Functional subtypes of renal α1-adrenoceptor in diabetic and non-diabetic 2K1C Goldblatt renovascular hypertension
Introduction
Multiple subtypes of α1-adrenoceptors have been identified in vascular tissues and are named α1A-, α1B-, and α1D-adrenoceptors[1-7]. An RNAse protection assay showed that the main renal arteries and branches of renal arteries have mRNA encoding α1A-adrenoceptors in much greater proportions than those encoding α1B- and α1D-adrenoceptor subtypes. A functional study demonstrated that α1A-adrenoceptors primarily mediate the responses to noradrenaline (NA) in these arteries[8]. However, several earlier studies reported that adrenergic vasoconstrictions in the kidneys of normotensive, stroke-prone spontaneously hypertensive rats, 2-kidney one clip (2K1C), and deoxycorticosterone acetate (DOCA)-salt hypertensive rats are mediated by either the α1A-adrenoceptor subtype[9-11] or by both α1A- and α1D-adrenoceptor subtypes[11-15]. Nevertheless, a large body of experimental evidence has implicated α1A-adrenoceptors as the major subtype modulating renal hemodynamics[15].
The kidney is damaged in diabetes as a result of somatic and autonomic neuropathies as well as vascular complications[16]. Hypertension and diabetes often occur together, and the 2 pathological states act synergistically with respect to cardiovascular damage associated with these disorders. As hypertension and diabetes frequently occur together, considerable interest in research has developed on diabetes and hypertension in relation to the pathophysiology and sequels of their combined occurrence. Several studies have reported altered vascular tone in diabetes in terms of enhanced reactivity of resistance vessels to adrenergic agonists and alteration in α1-adrenoceptor functionality. In our previous study, we provided evidence of the involvement of both post-synaptic α1A- and α1D-adrenoceptors along with presynaptic α1B-adrenoceptors in the kidneys of spontaneously hypertensive rats with diabetes[17] in mediating adrenergically-induced renal vasoconstrictions. We recently reported that in another pathological condition, nephrotoxic renal failure, both post-synaptic (α1A- and α1D-adrenoceptors) and presynaptic (α1B-adrenoceptors) were involved in mediating adrenergically-induced renal vasoconstrictions in rats, while in non-renal failure rats, only the functional contribution of post-synaptic α1-adrenoceptors was observed[5]. A similar disease-dependent change in the functional renal α1-adrenoceptors has also been reported in rats with experimental heart failure[18,19]. Collectively, these observations indicate a pathological state-dependent heterogeneity in the subtypes of functional α1-adrenoceptor subtypes in the kidneys of rats. and the presence of disease states have perhaps played a role in determining the functional subtypes of the α1-adrenoceptors involved.
With this background, this study set out to evaluate the functional α1-adrenoceptor subtypes that mediate renal vasoconstrictor response in diabetic 2K1C Goldblatt hypertensive rats. The present study is one of a few studies that focus on the elucidation of functional subtypes of the α1-adrenoceptor subtypes in a combined state of diabetes and hypertension, and is the first report on the functional subtypes of α1-adrenoceptors in a combined state of diabetes and experimental renovascular hypertension.
Material and methods
Preparation of 2K1C Goldblatt hypertensive rats The 2K1C Goldblatt hypertensive rats were prepared according to a method described earlier[10]. Male Sprague–Dawley rats, approximately 4 weeks old (120 g) were anaesthetized with pentobarbitone sodium 60 mg/kg intraperitoneally (ip; Sagatal, Rhone Merieux, Harlow, UK). The dorsal of the animal was shaved and a retroperitoneal incision was made. The right kidney was exposed and a piece of wet tissue paper was placed on the kidney to protect it from damage when it was retracted to the side using a self-retaining retractor. The renal artery was dissected, cleared from connective tissues, and a U-shaped silver clip with a 0.25 mm gap was slipped around it close to the junction with the aorta. The free edges of the clip were compressed firmly with Spencer Wells forceps to prevent it from dislodging. Before suturing the muscle and skin layers with a number 3 silk thread, benzylpenicillin G powder was sprinkled around the wounded area and also on the skin. The animals were allowed to recover in separate cages for several hours under an anglepoise lamp. The animals were then placed into single cages for 36–48 h to allow healing and then randomly grouped into groups of 6. The animals had commercial rat chow with water ad libitum, and were kept for 5-6 weeks before used in the acute studies. Only the animals that exhibited a direct mean arterial blood pressure of 150 mmHg or greater on the day of the acute experiments were included in the study.
