Chronic activation of neutral ceramidase protects β-cells against cytokine-induced apoptosis1
Introduction
Over the past decade, sphingolipid sphingomyelin and its metabolites, namely, ceramide (Cer), sphingosine (SPH), and sphingosine-1-phosphate (S1P), have emerged as a new class of signal-transduction molecules mediating a variety of cellular processes[1,2]. Cer is known to be associated with growth arrest and apoptosis[3], whereas S1P is thought to promote growth and survival[3,4]. It has also been proposed that the ratio of Cer to S1P within a cell determines the outcome of an apoptotic stimulus[3–5].
Ceramidase (CDase) deacylates Cer to form SPH, which can be further phosphorylated to S1P by the sphingosine kinase (SPHK). Thus far, 3 subtypes of CDase, namely, acid, neutral, and alkaline CDase, have been cloned and classified in mammalian cells according to the pH optima required for their activity. The current understanding is that the formation of SPH occurs via hydrolysis of Cer by CDase and not via de novo synthesis; therefore, CDase is vital not only for Cer degradation, but also for SPH/S1P generation[6]. Several lines of evidence support the role of CDase in sphingolipid-mediated cell regulation in response to a variety of stimuli, including cytokines[7–9], oxidized low density lipoprotein (oxLDL)[10], growth factors[11], and advanced glycation end products[12]. For example, the activation of neutral CDase (N-CDase) results in a decrease in the intracellular levels of Cer, thereby protecting rat mesangial cells from cytokine-induced apoptosis[8,9]. The overexpression of acid CDase provides protection against cytokine-induced apoptosis in murine fibrosarcoma L929 cells by the reduction of intracellular Cer, thereby directing the Cer/S1P rheostat towards cell survival[13]. Taken together, these results indicate that CDase may function as a key “switch” in determining the intracellular levels of Cer and S1P, its downstream active metabolite, thus, playing a pivotal role in the regulation of cell survival or apoptosis in response to external stimuli[6].
Cytokines, such as interleukin (IL)-1β, TNF-α, and interferon (IFN)-γ, are toxic to pancreatic β-cells and have been implicated in the pathogenesis of diabetes[14]. Recent data show that IL-1β and TNF-α induce early and sustained increases in SPHK activity and S1P levels in rat β-cell line INS-1 and pancreatic islets. Moreover, exogenous S1P provides protective effects against cytokine-induced apoptosis in β-cells[15,16]. Although CDase is known to act as a key “switch” in the sphingolipid signaling pathway and potentially protects the cells against cytokine-induced damage, the role of the CDase pathway in β-cells has not been elucidated. In this study, we investigated for the first time the activity and expression of N-CDase in β-cells and its role in the cellular response to cytokines.
Materials and methods
Reagents Recombinant rat IL-1β, TNF-α, and IFN-γ, protease inhibitor cocktail, o-phthaldehyde, and the mouse monoclonal anti-β-actin antibody were obtained from Sigma (St Louis, MO, USA). The Annexin V–fluorescein-isothiocyanate (FITC) kit was from Bender MedSystems (Vienna, Austria). TriPure isolation reagent was from Roche (Indianapolis, IN, USA) and the reverse transcription kit was from Toyobo (Osaka, Japan). C17-D-erythro-SPH and C12-Cer were purchased from Avanti Polar Lipids (Alabaster, AL, USA). C12-D-erythro-SPH was from Larodan (Malmö, Sweden). Secondary horseradish peroxidase-conjugated antibodies (swine antirabbit immunoglobulin [IgG] and rabbit antimouse IgG) were purchased from DakoCytomation (Glostrup, Denmark). Triton X-100 and the bicinchoninic acid (BCA) protein assay kit were from Pierce (Rockford, IL, USA).
Cell culture The rat insulin-secreting INS-1 cells (passages 16–33, a kind gift from Prof Xiao HAN, Key Laboratory of Human Functional Genomics of Jiangsu Province, Nanjing Medical University, Nanjing, China) were grown in RPMI-1640 medium supplemented with 10 mmol/L HEPES, 10% fetal calf serum (FCS), 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, 50 µmol/L 2-mercaptoethanol, 100 U/mL penicillin, and 100 mg/L streptomycin.
