Beneficial metabolic effects of nateglinide versus acarbose in patients with newly-diagnosed type 2 diabetes1
Introduction
Patients with type 2 diabetes frequently present with metabolic abnormalities such as postprandial hyperglycemia, postprandial lipemia, atherogenic dyslipidemia, and elevated levels of free fatty acids (FFA) and asymmetric dimethyl-arginine (ADMA). All of these alterations are known risk factors for atherosclerosis. Dyslipidemia in type 2 diabetic patients is characterized by increased levels of triglycerides and reduced levels of high-density lipoprotein cholesterol (HDL-C), while total cholesterol and low-density lipoprotein cholesterol (LDL-C) may be either normal or elevated[1]. Nateglinide, a D-phenylalanine derivative, has been shown to be effective in restoring early-phase insulin secretion and therefore reducing postprandial hyperglycemia and glucose excursion[2]. It has been demonstrated that the level of postprandial triglycerides is reduced by nateglinide in a diabetic animal model, but not in diabetic patients[3,4]. In a type 2 diabetic model, nateglinide reduced FFA levels in the portal blood after administration of a single dose[3]. However, the effects of nateglinide on FFA levels in diabetic patients have yet to be clarified.
Vascular endothelial dysfunction has been demonstrated as a risk factor for atherosclerosis in type 2 diabetic patients[5]. Although endothelial dysfunction was improved by using a single dose of nateglinide[6], the contribution of lowering postprandial hyperglycemia or improving early-phase insulin secretion to this effect remains unknown. ADMA, a specific endogenous inhibitor of nitric oxide (NO) synthase, has been found to be elevated with the condition of chronic hyperglycemia[7,8]. Moreover, high ADMA level is associated with either chronic or acute endothelial dysfunction in patients at risk for atherosclerosis[9,10]. We hypothesize, therefore, that the effect of nateglinide on insulin secretion may be beneficial to the postprandial lipid profiles and other metabolic parameters as is seen with postprandial hyper-glycemia. This crossover clinical trial aims to investigate the acute and chronic effects of nateglinide versus acarbose on plasma ADMA levels and lipid profiles in patients with newly-diagnosed type 2 diabetes.
Materials and methods
Study design Sixteen drug-naïve patients (5 males and 11 females, 49.4±1.6 years) with newly-diagnosed type 2 diabetes were screened and included in this study. Inclusion criteria were fasting plasma glucose ≤11 mmol/L, 6.5%–10.0% HbA1c, a body mass index of 22–30 kg/m2, and treatment with diet alone for at least 2 weeks and without any medication prior to enrolment for the study. In addition, 6 age-matched, healthy subjects with normal glucose tolerance were used as normal controls. The study protocol was approved by the Ethical Committee of Peking University Health Science Center. All subjects gave informed consent.
This study was conducted in a crossover open-labeled prospective design in order to diminish the statistical bias as much as possible. After 2 weeks of diet treatment, all subjects were randomized into group A and group B (8 subjects for each group). At the initiation of the study, the patients in group A were assigned to take nateglinide (120 mg, tid) just before meals; the patients in group B were assigned to take acarbose (50 mg, tid) together with meals. At the end of the fourth week, medication was discontinued. After a 1 week period of washout, the patients in group A were switched to acarbose (50 mg, tid); the patients in group B to nateglinide (120 mg, tid) for an additional 4 weeks (Figure 1).
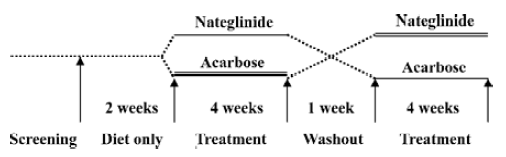
Sample collection Before and after treatment with nateglinide or acarbose, fasting blood samples were collected after 14 h of overnight fasting for all subjects. In order to assess the acute effects of nateglinide or acarbose on postprandial lipid profiles and the ADMA levels, a 75 g instant noodle ration served as a standard meal test and was consumed with the first dose of medication on the first day of treatment. Blood samples were collected from both arms at 30, 60, 120, and 240 min after the standard meal test.
