Effect of long-term partial bladder outlet obstruction on caldesmon isoforms and their correlation with contractile function
Introduction
Partial bladder outlet obstruction (PBOO) in human and animal models results in numerous changes in the detrusor. While in humans the degree, duration, and cause of obstruction varies from individual to individual, all animal models have the advantage of creating obstruction of the same degree, which is beneficial for studying the bladder at any given time. The rabbit bladder provides an excellent opportunity to understand the physiological, histological, and biochemical properties of a functioning bladder[1,2].
In both men[3] and rabbits[4] with PBOO, the detrusor smooth muscle (DSM) undergoes hypertrophy to compensate for the increased force required to expel urine against the obstruction. In some rabbits, as in men, DSM hypertrophy following PBOO compensates for the increased muscle contractility, which is required to overcome the increased urethral resistance during micturition (compensation), whereas other rabbits show bladder dysfunction (decompensation) despite smooth muscle (SM) hypertrophy[5,6]. With muscle strips from hypertrophied DSM from decompensated bladders, physiological studies show a decrease of force in response to carbachol (CCH) or field stimulation (FS)[4]. The molecular mechanisms responsible for these changes in contractility and emptying remain largely unknown.
The actin-associated protein caldesmon (CaD) is thought to modulate the regulation of SM contraction by myosin-mediated regulation via the phosphorylation of the myosin light chain[7]. CaD is expressed as 2 dominant isoforms, l-CaD and h-CaD with low and high molecular sizes (70 and 140 kDa), respectively. l-CaD is found in non-muscle cells of various tissue types[8], whereas h-CaD is present in SM cells from all sources. h-CaD is bound to thin filaments in SM, where it inhibits the actin-myosin interaction and actomyosin ATPase activity[9]. Both l-CaD and h-CaD have similar regions to bind actin and myosin[10].
Zhang et al[11] found that the expression of l-CaD increases significantly at the mRNA and protein levels in the decompensated bladders compared to normal and compensated bladders. The inability of decompensated bladders to empty, despite detrusor hypertrophy, is associated with an overexpression of l-CaD. The level of l-CaD overexpression might be a useful marker in estimating the degree of detrusor remodeling and contractile dysfunction in PBOO.
In the present study, we examined the expression of CaD isoforms in rabbit DSM during the progression of PBOO, and related them with time course of bladder obstruction.
Materials and methods
Partial bladder outlet obstruction The rabbit experiments, including the surgical procedure, were approved by the Institutional Animal Care and Use Committee of Xi’an Jiaotong University (Xi’an, China). Thirty-two adult male New Zealand white rabbits were divided into 4 groups, each consisting of 8 rabbits. Six rabbits in each group underwent partial outlet obstruction. The other 2 rabbits in each group underwent sham operations, in which the ligature was removed immediately after placing it around the urethra; these rabbits served as the control group. The rabbits in each group were evaluated after 1, 2, 4, and 8 weeks of obstruction, respectively. The data from all of the control animals showed no significant differences and were combined into 1 group.
Under anesthesia, an 8Fr catheter was inserted into the bladder via the urethra; the bladder neck was exposed through a small vertical abdominal incision. Once the ureters and vas deferens were identified, a 2-zero silk suture was placed below the bladder neck with right angle clamps. To maximize the standardization of the partial outlet obstruction, a second 8Fr catheter was placed outside the urethra, and the silk suture was tied around both catheters, which were then removed. In the sham-operated group, the silk suture was cut and removed after an identical dissection.
At 1, 2, 4, and 8 weeks’ postoperatively, the animal were euthanized and the bladder was opened via the same midline incision; a 1×1.5 cm section of the ventral bladder wall was excised with a scalpel. Three full-thickness strips were fixed for the histological studies. The mucosal and serosal layers were removed, and 3 longitutudinal bladder strips were obtained from each rabbit bladder for the contractile studies. The bladder muscle layer from the mid-body region was isolated and frozen in liquid nitrogen for protein extraction.