Induction of diabetes Male 2K1C Goldblatt hypertensive Sprague–Dawley rats (260–310 g) were treated with streptozotocin (55 mg/kg, ip) and caged in groups of 6 for 3 d. During the first 48 h of post-streptozotocin, the rats were given 5% glucose in drinking water and then plain water ad libitum. On d 3, the rats were individually placed in the custom-built, stainless steel metabolic cages. The 24 h water intake and urine output were measured on d 4 and continued until d 7. The body weight of the animals was measured on every alternate day until d 7 on which the acute study was conducted. Fasting blood glucose was measured by a commercially-available Peridochrom glucose kit (Boehringer Manheim, Germany) on the day of the acute experiment. Only the rats with blood glucose levels of ≥200 mg/dL were used in this study.
Hemodynamic study
General preparation of the animal Eight groups of animal were used in this study. Groups 1–4 were diabetic 2K1C Goldblatt hypertensive rats, while groups 5–8 were non-diabetic 2K1C Goldblatt hypertensive rats. The surgical preparation of the animals for the hemodynamic study was carried out as reported earlier[5,6,10]. In brief, the rats were anaesthetized (sodium pentobarbitone 60 mg/kg, ip) and supplemented with the same anesthetic (12.5 mg·kg–1·h–1) intra-arterially throughout the experiment, given as a continuous infusion of saline at 6 mL/h. After tracheotomy, the jugular vein was cannulated for saline infusion, bolus doses of anesthetic were administered as required, and the carotid artery was cannulated for blood pressure measurement using a pressure transducer attached to a computerized data acquisition system (Powerlab, ADInstrument, Sydney, NSW, Australia). The kidney was exposed via a midline abdominal incision. The renal artery was cleared and an electromagnetic flow probe (EP 100 series, Carolina Medical Instrument, King, NC, USA) connected to flowmeter (Carolina Medical Instrument, USA) was placed on the renal artery to allow direct recording of the renal blood flow with the data acquisition system. The renal nerves were isolated and carefully dissected for a short length and placed on fine bipolar electrodes, which were connected to an electrical stimulator (Grass S 48 stimulator, Grass Medical Instrument, Quincy, MA, USA). The iliac artery was cleared and a cannula was inserted so that its tip lay in the aorta just rostral to the left renal artery, which allowed for the close intrarenal arterial administration of saline and drugs.
Experimental protocols The whole experiment was carried out in 3 distinct phases starting with the saline-treatment phase. In this phase, saline was infused continuously and intrarenally, and the renal nerves were stimulated electrically at increasing frequencies of 1, 2, 4, 6, 8, and 10 Hz and then in reverse order. In the following experiments, graded bolus doses of NA (25, 50, 100, and 200 ng), phenylephrine (PE; 0.25, 0.5, 1, and 2 μg), and methoxamine (ME; 1, 2, 3, and 4 μg) were administered in ascending and then descending doses. Afterwards, the first dose of antagonist was given, and 15 min later, the sequence of renal nerve stimulation (RNS) and the bolus administration of adrenergic agonists were repeated. For the last phase, the close intrarenal administration of twice the initial dose of the antagonist was given, and 15 min later, the nerves were stimulated and the agonist administration was repeated as carried out in the previous 2 phases[5,9,10].
The 8 groups of rats, both non-diabetic and diabetic 2K1C Goldblatt hypertensive Sprague-Dawley rats, were used and were grouped according to the types of α1-adrenoceptor subtype-specific antagonists and calcium channel blockers used in the acute study. These drugs were given in bolus followed by maintenance doses in the continuous infusion of saline during the experiment. These drugs along with their doses used are showed in Table 1.