Laboratory evaluation Plasma glucose levels were measured using the glucose-oxidase method, while HbA1c values were assessed by HPLC. Insulin was measured by IMMULITE 1000 (DPC, Los Angeles, CA, USA). Trigly-cerides, total cholesterol, LDL-C, and HDL-C were analyzed using a standard technique. FFA levels were assayed using commercially available kits (Randox Laboratories Ltd, Co Antrim UK).
Plasma ADMA was determined with a modification of the HPLC method previously described[11] using pre-column derivatization with o-phthalaldehyde (OPA). Prior to analysis, plasma samples and standards (Sigma, St Louis, MO USA) were extracted on OASIS solid phase extraction cartridges (Waters, Milford, MA, USA). The eluents were dried over nitrogen and dissolved in 50% methanol for HPLC analysis. The analysis was carried out on a HPLC system (TSP, San Jose, CA, USA) consisting of Spectra-Physis SP8810 pumps, a spectral fluorescence detector FL3000, and ThermoQuest work station. Samples and standards were incubated for exactly 3 min with the OPA (Sigma, USA) reagent (5.4 mg/mL OPA in 1 mmol/L borate buffer, pH 9.5, containing 0.4% β-mercaptoethanol) before injection into the HPLC. The OPA-derivatives of ADMA were separated on a 5 µm Kromasil C18 column (Dalian Elite Analytical Instruments,Dalian, China) with the fluorescence monitor set at λex 338 nm and λem 425 nm. Samples were eluted from the column with 0.96% citric acid/methanol 2:1, pH 6.8, at a flow rate of 1 mL/min. The detection limit of the assay was 0.1 mmol/L.
Statistical analyses Values are expressed as the mean± SEM. The statistical significance of variation between means was tested using two-tailed paired Student’s t-test. Values of P<0.05 were considered statistically significant.
Results
Five male and 11 female patients with newly-diagnosed type 2 diabetes were selected to enter this study. The mean fasting plasma glucose and HbA1c levels of 16 diabetic patients were 8.2±0.4 mmol/L and 7.6%±0.3%, respectively. Six healthy subjects with normal glucose tolerance were enrolled as normal controls, and their mean fasting plasma glucose and HbA1c levels were 4.9±0.1 mmol/L and 5.0%±0.1%, respectively. The mean age of the 16 diabetic patients and 6 normal controls was 49.4±1.6 and 48.8±5.3 years, respectively.
Acute effects of nateglinide versus acarbose on plasma glucose and serum insulin levels The efficiencies of a single dose of nateglinide or acarbose for lowering postprandial hyperglycemia were similar in the patients with type 2 diabetes (Figure 2A). Nateglinide seemed to be slightly more efficacious at lowering postprandial 120 min hyperglycemia, while acarbose seemed to be better at lowering postprandial 60 min hyperglycemia after a standard meal test, but there was no significant difference between these 2 medications. Compared to acarbose, a single dose of nateglinide significantly increased postprandial insulin release in patients with type 2 diabetes (Figure 2B).

Acute effects of nateglinide versus acarbose on postprandial lipid profiles Triglyceride levels were slowly elevated with time after a standard meal test in normal subjects and in the type 2 diabetic patients taking a single dose of nateglinide or acarbose. There was no significant difference between nateglinide and acarbose treatment (Figure 3A). The levels of serum FFA were remarkably decreased and reached a nadir at 120 min after the standard meal test in normal subjects. Fasting FFA levels in type 2 diabetics were significantly higher than in normal subjects. Nateglinide decreased postprandial 120 min FFA concentration more profoundly than acarbose (181.7 mmol/L vs 257.8 mmol/L, P=0.019; Figure 3B). LDL-C and HDL-C levels after the standard meal test were unchanged by these 2 medications (data not shown).

