16].
As both baicalin and berberine have beneficial effects on brain ischemic damage, the opportunity of a combination of them or herbs containing them with NMD is increasing. A major concern is that a herb-drug interaction may occur. Thus, we investigated whether baicalin and berberine affected the transport of NMD across the blood-brain barrier (BBB) using the primary cultured rat brain microvessel endothelial cells (rBMEC) in the present study.
Materials and methods
Materials NMD and felodipine were provided by Shandong Xinhua Pharmaceutical Factory (Shandong, China). Baicalin and berberine standards were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Rhodamine123 (Rho123) was purchased from Sigma-Aldrich (St Louis, MO, USA). Cosmic calf serum was purchased from Hyclone (Tauranga, New Zealand). Gelatin, trypsin, and collagenase type II were purchased from Sigma-Aldrich (USA). Dulbecco’s modified Eagle’s medium (DMEM, high glucose) and Ham’s F-12 nutrient mixture were purchased from Gibco BRL (Rockville, MD, USA). Bovine serum albumin (BSA) was purchased from SABC (Fraction V; Luoyang, He-nan, China). Sprague-Dawley rats (7–10 d old) were supplied by the Center of Experimental Animals, China Pharmaceutical University (Nanjing, China). All other chemicals were of analytical grade and commercially available.
Isolation and culture of rBMEC Primary rBMEC isolation and culture were operated according to previous reports[17]. Briefly, the isolated cerebral gray matter was digested by trypsin (0.05%) at 37 °C for 20 min, then filtrated through a 149 µm nylon mesh before the filter was collected. The filtrate was filtered through a 79 µm nylon mesh before the matter on the nylon mesh was collected. Then the matter was digested by collagenase type II (0.1%) at 37 °C for 25 min, centrifuged at room temperature for 5 min (200×g), and the cells were collected. Cells were cultured in DMEM and F-12 supplemented with 20% cosmic calf serum under the conditions of 37 °C with 95% air and 5% CO2. Uptake and efflux experiments were performed in a 24-well plate when the cells reached confluence within 12–14 d.
Uptake experiment When the cells reached confluence within 12–14 d, uptake experiments were performed. Cultured rBMEC were pre-incubated at 37 °C in 1 mL Hanks’ balanced salt solution (HBSS) (0.137 mol/L NaCl, 5.37 mmol/L KCl, 1.26 mmol/L CaCl2, 0.81 mmol/L MgSO4·7H2O, 0.37 mmol/L Na2HPO4·H2O, 0.44 mmol/L KH2PO4, 4.17 mmol/L NaHCO3, and 2.92 mmol/L D-glucose) for 30 min. After the pre-incubation, the solution was removed and the HBSS (1 mL) containing NMD (10 mg/L) or both NMD (10 mg/L) and agents for testing was added to each incubation well. The steady state of NMD in rBMEC was recorded at 90 min[17]. The uptake was terminated at 90 min by washing the cells 3 times with 1 mL of ice-cold HBSS. Then the blank HBSS (0.3 mL) was added to each incubated well, frozen, and melted repeatedly 4 times to break down the cells. The uptake of Rho123 was performed in a similar way. After pre-incubation for 30 min with HBSS (1 mL), the solution was removed, and the HBSS (1 mL) containing Rho123 (0.1 mg/L) or both Rho123 (0.1 mg/L) and agents for testing was added to each incubation well. The steady state of Rho123 in rBMEC was recorded at 120 min according to previous study in our lab. So the uptake was terminated at 120 min by washing the cells 5 times with 1 mL of ice-cold HBSS. Then the blank HBSS (0.5 mL) was added to each incubated well, frozen, and melted repeatedly 4 times to break down the cells.