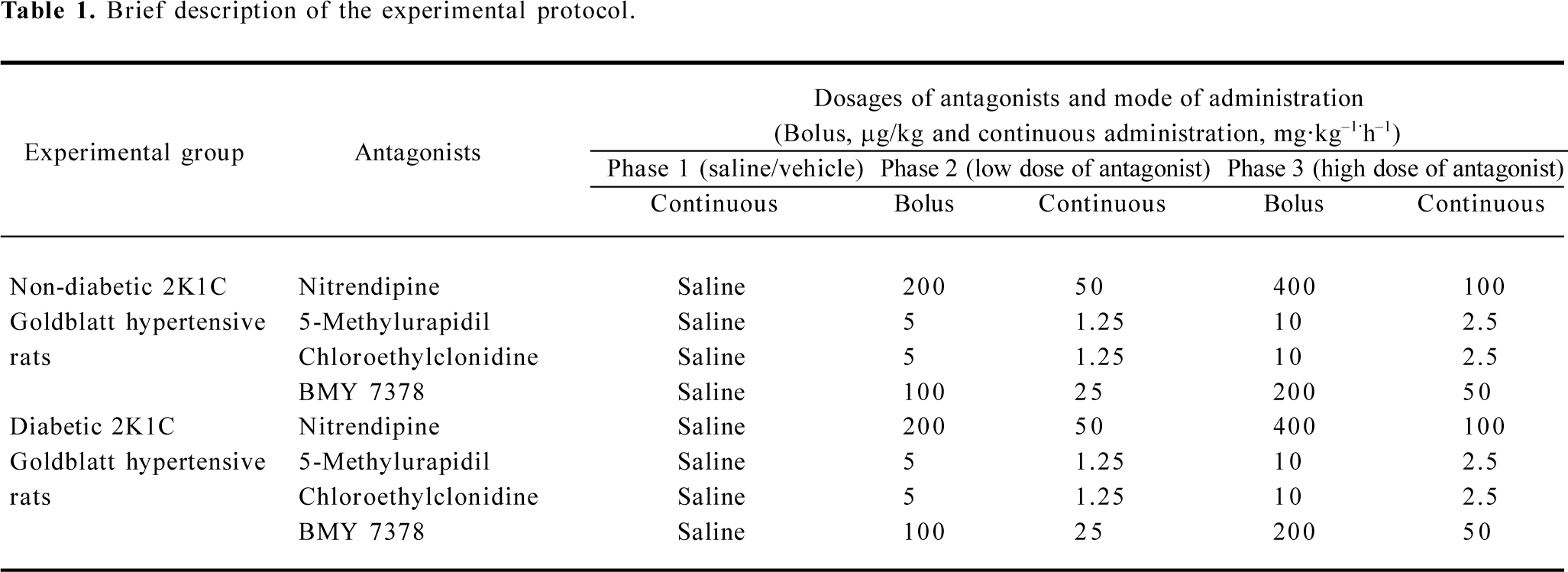
Full table
Drugs Streptozotocin (Sigma, St Louis, MO, USA) was dissolved in normal saline (0.9% NaCl) and used immediately. All antagonists and agonists used in this study were prepared (if required) as stock solutions. 5-Methylurapidil (Research Biochemicals International, Natick, MA, USA), a selective antagonist of the α1A-adrenoceptor subtype, was prepared in 0.04 mol/L lactic acid at a concentration of 100 μg/mL; chloroethylclonidine (Research Biochemicals International, USA), a selective antagonist of the α1B-adrenoceptor subtype, was prepared in saline at a concentration of 100 mg/mL. Nitrendipine (Research Biochemicals International, USA), an L-type Ca+ channel antagonist, was prepared in a mixture of PEG-400, glycerol, and distilled water (969:60:100) at a concentration of 1 mg/mL. Finally, BMY 7378 (Research Biochemicals International, USA), a selective antagonist of the α1D-adrenoceptor subtype, was prepared at a concentration of 1 mg/mL in normal saline.
All drugs were kept as frozen aliquots and used within 3 d of their preparation. These stock solutions were diluted in saline just before being used. The agonists used were NA (mixed agonist; Levophed, Sanofi Winthrop, London, England), PE (selective to α1-adrenoceptors; BASF Pharmaceuticals, Knoll, England, and ME (selective to α1A-adrenoceptors; (Vasoxine, Calmic Medical Division,Bristol,England). These drugs were freshly diluted from the frozen stocks on the day of the experiment.
Data presentation and statistical analysis The renal vasoconstrictor responses to all the adrenergic stimuli were taken as an average of 2 values of the decrease of the renal blood flow for each frequency (RNS) or dose (NA, PE, and ME) administered in ascending and descending orders. The basal values of the renal blood flow recorded in each phase was taken as 100%, and the percentage changes in the reductions of renal blood flow to each frequency or dose of adrenergic stimuli in each phase (vehicle and low and high dose of antagonist-treated phases) were calculated[5,9,10,17]. Data are presented as mean±SEM and were analyzed by two-way ANOVA followed by Duncan’s post-hoc and multi-range t-tests (Superanova, Abacus, Berkeley, CA, USA). The significance was taken at the 5% level[17].