Polyclonal antibody preparation An affinity-purified rabbit antirat N-CDase polyclonal antibody was raised against the peptide (KNRGYLPGQGPFVANFA) corresponding to amino acids 311–327 of rat N-CDase (GenBank accession N
RNA isolation and quantitative RT–PCR The INS-1 cells were seeded in 60 mm dishes and cultured for 2 d in complete medium. Then the medium was replaced with RPMI supplemented with 0.5% FCS for 4 h, followed by incubation with or without a cytokine mixture (5 ng/mL IL-1β, 10 ng/mL TNF-α, and 50 ng/mL IFN-γ). During the indicated time periods, total cellular RNA was extracted with TriPure isolation reagent, and 1 µg of RNA was reverse transcribed. Quantitative PCR was then performed using a 7900HT real-time PCR system (Applied Biosystems, Foster, CA, USA) and carried out in a 25 µL reaction mixture consisting of 2 µL cDNA, 12.5 µL 2×Master mix, 0.625 µL of the probe (20 µmol/L), and 1.125 µL of 20 µmol/L forward and reverse primers. The reactions were performed with 40 cycles (30 s at 95 °C and 1 min at 60 °C). The primers used for the real-time PCR were as follows: N-CDase, 5'-CCCAGGGCGGCTTTACA-3' (forward) and 5'-TGGGTGAACATGACGGATGT-3' (reverse), and GAPDH, 5'-CAAGTTCAACGGCACAGTCAA-3' (forward) and 5'-TGGTGAAGACGCCAGTAGACTC-3' (reverse). The GAPDH gene was amplified for each sample and used as an internal control gene to calculate the relative level of expression for the N-CDase gene using the 2-ΔΔCt method[17].
Assessment of apoptosis and necrosis For the apoptosis assessment, the INS-1 cells were cultured in medium without FCS for 4 h before exposure to cytokines for 24 h. After treatment, the cells were collected and washed with cold phosphate-buffered saline and incubated for 10 min with Annexin V, then propidium iodide (PI) in the appropriate buffer was added according to the manufacturer’s instructions. The analysis was performed with a FACScan flow cytometer (BD Biosciences, San Jose, CA, USA) using the CellQuest software (BD Biosciences). This method has been successfully used to assess apoptosis/necrosis in INS-1 cells and rat and human β-cells[18,19].
Western blotting analysis Cellular proteins were prepared from the INS-1 cells, and immunoblots were performed as previously described[12] with some modifications. Briefly, the INS-1 cells were collected and resuspended in buffer A (25 mmol/L HEPES, pH 7.5, 5 mmol/L EDTA, 0.5% Triton X-100, 1.5 mmol/L sodium fluoride, 1 mmol/L sodium vanadate, and 10 µL/mL protease inhibitor mixture) and homogenized by brief sonication. The homogenate was centrifuged for 10 min at 10 000×g at 4 °C, and the supernatant was taken for protein determination. The cellular proteins were then electrophoresed in a 7.5% SDS–PAGE gel and transferred onto Hybond ECL membranes (Amersham Biosciences, Piscataway, NJ, USA). The enhanced chemiluminescence (ECL) membranes were incubated with primary antibodies against N-CDase (1:500) overnight at 4 °C, followed by incubation with horseradish peroxidase-conjugated secondary antibodies (1:2000) for 2 h at room temperature. The signals were detected with the ECL system. To control lane loading, the same membranes were probed with anti-β-actin antibodies (1:5000) after being washed with washing buffer. The signals were quantified by scanning densitometry using National Institutes of Health Image J software (NIH, Bethesda, MD, USA). The results from each experimental group were expressed as relative integrated intensity compared with that of the control INS-1 cells measured with the same batch.