Efflux experiment For the efflux study, rBMEC were incubated with NMD (10 mg/L) or Rho123 (0.1 mg/L) for 90 min, then the cells were washed 5 times with 1 mL ice-cold HBSS. HBSS (1 mL) with or without test agents was added to initiate the efflux at 37 °C. The efflux was terminated at the designed time points by using the same procedure as used in the uptake study mentioned above. Efflux was estimated from the amount of NMD or Rho123 remaining in the cells.
Analytical method The concentration of NMD in the cell suspension was analyzed by a HPLC system (LC-10AT, Shimadzu, Kyoto, Japan) which was equipped with a VP-ODS column (4.6×150 mm, 5 µm, Shimadzu, Japan) and a UV-detector (SPD-10Avp, Shimadzu, Japan). The mobile phase consisted of MeOH: H2O (70:30, v/v, pH=7); the flow rate was set at 1.0 mL/min, and the analytical wavelength was 238 nm[18]. Internal standard (felodipine, 5 µg/mL, 10 µL) and 150 µL methanol were added to cell suspension (150 µL) and mixed vigorously for 10 min, then centrifuged (44 912×g for 10 min); 200 µL of supernatant was transferred to another clean tube and centrifuged (44 912×g for 10 min) again. After the second centrifugalization, 120 µL of supernatant was transferred to another clean tube and 20 µL was injected onto the HPLC system. For the standard sample, NMD was dissolved into a blank cell (cell without drug treatment) suspension at a concentration from 5 to 1200 ng/mL. The lowest limit of quantitation of NMD was 5 ng/mL in the cell suspension. A good linearity was obtained from 5–1200 ng/mL.
The concentrations of Rho123 in the cell suspension were determined by HPLC[19]. Twenty microliters of cell suspension was injected into a Shimadzu LC-10AVP system (Shimadzu, Japan) consisting of an LC-10A liquid pump, a CTO-10ASVP column oven, and a fluorescence detector (RF-10AXL) set at an excitation wavelength of 485 nm and emission wavelength of 565 nm. Conditions were as follows: column, a Shim-pack ODS (4.6 µm, 150 mm×4.6 mm id, Shimadzu, Japan); mobile phase, 0.1% HAc (pH 4.0)–acetonitrile=3:2 (v/v); column temperature, 40 °C; flow rate, 1 mL/min. Methanol was also used to remove the protein as in the case of NMD. For the standard sample, Rho123 was dissolved into the blank cell (cell without drug treatment) suspension at a concentration from 0.5 to 50 ng/mL. The lowest limit of quantitation of Rho123 was 0.5 ng/mL in the cell suspension. The linear range of Rho123 was 0.5–50 ng/mL.
The protein content in the cultured cells was measured by the method of Brandford MM[20] using BSA as the standard. Net uptake, expressed as the concentration ratio (ng/µg protein), was obtained by dividing the apparent uptake amount of NMD or Rho123 (ng/mL) by protein content (µg protein/mL). All data were expressed as mean±SD. Statistical analysis was performed by using Student’s t-test. A difference of P<0.05 was considered statistically significant.
Results
Effects of tested agents on the rBMEC cytoactivity In order to investigate whether tested agents affected cell cytoactivity, cells were incubated at 37 °C for 90 min with 10 mg/L NMD, 120 min with HBSS, 0.1 mg/L Rho123, 20 µg/mL baicalin, and 10 µg/mL berberine, respectively. Cell activity was measured using the 3-(4, 5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. The HBSS group was set as the control group. Data were shown in Table 1. No differences (P>0.05) were found relative to the control group. Thus, we believed that the tested agents did not damage the cells at the concentrations used.
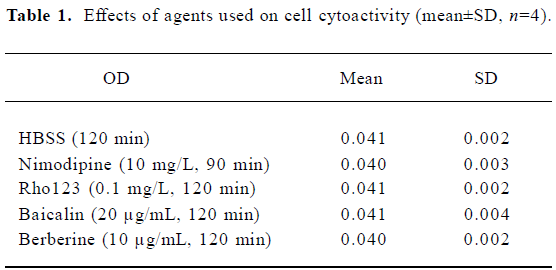
Full table
Effects of baicalin on the steady-state uptake of NMD The concentration-dependent, bidirectional effect of baicalin on the steady-state uptake of NMD was observed. Baicalin at 2, 5, and 10 µg/mL increased the steady-state uptake of NMD, whereas baicalin at 15 and 20 µg/mL decreased the uptake (Figure 1).