Chronic effects of nateglinide versus acarbose on fasting lipid profiles after 4 weeks of treatment Fasting HDL-C levels increased and LDL-C levels decreased significantly after 4 weeks of treatment with nateglinide in a sum of 2 arms of the study (P<0.05). The triglyceride and total cholesterol levels were unchanged by nateglinide treatment. Nateglinide led to the decrease of fasting FFA by about 10% after 4 weeks of treatment, but there was no statistically significant difference between pre- and post-treatment. Acarbose did not affect fasting lipid profiles after 4 weeks of treatment. In addition, there were no significant differences of fasting lipid profiles between nateglinide and acarbose treatments (Table 1).

Full table
Acute and chronic effects of nateglinide versus acarbose on plasma ADMA levels The fasting plasma ADMA level was significantly higher in the patients with type 2 diabetes than in normal subjects. Compared with a single dose of acarbose, a single dose of nateglinide decreased postprandial plasma ADMA concentration 240 min after the standard meal test in type 2 diabetic patients (P<0.05). However, the level of fasting ADMA was not significantly altered after 4 weeks of treatment with either nateglinide or acarbose (Figure 4).

Discussion
This crossover study showed that, as expected, insulin secretion after a standard meal test is delayed in type 2 diabetic patients, and that a single dose of nateglinide could partially restore early-phase insulin secretion. The data also indicated that nateglinide and acarbose had similar hypoglycemic effects on postprandial hyperglycemia.
It is well known that insulin inhibits the production of FFA from lipolysis. An increased hepatic FFA flux is postulated to be a major contributor of diabetic dyslipidemia because it leads to the overproduction of triglyceride-rich lipoproteins by the liver[12]. On the other hand, there are convincing data that show that plasma FFA levels modulate the severity of insulin resistance in type 2 diabetes[13,14]. Most humans are non-fasting throughout most of the day, and prolonged postprandial hyperlipidemia is an important characteristic of diabetic dyslipidemia[15]. Therefore, therapeutic effect of antihyperglycemic agents on postprandial lipemia, if any, may play a role in the prevention of atherosclerosis. In this study, a single dose of nateglinide significantly decreased the postprandial 120 min FFA level after a standard meal in comparison with acarbose. The level of fasting FFA after 4 weeks of treatment with nateglinide had a trend to decline from the baseline, although no significant difference was presented. In parallel with these results, nateglinide was found to acutely increase portal insulin levels and decrease portal FFA levels 15, 30, and 60 min after sucrose loading in diabetic rats[3]. Since nateglinide had similar effects on postprandial hyperglycemia as acarbose (which did not alter postprandial FFA levels), the reduction of postprandial 120 min FFA levels by nateglinide was likely to be the result of the partial restoration of early-phase insulin secretion rather than from the improvement of postprandial hyperglycemia. In fact, in a previous study, peripherally-administered insulin resulted in early insulin augmentation that reduced the glycemic and FFA responses to a meal in diabetic patients[16]. Furthermore, the improvement of postprandial FFA concentrations by nateglinide in diabetics was demonstrated to be associated with suppression of hormone-sensitive lipase, which induces FFA release from adipose tissue[17]. All these results suggest that the defect of early-phase insulin secretion might be one of the reasons why hyperlipidemia develops in type 2 diabetics.
In agreement with previous reports[4], this study showed that nateglinide had neither acute nor chronic effects on serum triglyceride levels in type 2 diabetics. The analogous results were also found in subjects at risk for type 2 diabetes[18]. Insulin has potential effects on postprandial lipid metabolism. It can affect either production or removal of triglycerides and triglyceride-rich lipoproteins[19,20]. So far, the reason why nateglinide has no effect on triglyceride metabolism, despite significantly increasing insulin secretion and improving postprandial hyperglycemia, remains unclear. It has been speculated that the acute increase of insulin by nateglinide might be not enough to overcome insulin resistance and then to substantially lower triglyceride concentrations in diabetic patients[18].