N-CDase activity assay N-CDase activity was determined by the released sphingoid base from Cer using a HPLC assay adopted from Xu et al[20]. The INS-1 cells were collected and resuspended in buffer A and centrifuged for 15 min at 10 000×g at 4 °C. The supernatant was collected, and the protein concentration was determined by the BCA method. Each sample containing 100 µg of protein was then assayed for N-CDase activity by incubation with 100 µmol/L C12-Cer in 0.2 mol/L HEPES buffer (pH 7.5) with 0.2% Triton X-100 and 5 mmol/L CaCl2 for 2 h at 37 °C. SPH formation was linear over this time of incubation and proportional to the amount of sample protein for up to 400 µg/assay. The reactions were stopped by extraction with chloroform and methanol. The o-phthaldehyde derivative of released SPH was determined by HPLC with C12-D-erythro-SPH as an external standard and C17-D-erythro-SPH as an internal standard. HPLC was conducted using the Agilent 1050-HPLC model fitted with a eclipse XDB-C18 column (Agilent Technologies, Palo Alto, CA, USA). The solvent was methanol–potassium phosphate buffer (90:10 v/v) and the flow rate was 0.8 mL/min. A HP1046 fluorescence detector was used with an excitation at 345 nm and emission at 455 nm.
RNA interference The gene silencing of rat N-CDase was performed essentially according to a standard protocol[21]. The efficiency of gene silencing was assessed by enzymatic activity or by measuring the protein level of the target gene using Western blotting. Briefly, the INS-1 cells were plated onto 60 mm dishes. The cells were then transfected with scrambled control siRNA (SCR; Ambion catalog N
Statistical analysis Data are expressed as mean±SD. Significance was tested by Student’s t-test or ANOVA, and P<0.05 was considered to be statistically significant.
Results
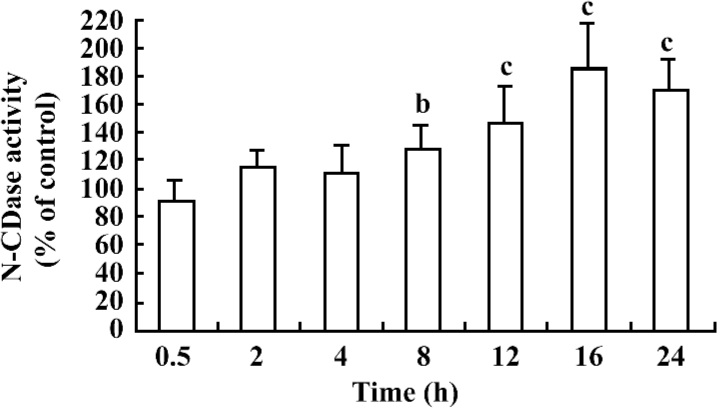
Cytokines induced the chronic activation of N-CDase To investigate whether N-CDase activity was present in the INS-1 cells and affected by cytokines, the INS-1 cells were stimulated for different time periods with a cytokine mixture (5 ng/mL IL-1β, 10 ng/mL TNF-α, and 50 ng/mL IFN-γ) that was reported to induce apoptosis in pancreatic β-cells and INS-1 cells[18,22]. N-CDase activity was measured by quantitating the released SPH. The results showed that N-CDase was active in the INS-1 cells (18.2±2.5 pmol SPH·min–1·mg–1 protein in the control cells). Moreover, the treatment of the INS-1 cells with cytokines resulted in a time-dependent delay in the activation of N-CDase. As a result, the activation of N-CDase was first observed at 8 h after stimulation (129%±16% of that of the control), reached a maximum at 16 h (183%±33% of that of the control), and remained elevated at 24 h (170%±21% of that of the control; Figure 1).