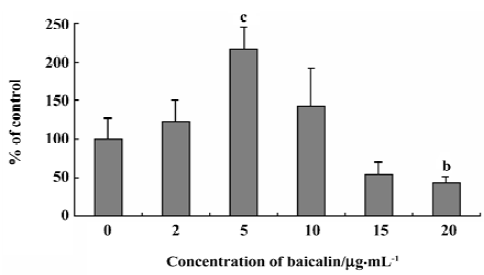
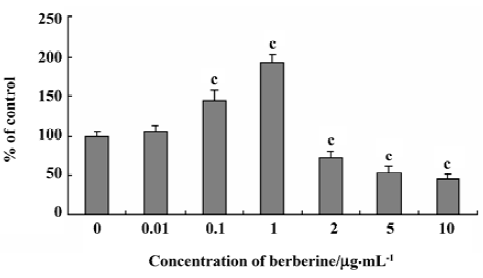
Effects of berberine on the steady-state uptake of NMD Similar to baicalin, berberine also affected the uptake of NMD in a dose-dependent, biphase manner. Figure 2 shows the profiles of the steady-state uptake of NMD by rBMEC in the presence or absence of berberine. Berberine increased the uptake of NMD by primary cultured rBMEC in a dose-dependent manner from 0.01 to 1 µg/mL, but decreased the uptake in a dose range from 2 to 10 µg/mL. The steady-state uptake of NMD was increased to 109%, 151%, and 202% of the control group in the presence of 0.01, 0.1, and 1 µg/mL berberine, respectively. The uptake of NMD decreased to 72%, 53%, and 46% of the control group by 2, 5, 10 µg/mL berberine, respectively.
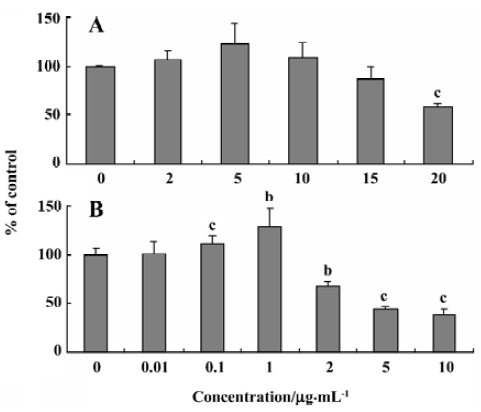
Effects of baicalin and berberine on the steady-state uptake of Rho123 The transport of NMD across rBMEC was reported to be restricted by P-glycoprotein (P-gp)[17]. To clarify whether the observed effects of baicalin and berberine on the uptake of NMD were via P-gp modulation, we used Rho123, a typical P-gp substrate, as the positive control. The results were illustrated in Figure 3. The steady-state uptake of Rho123 was 106%, 123%, 108%, 86%, and 59% of the control group in the presence of 2, 5, 10, 15, and 20 µg/mL baicalin, respectively (Figure 3A). This result was similar to the observations in the uptake of NMD. Similar to the effect on the uptake of NMD, berberine exhibited a dose-depen-dent, bidirectional effect on the steady-state uptake of Rho123 by rBMEC. The steady-state uptake of Rho123 by primary cultured rBMEC was increased by berberine (0.01–1 µg/mL), but decreased by berberine (2–10 µg/mL; Figure 3B).