Physiological studies The muscle strips were attached by a 4-zero silk suture to a post in a 50 mL tissue organ bath at one end and an isometric force transducer at the other end. The transducer was calibrated with known weights, and its output was directed to a polygraph. The tissue organ baths were maintained at 37 °C and contained Tyrode’s solution (125 mmol/L sodium chloride, 2.7 mmol/L potassium chloride, 1.8 mmol/L calcium chloride, 0.5 mmol/L magnesium chloride, 23.8 mmol/L sodium bicarbonate, 0.4 mmol/L sodium phosphoric acid, and 5.6 mmol/L glucose) perfused by a bubbled mixture of 95% oxygen and 5% carbon dioxide. After equilibration for 30 min at slack length, the strips were gradually stretched to the length where optimal force was generated, and the maximal response to FS (32 Hz, 80 V, 1 ms duration) was determined. After FS, the response to 200 µmol CCH was determined. Peak tension was recorded for each of the different stimuli. Between the additions of pharmacological agents, each strip was washed 3 times with fresh buffer at 10 min intervals. After the completion of the experiments, the muscle between the 2 silk sutures was weighed. All of the results are expressed in gram tension/100 mg tissue. The mean of the 3 strips from 1 animal represented 1 individual preparation. The SEM for each experiment was based on the 6 animals from each group.
The rabbits were divided into the control (sham operated) and 1, 2, 4, and 8-week obstructed groups. Muscle strip studies subcategorized the obstructed group of bladders into compensated (force more than 10 g tension/100 mg tissue at cholinergic stimulation) and decompensated (force less than 10 g tension/100 mg tissue)[5]. All of the results are presented with these definitions.
Histological studies Full-thickness strips from the detrusor obtained from the bladder were fixed in 4% buffered formalin. The strips were embedded in paraffin, and 6 mm tissue sections were cut. The sections were stained with conventional hematoxylin-eosin and Masson’s trichrome preparations. The muscle fraction was analyzed with a microscope equipped with a digital camera and Image-Pro Plus 5.0 image analysis software. The percentage area was selected in the program and generated automatically for each image. Twenty random images per tissue were analyzed for each tissue sample. The averages were then calculated for each group.
Protein extraction and Western blotting The total protein was extracted in extraction buffer [20% glycerol, 50 mmol/L Tris-HCl (pH 6.8) and 0.5% (v/v) Tween-20] and protease inhibitor cocktail (Sigma, St Louis, MO, USA). After adding 10% SDS, the sample was mixed, boiled for 4 min, and centrifuged at 11 000×g for 15 min at 4 °C to remove the undissolved material. The protein concentration in the supernatant was measured with the Bradford method. Aliquots of the protein extract containing 30 µg of total protein were electrophoresed in a 6% SDS-PAGE gel, and blotted overnight at 4 °C to a P-membrane with Towbin buffer [25 mmol/L Tris, 192 mmol/L glycine, and 20% (v/v) methanol]. The membrane was blocked with 5% dry milk, and incubated with an antibody against CaD (Santa Cruz Biotechnology, Santa Cruz, CA, USA; catalog No sc-7574). After treatment with the primary antibody, the membrane was washed in TBST buffer (20 mmol/L Tris, 500 mmol/L NaCl, and 0.05% Tween-20), and incubated with a secondary antibody (mouse antigoat immunoglobulin G at 1:5000). The substrates were visualized by ECL (Amersham Pharmacia Biotech) and by exposing the membranes to autoradiographic films. The films were scanned and analyzed with Image J Software (National Institutes of Health). Standard curves were constructed to establish the protein concentrations for the analysis fall within the linear range.
Statistical analysis All data are expressed as the mean±SEM with P<0.05 considered statistically significant. ANOVA, followed by the Bonferonni test for individual differences, were used for comparative purposes.