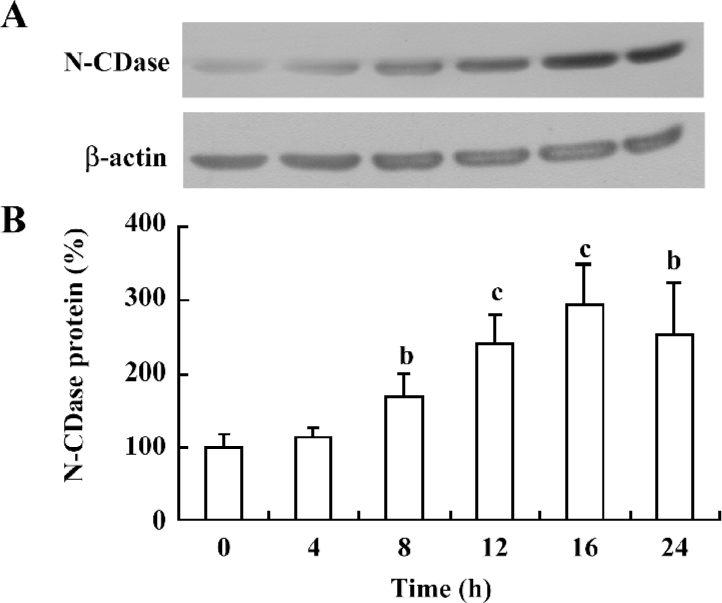
Cytokines upregulated N-CDase protein expression To test whether the effect of cytokines on N-CDase activity could be attributed to an increased N-CDase protein content, the INS-1 cells were treated with cytokines at different time points, and the N-CDase protein levels were quantified. Western blotting showed that the antibody against N-CDase recognized a protein with an apparent molecular mass of 110–120 kDa, as previously described in rat mesangial cells[8,12]. The N-CDase expression increased significantly at 8 h (171%±30% of that of the control) and further increased at 16 h (293%±54% of control), and remained elevated at 24 h (255%±67% of that of the control; Figure 2).
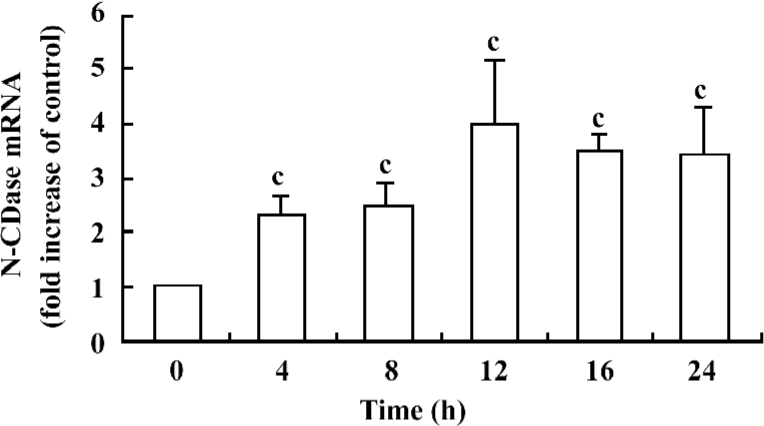
Cytokines upregulated N-CDase mRNA expression To confirm whether there was also an upregulation of the N-CDase mRNA induced by cytokines, total RNA was isolated from the INS-1 cells at the indicated time points and subjected to quantitative RT–PCR. A marked increase in N-CDase mRNA was observed as early as 4 h after treatment. The mRNA levels peaked at 12 h and remained elevated at 24 h (Figure 3).
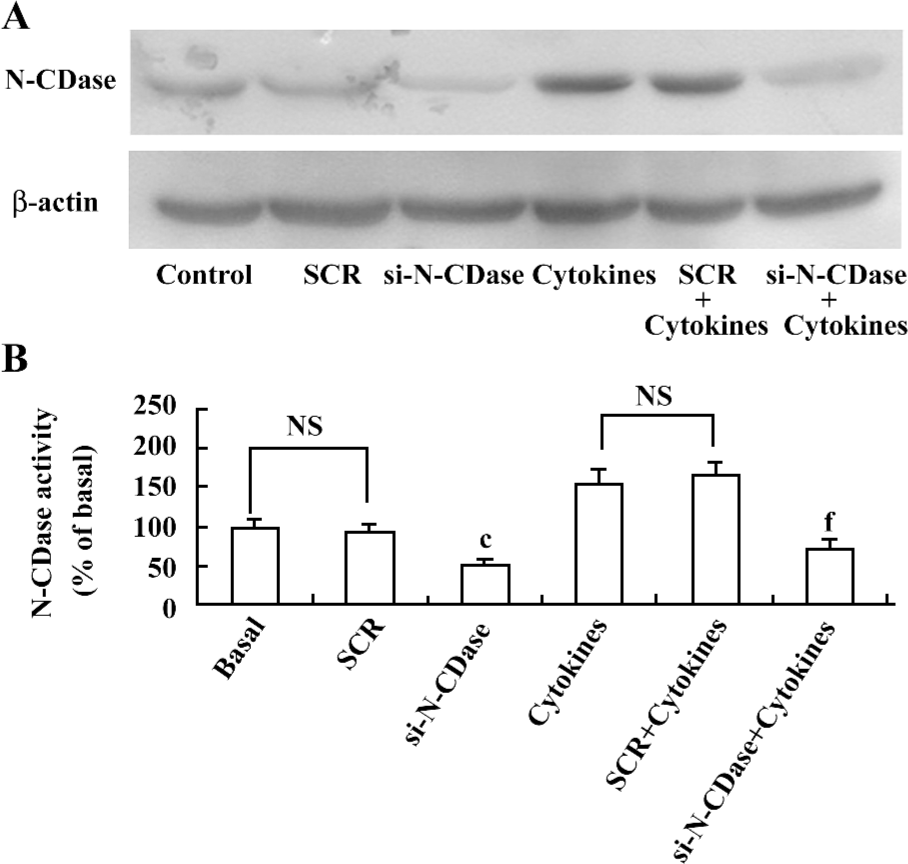
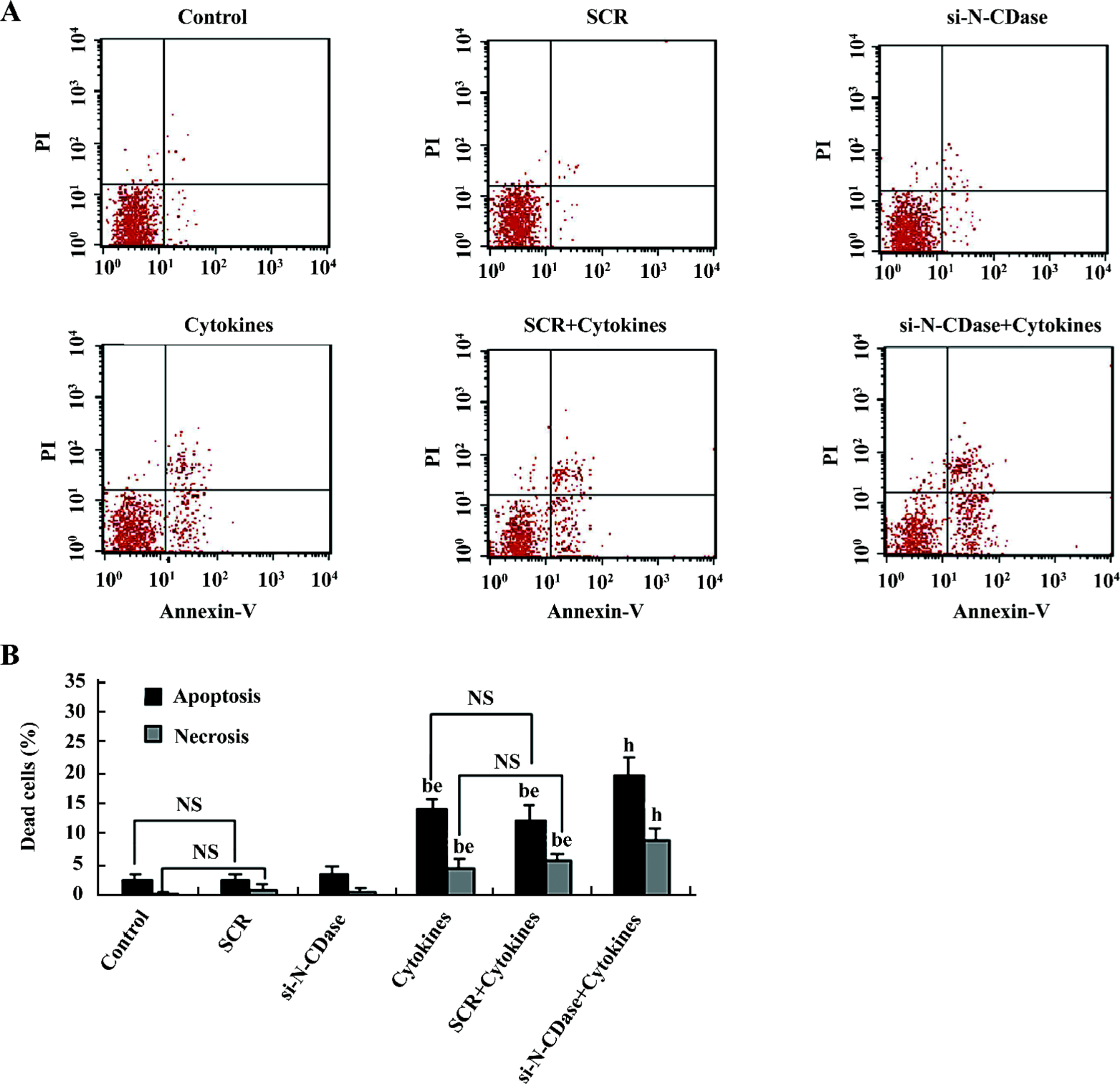
Inhibition of N-CDase activity by siRNA enhanced cytokine-induced apoptosis To determine whether the inhibition of CDase activity potentiated the cytotoxic response induced by cytokines, N-CDase was inhibited by RNA interference in the presence and