Effects of baicalin and berberine on the efflux of NMD To further understand the mechanism of the effect baicalin and berberine showed on the transport of NMD, we examined the effects of baicalin and berberine on the efflux of NMD from rBMEC. In the presence of baicalin (5 µg/mL), the amount of NMD remaining in the cells was significantly higher than the control group. When the cells were incubated with 20 µg/mL baicalin, the amount of NMD remaining in the cells significantly decreased (Figure 4). These results suggested that 5 µg/mL baicalin inhibited the efflux of NMD, while 20 µg/mL baicalin stimulated the efflux of NMD from rBMEC. Similarly, the resident amount of NMD in the cells was significantly increased by berberine (1 µg/mL), but decreased with berberine (10 µg/mL; Figure 4), which indicated that the efflux of NMD from rBMEC was inhibited by 1 µg/mL berberine, but promoted by 10 µg/mL berberine.
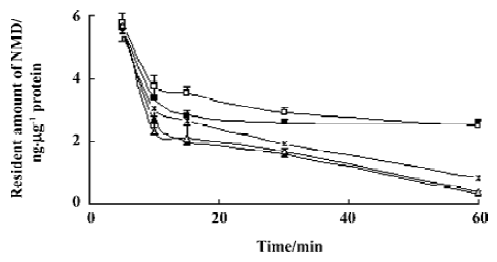
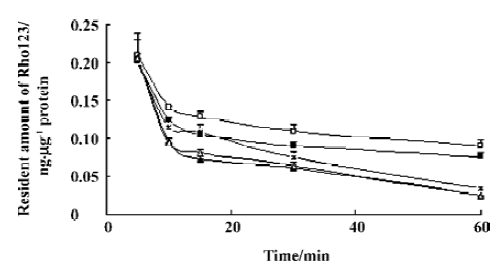
Effects of baicalin and berberine on the efflux of Rho123 As in the uptake experiment, the effects of baicalin and berberine on the efflux of Rho123 were also examined to provide a positive control. Similar with the effect on the efflux of NMD, 5 µg/mL baicalin and 1 µg/mL berberine inhibited the efflux of Rho123, while 20 µg/mL baicalin and 10 µg/mL berberine stimulated the efflux (data were shown in Figure 5).
Discussion
The present study demonstrated that both baicalin and berberine affected the transport of NMD across the BBB in a bidirectional manner.
The MTT assay demonstrated that tested agents did not damage cells at the concentrations used; we believed that the observed changes were not the result of nonspecific cytotoxicity.
We observed a concentration-dependent, bidirectional effect of baicalin on the steady-state uptake of NMD by primary cultured rBMEC; the uptake was significantly increased by 5 µg/mL baicalin, but decreased with 20 µg/mL baicalin. In the efflux experiment, baicalin (5 µg/mL) inhibited the efflux of NMD while baicalin (20 µg/mL) stimulated the efflux. Baicalin belongs to the class of flavonoids and in recent years, flavonoids had been described as P-gp modu-lators, however, contradictory effects have been reported. Critchfield et al[21] reported that quercetin increased adria-mycin efflux from HCT-15 colon cells, whereas Scambia et al[22] found that quercetin inhibited Rho123 efflux in MCF-7 breast cells. Gwenaelle et al[23] demonstrated flavonoids as a new class of bifunctional modulators of P-gp and Yoshiharu et al[24] reported a concentration-dependent biphasic effect of quercetin on the uptake of [3H]vincristine, which was a similar phenomenon to our result. The effect of baicalin on the uptake and efflux of Rho123, the positive control, has the same tendency as the effect on NMD uptake; baicalin (5 µg/mL) inhibited the efflux and increased the uptake of Rho123, whereas 20 µg/mL baicalin stimulated the efflux and significantly decreased the uptake of Rho123. These results suggested that the effect of baicalin on the transport of NMD across the BBB might due to the modulation of P-gp function.