Results
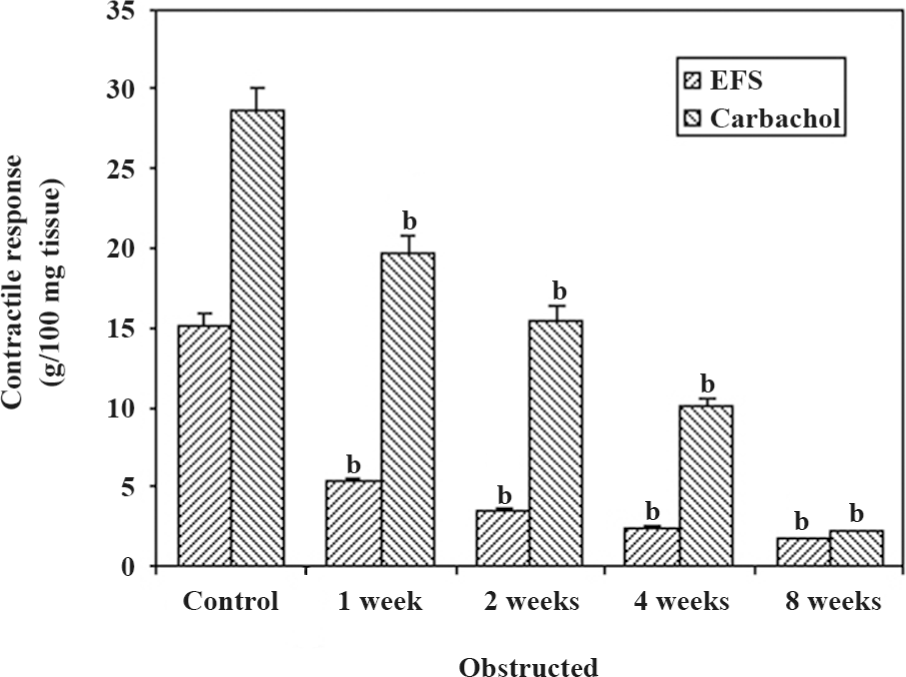
Physiological studies The maximal contractile responses to FS and CCH are presented in Figure 1. The responses to all forms of stimulation decreased progressively in relation to the duration of obstruction. The contractile responses to FS were significantly sensitive to obstruction in comparison to the contractile responses to CCH. The responses to CCH decreased progressively, but significantly at 1 week (P<0.05), and then decreased progressively, reaching a response less than 10 g tension/100 mg tissue at 4 weeks of obstruction. It should be noted that the 8-week obstructed group had the lowest responses to FS and CCH.
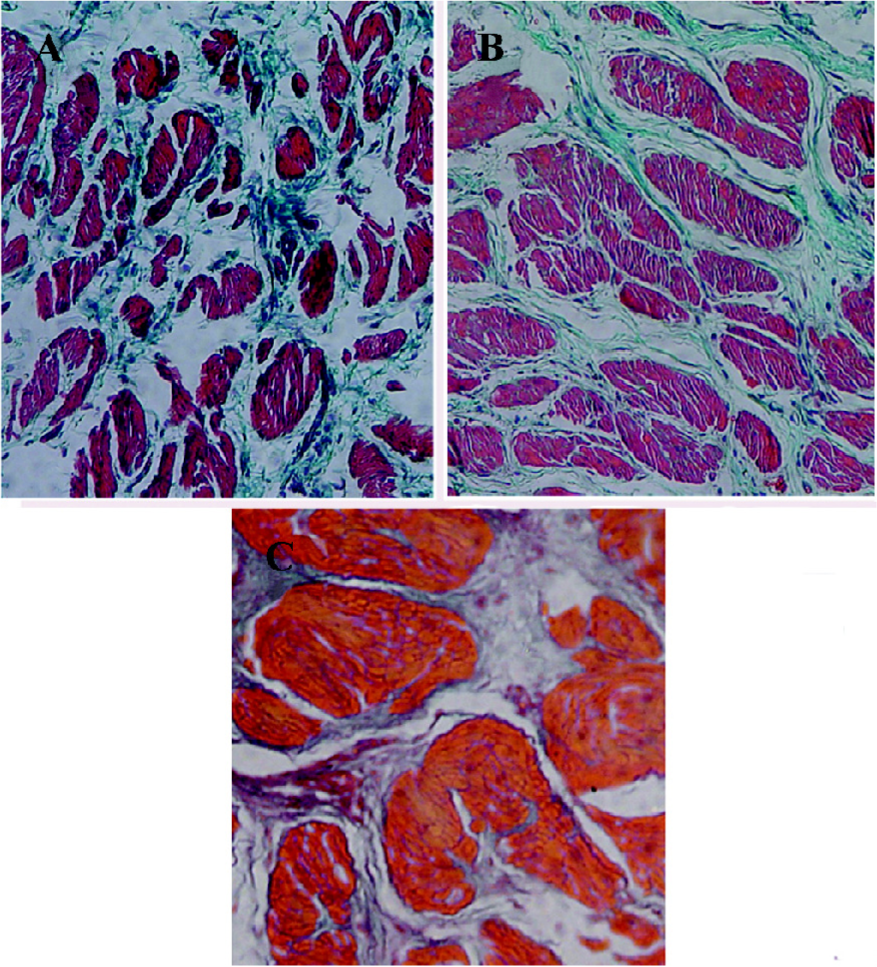
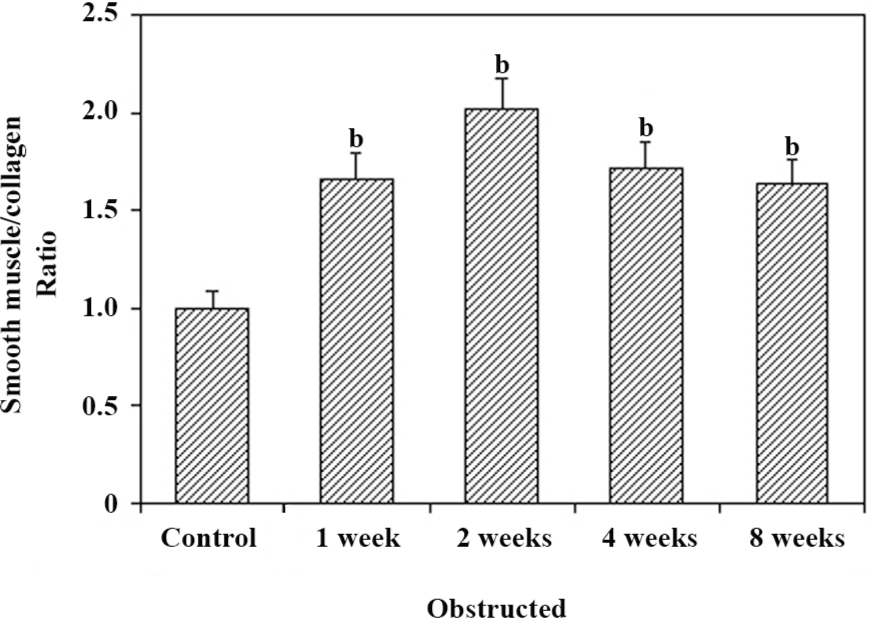
Histological studies Figure 2 shows the representative images of the control and 2 and 8-week obstructed rabbit bladder body detrusor tissue sections showing SM and collagen using Masson trichrome staining. The effect of obstruction on the SM/collagen ratio is shown in Figure 3. Obstructed bladders showed significantly hypertrophied muscle bundles, and the collagen infiltration increased progressively. The SM/collagen ratio increased progressively before 2 weeks of obstruction, and decreased gradually thereafter. After 1 week of obstruction, the ratio increased significantly compared with the control (P<0.05). After 4 and 8 weeks of obstruction, although significantly lower than the earlier time points, the ratio was still higher than that of the controls (Figure 3).