The present study also showed that nateglinide increased HDL-C levels and decreased LDL-C levels after 4 weeks of treatment. Similarly, repaglinide, another rapidly-acting prandial glucose regulator, was reported to decrease serum total cholesterol concentration in type 2 diabetic patients[21]. Whether nateglinide affects serum HDL-C and LDL-C levels in this manner deserves further investigation as it may indicate a cardiovascular advantage if it can be confirmed.
In the present study, nateglinide was found to decrease postprandial ADMA concentrations. To our knowledge, this is the first description of the effect of nateglinide on ADMA in type 2 diabetics. ADMA, an endogenous competitive inhibitor of NO synthase, is synthesized in many tissues, including vascular endothelial cells. Inconsistent changes in ADMA levels in diabetes have been described in the literature, most of which showed that ADMA levels were elevated[7,22], as seen in our study. Moreover, the improvement of hyperglycemia was associated with lowered plasma ADMA concentrations[23,24]. However, there was 1 study reporting that ADMA levels in diabetic patients were lower than in healthy subjects and were inversely correlated with HbA1c levels[25]. In that study, the selected diabetic patients had increased glomerular filtration rates compared to healthy subjects, and the lowered ADMA in type 2 diabetic patients was accordingly attributable to increased GFR. This speculation, however, needed to be clarified, as the difference of GFR between 90 mL/min/1.73 m2 and 98 mL/min/1.73 m2 might have no clinical significance. Therefore, the reason of inconsistent results of ADMA levels in diabetes was unclear. There is an intriguing relationship between insulin resistance and ADMA levels reported in some of the literature. Plasma concentrations of ADMA have been demonstrated to be elevated in several clinical syndromes associated with insulin resistance and in apparently-healthy insulin resistant individuals[26,27]. Rosiglitazone treatment or weight loss by non-pharmacological means in these subjects resulted in both an enhancement in insulin sensitivity and a fall in plasma ADMA concentrations[27,28]. On the basis of the above-mentioned data, it is reasonable to think that the plasma levels of ADMA were elevated rather than decreased in the patients with type 2 diabetes characterized by hyperglycemia and insulin resistance.
Reduced production of NO in endothelial cells by elevated ADMA leads to the abnormality of endothelial cell-mediated vasodilation[29], which has been demonstrated in patients with established atherosclerosis[30]. Interestingly, it has been shown that post-challenge hyperglycemia caused the alteration of vascular function, but did not lead to elevation in ADMA concentration in patients with impaired glucose tolerance[31]. Indeed, our data also suggested that acarbose did not affect postprandial ADMA concentration. In this regard, we speculate that diminished postprandial ADMA levels produced from nateglinide might result from the partial restoration of early-phase insulin secretion or from nateglinide per se. The latter is yet to be elucidated. More-over, several clinical studies have demonstrated that nate-glinide is capable of improving insulin sensitivity in patients with established diabetes[32,33]. Therefore, it is conceivable that the effect of nateglinide on postprandial plasma ADMA concentration is possibly associated with its ability to improve insulin sensitivity as well. However, the results of our study also indicated that nateglinide did not alter the fasting ADMA concentration after 4 weeks of treatment. The reason for this phenomenon remains unknown. The results of this study need to be confirmed in a larger clinical trial. Nevertheless, the fact that nateglinide not only lowers postprandial ADMA level, but also improves endothelial dysfunction[34] suggests its therapeutic advantage in the prevention of atherosclerosis.
Taken together, the results of our study demonstrate that nateglinide partially restores early-phase insulin secretion, decreases postprandial serum FFA and ADMA concentra-tions, and possibly modulates serum HDL-C and LDL-C abnormalities indicating that nateglinide has a cardiovascular advantage over acarbose.
Acknowledgments
We are grateful to Qiu-ming GENG, Lu ZHANG, Zheng MA, and Guo-quan LI for their excellent technical assistance. Nateglinide and acarbose were kindly provided by Beijing Novartis Pharma, Ltd (Beijing, China) and Bayer Healthcare Company, Ltd (Beijing, China) respectively.