Berberine also showed a dose-dependent bidirectional effect on the transport of NMD across the BBB. Berberine increased the uptake of NMD from 0.01 to 1 µg/mL and decreased the uptake from 2 to 10 µg/mL. The efflux of NMD was inhibited by 1 µg/mL berberine and was promoted by 10 µg/mL berberine. We also adopted Rho123 as a positive control. Berberine demonstrated the similar effect on the transport of Rho123 as on the transport of NMD. Our results suggested that the effect of berberine on the transport of NMD across the BBB might result from the modulation of berberine on P-gp function. Sun et al[25] reported that berberine (30 µmol/L, corresponding to 10 µg/mL) decreased the uptake of carbamazepine by rBMEC, which was in agreement with our result. Verapamil had been reported to modulate P-gp ATPase activity in a bidirectional manner[26]. Whether berberine affected P-gp function via the same path still needs further investigation.
As the concentrations of baicalin (<10 µg/mL)[27] and berberine (<100 ng/mL level)[28] in vivo were so low, they might only be able to demonstrate inhibitory effects on P-gp function in in vivo studies. In addition, the detail mechanism of the bidirectional effect observed in the present study still needs further investigation.
References
- Ljunggren B, Brandt L, Saveland H, Romner B, Ryman T, Anderson KE. Aneurysmal subarachnoid hemorrhage: Prevention of delayed ischemic dysfunction with intravenous nimodipine. Neurosurg Rev 1987;10:255-63.
- Mohr JP, Orgogozo JM, Harrison MJG, Hennerici M, Wahlgren NG, Gelmers HI. Meta-analysis of oral nimodipine trials in acute ischemic stroke. Cerebrovasc Dis 1994;4:177-210.
- Pantoni L, Bianchi C, Beneke M, Inzitari D, Wallin A, Erkinjuntti T. The Scandinavian multi-infarct dementia trial: a double-blind, placebo-controlled trial on nimodipine in multi-infact dementia. J Neurol Sci 2000;175:116-23.
- Duarte J, Perez-Vizacaino F, Zarzuelo A, Jimenez J, Tanargo J. Vasodilator effects of quercetin in isolated rat vascular smooth muscle. Eur J Pharmacol 1993;239:1-7.
- Gao ZH, Huang KX, Yang XL, Xu HB. Free-radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria-Baicalensis Geo. Biochim Biophys Acta 1999;1472:643-50.
- Fukutake M, Yokota S, Kawamura H, Lizuka A, Amagaya S, Fukuda K, et al. Inhibitory effect of Coptidis rhizoma and Scutellaria radix on azoxy-methane induced aberrant crypt foci formation in rat colon. Biol Pharm Bull 1998;21:814-17.
- Chan FL, Choi HL, Chen ZY, Chan PSF, Huang Y. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett 2000;160:219-28.
- Li H, Wang H, Chen JH, Wang LH, Zhang HS, Fan Y. Determination of amino acid neurotransmitters in cerebral cortex of rats administered with baicalin prior to cerebral ischemia by capillary electrophoresis-laser-induced fluorescence detection. J Chromatogr B 2003;788:93-101.
- Zhang ZJ, Wang Z, Zhang XY, Ying K, Liu JX, Wang YY. Gene expression profile induced by oral administration of baicalin and gardenin after focal brain ischemia in rats. Acta Pharmacol Sin 2005;26:307-14.
- Lee H, Yang LL, Wang CC, Hu SY, Chang SF, Leec YH. Differential effects of natural polyphenols on neuronal survival in primary cultured central neurons against glutamate- and glucose deprivation-induced neuronal death. Brain Res 2003;986:103-13.
- Taylor CT, Baird AW. Berberine inhibition of electrogenic ion transport in rat colon. Br J Pharmacol 1995;116:2667-72.
- Kaneda Y, Torii M, Tanaka T, Aikawa M. In vitro effects of berberine sulphate on the growth and structure of Entamoeba histolytica, Giardia lamblia and Trichomonas vaginalis. Ann Trop Med Parasitol 1991;85:417-25.