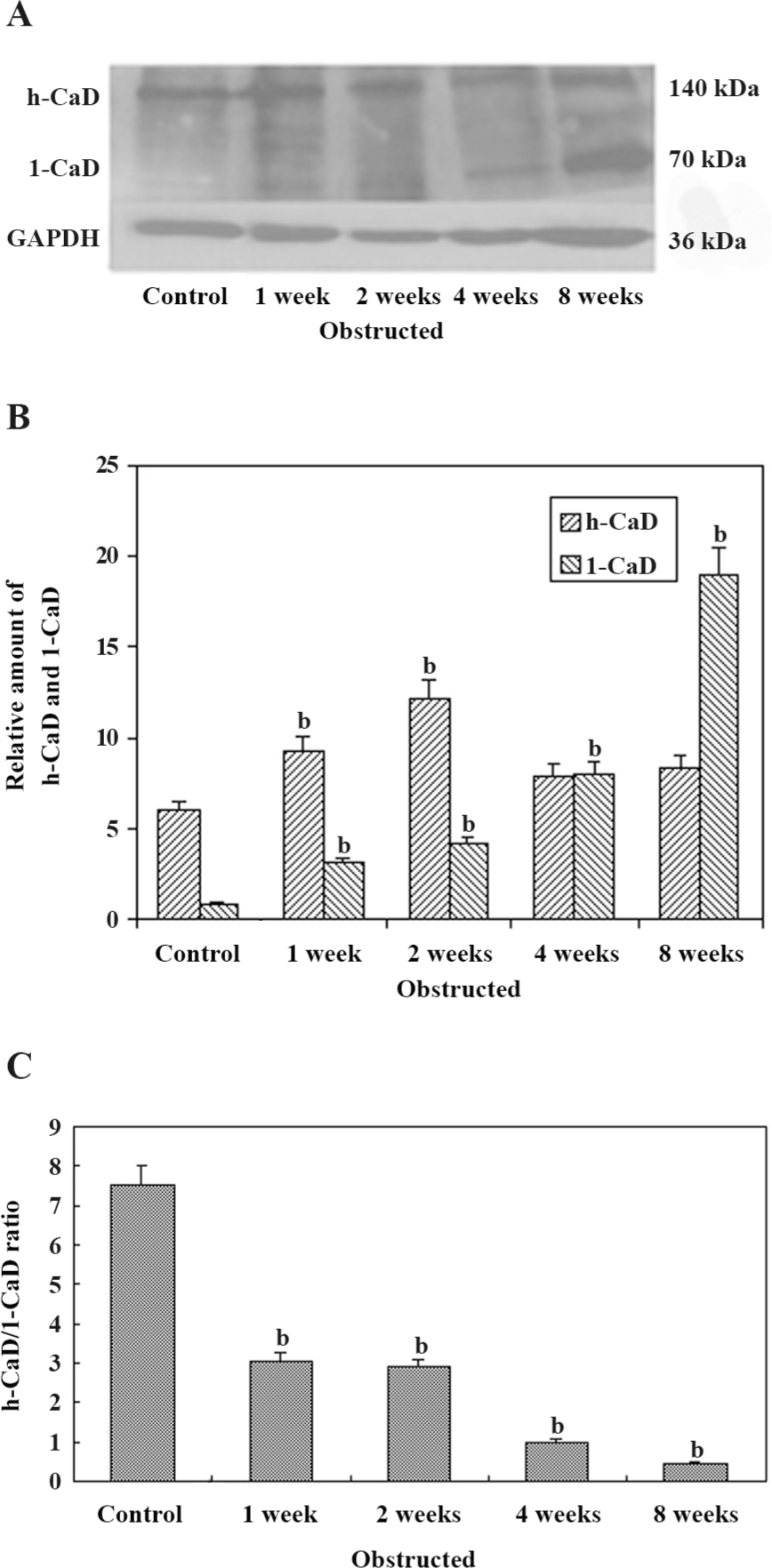
Western blot analysis of CaD isoforms in the bladder Figure 4A shows the expression of h-CaD and l-CaD from the control and obstructed rabbit bladders by Western blotting. Data obtained from the Western blot analysis of samples from the different groups are shown in Figure 4B. The expression of l-CaD was very low in the control bladder. Following obstruction-induced hypertrophy, the expression of l-CaD increased significantly to approximately the same extent as that of the 1–2-week obstructed groups and increased further in the 4–8-week obstructed groups. However, the increase was almost 8-fold higher in the 4-week obstructed bladders and almost 25-fold higher in the 8-week obstructed bladders. The expression of h-CaD increased in all of the obstructed bladders, but at significantly higher levels in the 1–2-week obstructed bladders versus the control and 4–8-week obstructed bladders (P<0.05). The h-CaD/l-CaD ratio decreased significantly in the 1–2-week obstructed groups and decreased further in the 4–8-week obstructed groups.