Results
General observations There was no change (P>0.05) in the baseline values of renal blood flow among the diabetic (25.3±2.1 mL·min–1·kg–1) and non-diabetic 2K1C Goldblatt hypertensive rats (27.6±1.9 mL·min–1·kg–1). However, there was a change in the mean arterial blood pressure among these experimental groups. The mean arterial blood pressure was lower (P<0.05) in the diabetic 2K1C rats compared to that of the non-diabetic and diabetic 2K1C Goldblatt hypertensive rats (130.0±5 mmHg vs 143.0±5 mmHg, diabetic vs non-diabetic 2K1C Goldblatt hypertensive rats) in our observations. There was a significant difference in the glycemic status of the diabetic and non-diabetic 2K1C Goldblatt hypertensive rats (P<0.05). The diabetic 2K1C Goldblatt hypertensive rats were hyperglycemic, while the non-diabetic 2K1C Goldblatt hypertensive rats had a normal glycemic status (diabetic 2K1C Goldblatt hypertensive rats 298.0±11.6 mg/dL vs non-diabetic 2K1C Goldblatt hypertensive rats 138.2± 8.5 mg/dL).
Renal vasoconstrictor responses
RNS RNS produced frequency-dependent renal vasoconstrictions in both the non-diabetic and diabetic 2K1C Goldblatt hypertensive rats (all P<0.05). Nitrendipine and 5-methylurapidil significantly attenuated the