absence of cytokines for 24 h. The basal and cytokine-induced expression and activity of N-CDase were markedly inhibited in the INS-1 cells transfected with N-CDase siRNA compared to the untransfected cells or the cells transfected with SCR (Figure 4A, 4B). Furthermore, although the treatment of the INS-1 cells with N-CDase siRNA alone did not induce significant apoptosis, it enhanced cytokine-induced apoptosis (20.2%±2.9%) and necrosis (9.5%±1.9%) compared to the untransfected cells (apoptosis: 14.6%±1.7% and necrosis: 5.0%±1.6%) and the negative control transfected cells (apoptosis: 12.7%±2.5% and necrosis: 6.3%±1.3%; (Figure 5A, 5B).
Discussion
CDase is an essential enzyme that catalyzes the formation of SPH from Cer and plays a crucial role in sphingolipid-mediated cell regulation. To our knowledge, this study is the first to characterize N-CDase expression and activity, as well as its role in the cellular response to cytokines in the pancreatic β-cell line INS-1, a known in vitro model currently used for the study of pancreatic β-cell function and apoptosis.
A recent study revealed that in INS-1 cells and islets, IL-1β and TNF-α induced early and sustained increases in SPHK activity and S1P levels, a downstream metabolite in the sphingolipid metabolic pathway[15]. Recently, Laychock et al reported that exogenous S1P provides protective effects against cytokine-induced apoptosis in β-cells[16]. These findings prompted us to further investigate whether CDase, the rate-limiting enzyme in the sphingolipid pathway, also played a role in the pathological response of β-cells to cytokines. CDase, although not described in β-cells, is believed to function as a key “switch”, determining the intracellular levels of Cer and S1P and providing protection against cytokine toxicity[6]. In contrast to early increased SPHK activity and S1P levels as previously reported[15], our results showed that cytokines did not induce rapid activation of N-CDase. The prolonged treatment of the INS-1 cells with cytokines resulted in the delayed activation of N-CDase, and this activity increased from 8 to 24 h, reaching a peak at 16 h.
It has been shown that N-CDase is stimulated in response to cytokines in various cell types[7–9]. Here, we confirmed the chronic activation of N-CDase induced by cytokines in INS-1 cells, which might result from the increased N-CDase mRNA and protein expression. Additionally, a quantitative discrepancy between the increase in the activity and the elevation in the protein level of N-CDase was observed in this study. We speculated that it might be related to the regulation mechanism of N-CDase. In addition to being regulated by gene transcription, the N-CDase expression and activity were probably also modulated by phosphorylation per se, either by protein kinase C or tyrosine kinase[6,7,23]. Thus, although not described in β-cells, the possibility that N-CDase was also regulated by phosphorylation/dephosphorylation cannot be ruled out in this study.