Discussion
Previous studies on the rabbit model for bladder outlet obstruction have identified several changes in whole bladder function and contractile performance of the muscle strip[11,13]. In the present study, we analyzed the molecular changes in CaD isoform expression and correlated them with the time course of obstruction. We found that bladder contractile function decreased over the course of the obstruction, and the 8-week obstructed group was the most severely compromised. In addition, we found that l-CaD and h-CaD in DSM showed significant differences during the course of PBOO. In particular, there was a progressive increase in l-CaD and a significant overexpression in the 8-week obstructed bladders compared to the normal and compensated bladders, which indicated a close relationship with bladder contractile function. The expression of h-CaD increased in all obstructed bladders, but only at significantly higher levels in the 1–2-week obstructed bladders.
Despite the acute onset, partial bladder outlet obstruction in rabbits induces detrusor remodeling similar to that in men with benign prostatic hyperplasia in terms of its impact on structural and functional alterations in SM[14]. In the animal model, the level of decompensation may be based on more objective criteria, such as the contractile responses to various forms of stimulation. In the present study, we defined the muscle strips that force less than 10 g tension/100 mg tissue at cholinergic stimulation as decompensated[5]. The DSM undergo compensatory hypertrophy required to overcome the increased outlet resistance for the increased force production. In some obstructed rabbits, as in humans, detrusor hypertrophy and the associated remodeling are sufficient to maintain a close to normal (compensated) bladder function, whereas other rabbits show severe bladder dysfunction (decompensated). Mannikarottu et al[15,16] reported that bladder decompensation progresses over 28 d based on bladder weight and response to various stimuli. The responses of the rabbit bladders to partial outlet obstruction may be divided into initial, compensated, and decompensated phases[17,18]. The initial phase occurs immediately after partial outlet obstruction. It is characterized by a rapid increase in bladder mass until it becomes relatively stable after surgery. The compensated state begins after this initial growth period and is characterized by normal or slightly lower than normal contractility. In some periods after compensation, bladder function begins to deteriorate, resulting in progressive decreases in contractile response to all forms of stimulation, increased bladder mass, and decreased compliance. In the final phase, decompensation is characterized by connective tissue replacement of SM, which finally results in a fibrous organ with little or no contractile function[17,18]. Based on the morphological and physiological studies, it can be concluded that the 1–2-week obstructed bladders were in the compensated phase. At 4 weeks, the obstructed bladders were in the decompensated phase, and the 8-week obstructed bladders were severely decompensated, but not in the final decompensated phase. Hence our findings on changes in CaD isoforms can be correlated with these well-accepted time points in bladder function after obstruction.
The SM is hypertrophied throughout the obstruction period. Although hypertrophied, the response to FS and CCH decreased, which indicates that the molecular characteristics of SM have changed. An altered regulation of contractile proteins is responsible for the decompensation of detrusor muscles secondary to obstruction.
In the present study, we focused on the relationship between CaD and the progression of PBOO. The major mechanism that regulates SM contraction is mediated via myosin light chain phosphorylation. However, physiological data indicate that there is also a secondary, actin-linked system of SM regulation involving CaD, tropomyosin, and calmodulin[19,20]. The thin filament-associated proteins h-CaD have been known to modulate actomyosin ATPase and contraction in SM[21]. h-CaD inhibits the in vitro motility of actin filaments over myosin heads, but the role of l-CaD in contractility is less clear. In the present study, we proved that the expression of l-CaD increased significantly to approximately the same extent at that of the 1–2-week obstructed groups and increased further in the 4–8-week obstructed groups. The expression of h-CaD increased in all of obstructed bladders, but at significantly higher levels in the 1–2-week obstructed bladders compared to the control and 4–8-week obstructed bladders. It is possible that excess h-CaD prevents its replacement with l-CaD in the thin filaments and the alteration of contractile characteristics in the 1–2-week obstructed groups. Yet the role of l-CaD in contractility or cell motility is less clear; the overexpressed l-CaD may be associated with remodeling of the cytoskeletal structure, which enables the SM cells to resist high intravesical pressure during micturition. The overexpressed l-CaD displaces h-CaD from the thin filaments, since the binding sites for both l-CaD and h-CaD are the same, and the thin filaments containing l-CaD may interfere with the generation and/or maintenance of the additional force required for compensating the obstruct-induced contractile dysfunction. Therefore, the overexpression of l-CaD is associated with bladder decompensation and could be a marker for the status of detrusor muscle remodeling and dysfunction.