- Ckless K, Schlottfeldt JL, Pasqual M, Moyna P, Henriques JA, Wajner M. Inhibition of in-vitro lymphocyte transformation by the isoquinoline alkaloid berberine. J Pharm Pharmacol 1995;47:1029-31.
- Wei YZ, Yao ZB, Yuan QF, Chen YC. Protective effect of berberine on rat hippocampus against cerebral ischemia. Chin J Chem Neuroanat 1995;11:315-21.
- Wu JF, Shi YJ, Liu TP. Protective effects of berberine on cerebral ischemia in mice and rats. Chin J Pharmacol Toxicol Methods 1995;9:100-3.
- Yoo KY, Hwang IK, Lim BO, Kang TC, Kim DW, Kim SM, et al. Berberry extract reduces neuronal damage and N-methyl-D-aspartate receptor 1 immunoreactivity in the gerbil hippocampus after transient forebrain ischemia. Biol Pharm Bull 2006;29:623-8.
- Zhang L, Liu XD, Xie L, Wang GJ. P-glycoprotein restricted transport of nimodipine across blood-brain barrier. Acta Pharmacol Sin 2003;24:903-6.
- Liu XD, Zhang L, Xie L. Effect of P-glycoprotein inhibitors erythromycin and cyclosporin A on brain pharmacokietics of nimodipine in rats. Eur J Drug Metab Pharmacokinet 2003;28:309-13.
- Ando H, Nishio Y, Ito K, Nakao A, Wang L, Zhao YL, et al. Effect of endotoxin on P-glycoprotein mediated biliary and renal excretion of rhodamine-123 in rats. Antimicrob Agents Chemother 2001;45:3462-67.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-54.
- Critchfield JW, Welsh CJ, Phang JM, Yeh GC. Modulation of adriamycin accumulation and efflux by flavonoids in HCT-15 colon cells. Biochem Pharmacol 1994;48:1437-45.
- Scambia G, Ranelletti FO, Benedetti Panici P, De Vincenzo R, Bonanno G, Ferrandina G, et al. Quercetin potentiates the effect of adriamycin in a multidrug-resistant MCF-7 human breast-cancer cell line: p-glycoprotein as a possible target. Cancer Chemother Pharmacol 1994;34:459-64.
- Gwenaelle C, Helene BC, Guila D, Jean MJ, Denis B, Attilio DP. Flavonoids: a class of modulators with bifunctional interactions at vicinal ATP- and steroid-binding sites on mouse P-glycoprotein. Proc Natl Acad Sci USA 1998;95:9831-6.
- Yoshiharu M, Hitomi T, Hirotami M, Mikihiko N, Takashi T, Hisakazu O, et al. Effect of bioflavonoids on vincristine transport across blood-brain barrier. Eur J Pharmacol 2000;395:193-201.
- Sun JJ, Xie L, Liu XD. Transport of carbamazepine and drug interactions at the blood-brain barrier. Acta Pharmacol Sin 2006;27:249-53.
- Stephane O, Lluis MM, Jean BJ, Manuel G. Effects of steroids and verapamil on P-glycoprotein ATPase activity: progesterone, desoxycorticosterone, corticosterone and verapamil are mutually non-exclusive modulators. Biochem J 1996;317:515-22.
- Lu T, Song J, Xie L, Wang GJ, Liu XD. Simultaneous determination of baicalin and wogonoside by HPLC in rat plasma administrated with Huangqin decoction and its pharmacokinetic study. Zhong Cao Yao 2005;36:870-3.
- Lu T, Liang Y, Song J, Xie L, Wang GJ, Liu X. Simultaneous determination of berberine and palmatine in rat plasma by HPLC-ESI-MS after oral administration of traditional Chinese medicinal preparation Huang-Lian-Jie-Du decoction and the pharmacokinetic application of the method. J Pharm Biomed Anal 2006;40:1218-24.