References
- Buse JB, Tan MH, Prince MJ, Erickson PP. The effects of oral anti-hyperglycemic medications on serum lipid profiles in patients with type 2 diabetes. Diabetes Obes Metab 2004;6:133-56.
- Ikenoue T, Akiyoshi M, Fujitani S, Okazaki K, Kondo N, Maki T. Hypoglycemic and insulinotropic effects of a novel oral anti-diabetic agent, (-)-N-(trans-4-isopropylcyclohexane-carbonyl)-D-phenylalanine (A-4166). Br J Pharmacol 1997;120:137-45.
- Mori Y, Kitahara Y, Miura K, Tajima N. Comparison of voglibose and nateglinide for their acute effects on insulin secretion and free fatty acid levels in OLETF rat portal blood after sucrose loading. Endocrine 2004;23:39-43.
- Vakkilainen J, Mero N, Schweizer A, Foley JE, Taskinen MR. Effects of nateglinide and glibenclamide on postprandial lipid and glucose metabolism in type 2 diabetes. Diabetes Metab Res Rev 2002;18:484-90.
- de Koning EJ, Rabelink TJ. Endothelial function in the postprandial state. Atheroscler Suppl 2002;3:11-6.
- Shimabukuro M, Higa N, Takasu N, Tagawa T, Ueda S. A single dose of nateglinide improves post-challenge glucose metabolism and endothelial dysfunction in type 2 diabetic patients. Diabet Med 2004;21:983-6.
- Abbasi F, Asagmi T, Cooke JP, Lamendola C, McLaughlin T, Reaven GM, et al. Plasma concentrations of asymmetric dim-ethylarginine are increased in patients with type 2 diabetes mellitus. Am J Cardiol 2001;88:1201-3.
- Xiong Y, Lei M, Fu S, Fu Y. Effect of diabetic duration on serum concentrations of endogenous inhibitor of nitric oxide synthase in patients and rats with diabetes. Life Sci 2005;77:149-59.
- Annuk M, Zilmer M, Fellstrom B. Endothelium-dependent vasodilation and oxidative stress in chronic renal failure: impact on cardiovascular disease. Kidney Int Suppl 2003;84:S50-3.
- Stuhlinger MC, Oka RK, Graf EE, Schmolzer I, Upson BM, Kapoor O, et al. Endothelial dysfunction induced by hyperhomo-cysteinemia: role of asymmetric dimethylarginine. Circulation 2003;108:933-8.
- Bode-Boger SM, Boger RH, Kienke S, Junker W, Frolich JC. Elevated L-arginine/dimethylarginine ratio contributes to enhanced systemic NO production by dietary L-arginine in hypercholesterolemic rabbits. Biochem Biophys Res Commun 1996;219:598-603.
- Taskinen MR. Diabetic dyslipidemia: from basic research to clinical practice. Diabetologia 2003;46:733-49.
- Kelley D, Williams K, Price J, McKolanis T, Goodpaster B, Thaete F. Plasma fatty acids, adiposity and variance of skeletal muscle insulin resistance in type 2 diabetes mellitus. J Clin Endocrinol Metab 2001;86:5412-9.
- Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, et al. Overnight lowering of free fatty acids with acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 1999;48:1836-41.
- Karpe F. Postprandial lipemia - effect of lipid-lowering drugs. Atheroscler Suppl 2002;3:41-6.
- Bruce D, Chisholm D, Storlien L, Kraegen E. Physiological importance of deficiency in early prandial insulin secretion in non-insulin dependent diabetes. Diabetes 1988;37:736-44.
- Dimitriadis G, Boutati E, Lambadiari V, Mitrou P, Maratou E, Brunel P, et al. Restoration of early insulin secretion after a meal in type 2 diabetes: effects on lipid and glucose metabolism. Eur J Clin Invest 2004;34:490-7.