Control bladders show very little l-CaD, and l-CaD may be from the interstitial cells. Compensated bladders of the 1–2-week obstructed groups showed an increased expression of both l-CaD and h-CaD, whereas the decompensated bladders of the 4–8-week obstructed groups showed an overexpression of l-CaD, but very little change in h-CaD compared to the controls. Therefore, the h-CaD/l-CaD ratio in the control groups was approximately 9:1, in the compensated bladders of the 1–2-week obstructed groups, the ratio decreased significantly to 3:1. In the decompensated bladders of the 4–8-week obstructed groups, the ratio decreased to less than 1:1. Therefore, the h-CaD/l-CaD ratio has a close relationship with bladder function. h-CaD and l-CaD have the same structure, so they can be analyzed by 1 polyclonal antibody simultaneously, which makes it possible to measure the h-CaD/l-CaD ratio more objectively. Although the implication of the h-CaD/l-CaD ratio is unclear, the h-CaD/l-CaD ratio could be a relatively precise marker for bladder function after PBOO.
We clearly show the incremental changes in CaD with the progression from compensated to decompensated function. In the only comparable study, Zhang et al[11] reported that h-CaD was unchanged and l-CaD was overexpressed in the SM of the decompensated bladders in response to partial bladder outlet obstruction for 2 weeks. Our study is different. The time course of obstruction in our study was much longer, and we clearly documented deterioration into decompensation over time.
An additional characteristic response of the rabbit bladder to obstruction is the increased collagen deposition between and within SM bundles[22] (Figure 2). In the present study, we noticed that the relative area of collagen increased significantly after 2 weeks’ obstruction, and the SM/collagen ratio was downregulated from then on. We can conclude that before the 2-week obstruction, the increase in bladder mass was mainly due to SM hypertrophy, and that the increase in bladder mass after 2 weeks’ obstruction was mainly due to the increased deposition of collagen. The fibroblast was hyperplastic in the obstructed groups and produced more collagens, which induced the bladder and resulted in decompensation. One would suspect that the overexpression of l-CaD may be from the hyperplastic fibroblast; however, Zhang et al[11] proved that the CaD and SM myosins were colocalized in the bladder myocytes from the decompensated bladders, and SM cells outnumbered fibroblasts, which indicated that a large portion of the overexpressed l-CaD in the hypertrophied detrusor were derived from SM cells.
The present study demonstrates an association between alterations in the contraction of the rabbit bladder after obstruction with l-CaD and h-CaD in the bladder muscle. The expression of l-CaD increased significantly to approximately the same extent as that of the 1–2-week obstructed groups and increased further in the 4–8-week obstructed groups. The expression of h-CaD increased in all of the obstructed bladders, but at significantly higher levels in the 1–2-week obstructed bladders compared to the control and 8-week obstructed bladders. The h-CaD/l-CaD ratio decreased significantly in the 1–2-week obstructed groups and decreased further in the 4–8-week obstructed groups. This suggests that the overexpression of l-CaD and the h-CaD/l-CaD ratio could be markers for the status of detrusor muscle remodeling and dysfunction.
References
- Stanton MC, Austin JC, Delaney DP, Gosfield A, Marx JO, Zderic SA, et al. Partial bladder outlet obstruction selectively abolishes protein kinase C induced contraction of rabbit detrusor smooth muscle. J Urol 2006;176:2716-21.
- Levin RM, Haugaard N, O’Connor L. Obstructive response of human bladder to BPH vs., rabbit bladder response to partial outlet obstruction: A direct comparison. Neurourol Urodyn 2000;19:609-29.