Previous data show that cytokine-induced increases in SPHK activity and S1P levels in INS-1 cells appear to occur in the following 2 phases: an early phase independent of new protein synthesis, and a late phase (after treatment for 8 h) dependent on new protein synthesis, as well as the induction of new enzymes[15]. Current understanding indicates that CDase is vital not only for Cer degradation, but also for SPH/S1P generation. Further, the concomitant activation of CDase and SPHK has been observed in response to several stimuli, including cytokines, oxLDL, growth factors, and advanced glycation end products in a variety of cell types[7–12]. Thus, our findings regarding the cytokine-induced chronic activation of N-CDase in INS-1 cells, which was associated with an increased amount of N-CDase protein, raised the possibility that N-CDase activation may be involved in the increase in SPHK activity and S1P levels induced by cytokines in the late phase. Further elucidation of the quantitative relationship between the N-CDase activation and S1P generation in response to cytokines is clearly warranted.
Furthermore, we observed that when N-CDase activity was inhibited by N-CDase siRNA transfection, cytokine-induced apoptosis in INS-1 cells markedly increased. These results suggest that N-CDase potentially provides protection against cytokine toxicity. Our findings are also consistent with previous reports stating that TNF-α/IL-1β induces the activation of N-CDase in rat mesangial cells, and when its activity is inhibited, cytokine-induced cell apoptosis can occur[8,9]. Cer has been implicated in cell death following a myriad of cellular stresses as it mediates several apoptosis signaling pathways, such as the activation of caspase-3, inhibition of phosphatidylinositol 3-kinase/the protein kinase B (PKB) pathway either by the activation of a phosphatase and subsequent dephosphorylation of PKB, and an increase in mitochondrial permeability[3,5,24]. However, CDase activation results in a decrease in the intracellular Cer levels and a concomitant increase in S1P, which promotes growth and survival and opposes the pro-apoptotic effects of Cer, leading to apoptosis suppression. Thus, the mechanism by which CDase exerts its protective effects appears to involve the regulation of the cellular Cer/S1P ratio[6,24]. Osawa et al further demonstrated that the overexpression of N-CDase prevents hepatocyte apoptosis induced by TNF-α through reducing C16-Cer and promoting the activation of AKT through S1P formation[25]. Therefore, the chronic activation of N-CDase may reduce intracellular Cer and the ratio of Cer/S1P, mediating the protective effects against cytokine-induced apoptosis in INS-1 cells.
In summary, N-CDase is active in INS-1 cells, and its activity is chronically increased by cytokine treatment. Furthermore, the finding that the inhibition of N-CDase activity enhances cytokine-induced apoptosis suggests that it potentially protects β-cells from cytokine toxicity. However, the regulatory mechanisms underlying N-CDase activity and cellular Cer/S1P levels in response to cytokines remain to be determined.
Acknowledgment
We thank Yi-te HSU (Medical University of South Carolina, USA) for carefully reading the manuscript.
References
- Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science 1996;274:1855-9.
- Kolesnick R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J Clin Invest 2002;110:3-8.
- Taha TA, Mullen TD, Obeid LM. A house divided: ceramide, sphingosine, and sphingosine 1-phosphate in programmed cell death. Biochim Biophys Acta 2006; 1758: 2027–36.
- Spiegel S, Milstien S. Sphingosine-1-phosphate, a key cell signaling molecule. J Biol Chem 2002; 277: 25 851–4.
- Huwiler A, Zangemeister-Wittke U. Targeting the conversion of ceramide to sphingosine 1-phosphate as a novel strategy for cancer therapy. Crit Rev Oncol Hematol 2007;63:150-9.
- El Bawab S, Mao C, Obeid LM, Hannun YA. Ceramidases in the regulation of ceramide levels and function. Subcell Biochem 2002;36:187-205.
- Nikolova-Karakashian M, Morgan ET, Alexander C, Liotta DC, Merrill AH. Bimodal regulation of ceramidase by interleukin-1β, implications for the regulation of cytochrome p450 2C11. J Biol Chem 1997; 272: 18 718–24.