RNS-induced renal vasoconstrictions in the non-diabetic and diabetic 2K1C rats (P<0.05). BMY 7378 also attenuated these responses, but only in the non-diabetic 2K1C rats (P<0.05), while in the diabetic 2K1C rats, it accentuated these responses (P<0.05). Chloroethylclonidine accentuated these responses in both the diabetic and non-diabetic 2K1C Goldblatt hypertensive rats (all P<0.05; Figure 1; Table 2).
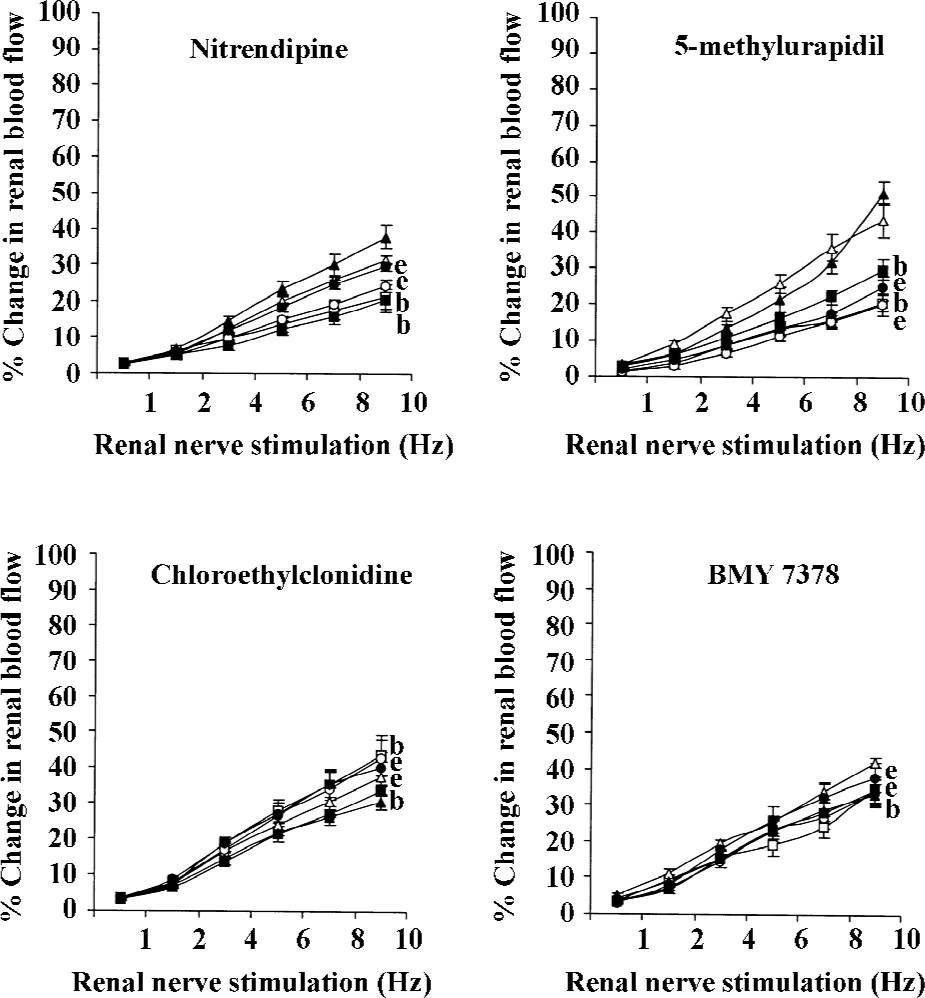
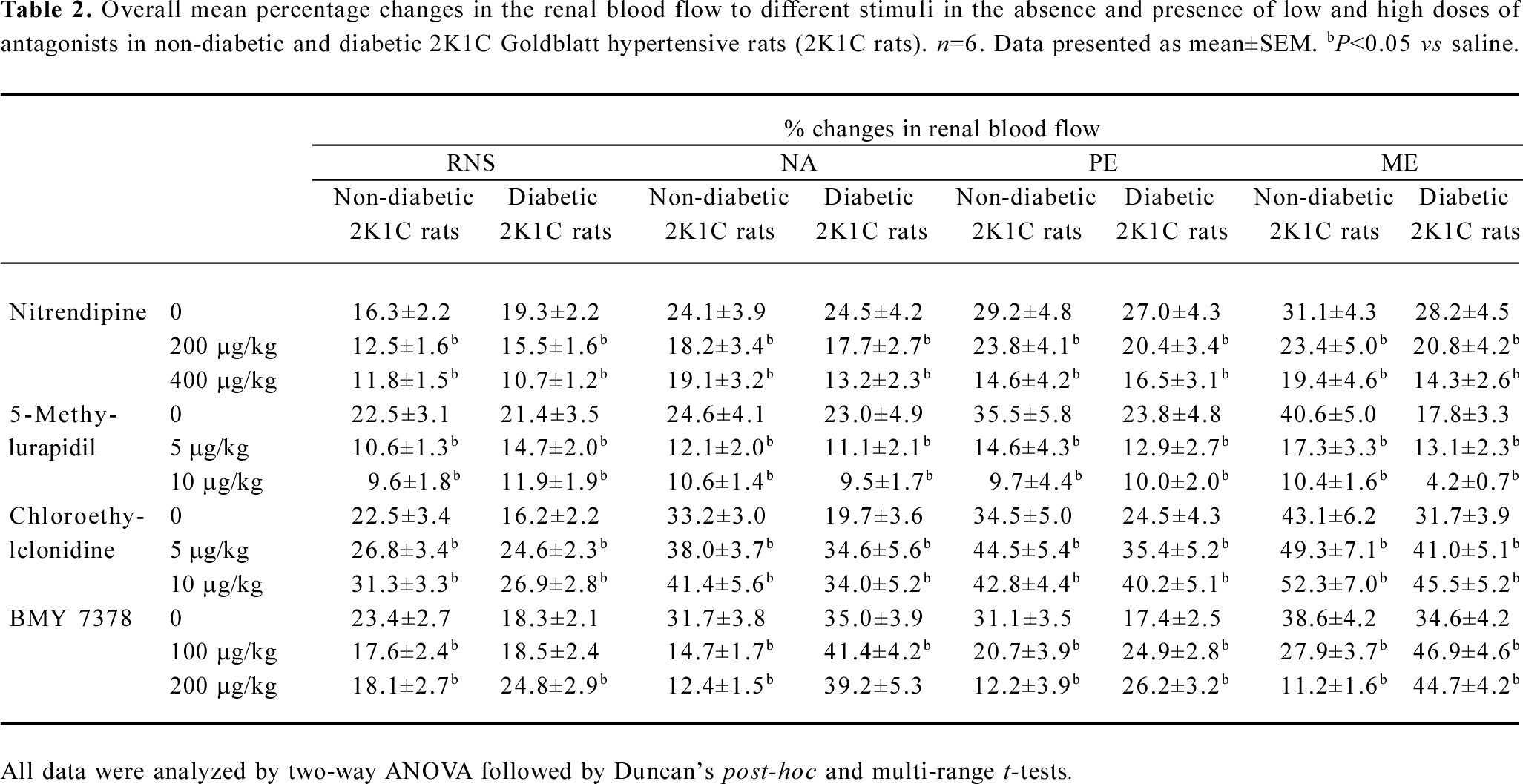
Full table
NA NA-induced renal vasoconstrictor responses were dose dependent in both experimental groups (P<0.05). In the diabetic and non-diabetic 2K1C Goldblatt hypertensive rats, nitrendipine and 5-methylurapidil significantly attenuated the NA-induced renal vasoconstrictor responses (all P<0.05). Interestingly, chloroethylclonidine significantly accentuated these responses in the diabetic and non-diabetic 2K1C Goldblatt hypertensive rats (P<0.05). BMY 7378 it was also of interest as it caused significant attenuation of the NA-induced renal vasoconstrictor responses in the non-diabetic 2K1C Goldblatt hypertensive rats (P<0.05), while in the diabetic 2K1C Goldblatt hypertensive rats, BMY 7378 accentuated these responses (P<0.05; Table 2; Figure 2).