- Tse V, Wills E, Szonyi G, Khadra MH. The application of ultrastructural studies in the diagnosis of bladder dysfunction in a clinical setting. J Urol 2000;163:535-9.
- AJ. Effect of bladder outlet obstruction on the morphology, physiology, and pharmacology of the bladder. Prostate Suppl 1990;3:9-26.
- Kato K, Monson FC, Longhurst PA, Wein AJ, Haugaard N, Levin RM. The functional effects of long-term outlet obstruction on the rabbit urinary bladder. J Urol 1990;143:600-6.
- Stein R, Gong C, Hutcheson JC, Canning DA, Zderic SA. The decompensated detrusor III: impact of bladder outlet obstruction on sarcoplasmic endoplasmic reticulum protein and gene expression. J Urol 2000; 164: 1026?–30.
- Marston SB, Redwood CS. The molecular anatomy of caldesmon. Biochem J 1991;279:1-16.
- Bryan J, Imai M, Lee R, Moore P, Cook RG, Lin WG. Cloning and expression of a smooth muscle caldesmon. J Biol Chem 1989; 264: 13 873–9.
- Sobue K, Muramoto Y, Fujita M, Kakiuchi S. Purification of a calmodulin-binding protein from chicken gizzard that interacts with F-actin. Proc Natl Acad Sci USA 1981;78:5652-5.
- Helfman DM, Levy ET, Berthier C, Shtutman M, Riveline D, Grosheva I, et al. Caldesmon inhibits nonmuscle cell contractility and interferes with the formation of focal adhesions. Mol Biol Cell 1999;10:3097-112.
- Zhang EY, Stein R, Chang S, Zheng Y, Zderic SA, Wein AJ, et al. Smooth muscle hypertrophy following partial bladder outlet obstruction is associated with overexpression of non-muscle caldesmon. Am J Pathol 2004;164:601-12.
- Stein R, Hutcheson JC, Krasnopolsky L, Canning DA, Carr MC, Zderic SA. The decompensated detrusor V: molecular correlates of bladder function after reversal of experimental outlet obstruction. J Urol 2001;166:651-7.
- Chacko S, Chang S, Hypolite J, Disanto M, Wein A. Alteration of contractile and regulatory proteins following partial bladder outlet obstruction. Scand J Urol Nephrol Suppl 2004;215:26-36.
- Stanton MC, Austin JC, Delaney DP, Gosfield A, Marx JO, Zderic SA, et al. Partial bladder outlet obstruction selectively abolishes protein kinase C induced contraction of rabbit detrusor smooth muscle. J Urol 2006;176:2716-21.
- Mannikarottu A, Lin AD, Whitebeck C, Leggett R, Kogan B, Levin R. Effect of partial bladder outlet obstruction on nitrotyrosine levels and their correlation with contractile function. Neurourol Urodyn 2006;25:397-401.
- Gosling JA, Kung LS, Dixon JS, Horan P, Whitbeck C, Levin RM. Correlation between the structure and function of the rabbit urinary bladder following partial outlet obstruction. J Urol 2000;163:1349-56.
- Kato K, Monson FC, Longhurst PA. The functional effects of long-term outlet obstruction on the rabbit urinary bladder. J Urol 1990;143:600-7.
- Schroder A, Chichester P, Kogan BA, Longhurst PA, Lieb J, Das AK, et al. Effect of chronic bladder outlet obstruction on blood flow of the rabbit bladder. J Urol 2001;165:640-6.
- Chacko S, Longhurst PA. Regulation of actomyosin and contraction in smooth muscle. World J Urol 1994;12:292-9.
- Haeberle JR. Thin-filament linked regulation of smooth muscle myosin. J Muscle Res Cell Motil 1999;20:363-70.
- Gusev NB, Vorotnikov AV, Biriukov KG, Shirinskii VP. Caldesmon and calponin are proteins, participating in the regulation of myosin and actin interaction in nonmuscle and smooth muscle cells. Biokhimiia 1991;56:1347-68.
- Levin RM, Chichester P, Hass MA. Obstructive bladder dysfunction: morphological, biochemical, and molecular changes. Eur Urol Suppl 2002;1:14-20.