- Franzen R, Pautz A, Bräutigam L, Geisslinger G, Pfeilschifter J, Huwiler A. Interleukin-1β induces chronic activation and de novo synthesis of neutral ceramidase in renal mesangial cells. J Biol Chem 2001; 276: 35 382–9.
- Huwiler A, Pfeilschifter J, Van Den Bosch H. Nitric oxide donors induce stress signaling via ceramide formation in rat renal mesangial cells. J Biol Chem 1999;274:7190-5.
- Auge N, Nikolova-Karakashian M, Carpentier S, Parthasarathy S, Negre-Salvayre A, Salvayre R, . Role of sphingosine 1-phosphate in the mitogenesis induced by oxidized low density lipoprotein in smooth muscle cells via activation of sphingomyelinase, ceramidase, and sphingosine kinase. J Biol Chem 1999; 274: 21 533–8.
- Coroneos E, Martinez M, McKenna S, Kester M. Differential regulation of sphingomyelinase and ceramidase activities by growth factors and cytokines, implications for cellular proliferation and differentiation. J Biol Chem 1995; 270: 23 305–9.
- Geoffroy K, Wiernsperger N, Lagarde M, El Bawab S. Bimodal effect of advanced glycation end products on mesangial cell proliferation is mediated by neutral ceramidase regulation and endogenous sphingolipids. J Biol Chem 2004; 279: 34 343–52.
- Strelow A, Bernardo K, Adam-Klages S, Linke T, Sandhoff K, Krönke M, et al. Overexpression of acid ceramidase protects from tumor necrosis factor-induced cell death. J Exp Med 2000;192:601-12.
- Eizirik DL, Mandrup-Poulsen TA. Choice of death-the signal-transduction of immune-mediated β-cell apoptosis. Diabetologia 2001;44:2115-33.
- Mastrandrea LD, Sessanna SM, Laychock SG. Sphingosine kinase activity and sphingosine-1 phosphate production in rat pancreatic islets and INS-1 cells: response to cytokines. Diabetes 2005;54:1429-36.
- Laychock SG, Sessanna SM, Lin MH, Mastrandrea LD. Sphingosine 1-phosphate affects cytokine-induced apoptosis in rat pancreatic islet beta-cells. Endocrinology 2006;147:4705-12.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 2001;25:402-8.
- Li L, El Kholy W, Rhodes CJ, Brubaker PL. Glucagon-like peptide-1 protects β-cells from cytokine-induced apoptosis and necrosis: role of protein kinase B. Diabetologia 2005;48:1339-49.
- Delaney CA, Pavlovic D, Hoorens A, Pipeleers DG, Eizirik DL. Cytokines induce deoxyribonucleic acid strand breaks and apoptosis in human pancreatic islet cells. Endocrinology 1997;138:2610-4.
- Xu R, Jin J, Hu W, Sun W, Bielawski J, Szulc Z, et al. Golgi alkaline ceramidase regulates cell proliferation and survival by controlling levels of sphingosine and S1P. FASEB J 2006;20:1813-25.
- Zeidan YH, Pettus BJ, Elojeimy S, Taha T, Obeid LM, Kawamori T, . Acid ceramidase but not acid sphingomyelinase is required for tumor necrosis factor-á-induced PGE2 production. J Biol Chem 2006; 281: 24 695–703.
- Chen M, Yang Z, Wu R, Nadler JL. Lisofylline, a novel antiinflammatory agent, protects pancreatic β-cells from proinflammatory cytokine damage by promoting mitochondrial metabolism. Endocrinology 2002;143:2341-8.
- Franzen R, Fabbro D, Aschrafi A, Pfeilschifter J, Huwiler A. Nitric oxide induces degradation of the neutral ceramidase in rat renal mesangial cells and is counterregulated by protein kinase C. J Biol Chem 2002; 277: 46 184–90.
- Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta 2002;1585:114-5.
- Osawa Y, Uchinami H, Bielawski J, Schwabe RF, Hannun YA, Brenner DA. Roles for C-ceramide and sphingosine 1-phosphate in regulating hepatocyte apoptosis in response to tumor necrosis factor-alpha. J Biol Chem 2005; 280: 27 879–87.