- Johanson EH, Jansson PA, Gustafson B, Sandqvist M, Taskinen MR, Smith U, et al. No acute effect of nateglinide on postprandial lipid and lipoprotein responses in subjects at risk for type 2 diabetes. Diabetes Metab Res Rev 2005;21:376-81.
- Malmstrom R, Packard CJ, Caslake M, Bedford D, Stewart P, Yki-Jarvinen H, et al. Defective regulation of triglyceride metabolism by insulin in the liver in NIDDM. Diabetologia 1997;40:454-62.
- Panarotto D, Remillard P, Bouffard L, Maheux P. Insulin resistance affects the regulation of lipoprotein lipase in the postprandial period and in an adipose tissue-specific manner. Eur J Clin Invest 2002;32:84-92.
- Damsbo P, Marbury TC, Hatorp V, Clauson P, Muller PG. Flexible prandial glucose regulation with repaglinide in patients with type 2 diabetes. Diabetes Res Clin Pract 1999;45:31-9.
- Masuda H, Goto M, Tamaoki S, Azuma H. Accelerated intimal hyperplasia and increased endogenous inhibitors for NO synthesis in rabbits with alloxan-induced hyperglycaemia. Br J Pharmacol 1999;126:211-8.
- Asagami T, Abbasi F, Stuelinger M, Lamendola C, McLaughlin T, Cooke JP, et al. Metformin treatment lowers asymmetric dimethylarginine concentrations in patients with type 2 diabetes. Metabolism 2002;51:843-6.
- Yasuda S, Miyazaki S, Kanda M, Goto Y, Suzuki M, Harano Y, et al. Intensive treatment of risk factors in patients with type-2 diabetes mellitus is associated with improvement of endothelial function coupled with a reduction in the levels of plasma asymmetric dimethylarginine and endogenous inhibitor of nitric oxide synthase. Eur Heart J 2006;27:1159-65.
- Paiva H, Lehtimaki T, Laakso J, Ruokonen I, Rantalaiho V, Wirta O, et al. Plasma concentrations of asymmetric-dimethyl-arginine in type 2 diabetes associate with glycemic control and glomerular filtration rate but not with risk factors of vasculopathy. Metabolism 2003;52:303-7.
- Vallance P. Importance of asymmetrical dimethylarginine in cardiovascular risk. Lancet 2001;358:2096-7.
- Stuhlinger MC, Abbasi F, Chu JW, Lamendola C, McLaughlin TL, Cooke JP, et al. Relationship between insulin resistance and an endogenous nitric oxide synthase inhibitor. JAMA 2002;287:1420-6.
- McLaughlin T, Stuhlinger M, Lamendola C, Abbasi F, Bialek J, Reaven GM, et al. Plasma asymmetric dimethylarginine concentrations are elevated in obese insulin-resistant women and fall with weight loss. J Clin Endocrin Metab 2006;91:1896-900.
- Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest 1997;100:2153-7.
- Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med 1999;340:115-26.
- Wascher TC, Schmoelzer I, Wiegratz A, Stuehlinger M, Mueller-Wieland D, Kotzka J, et al. Reduction of postchallenge hyperglycaemia prevents acute endothelial dysfunction in subjects with impaired glucose tolerance. Eur J Clin Invest 2005;35:551-7.
- Hazama Y, Matsuhisa M, Ohtoshi K, Gorogawa S, Kato K, Kawamori D, et al. Beneficial effects of nateglinide on insulin resistance in type 2 diabetes. Diabetes Res Clin Pract 2006;71:251-5.
- Mari A, Gastaldelli A, Foley JE, Pratley RE, Ferrannini E. Beta-cell function in mild type 2 diabetic patients: effects of 6-month glucose lowering with nateglinide. Diabetes Care 2005;28:1132-8.
- Shimabukuro M, Higa N, Takasu N, Tagawa T, Ueda S. A single dose of nateglinide improves post-challenge glucose metabolism and endothelial dysfunction in type 2 diabetic patients. Diabet Med 2004;21:983-6.