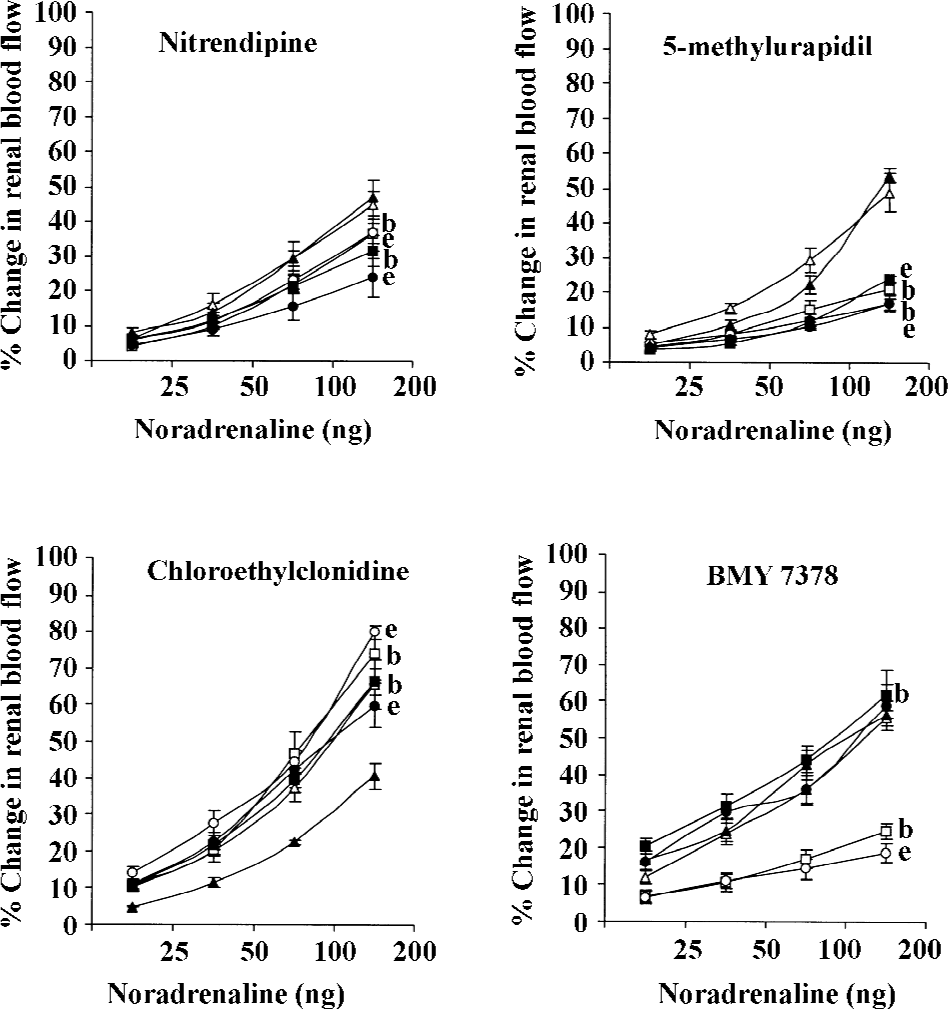
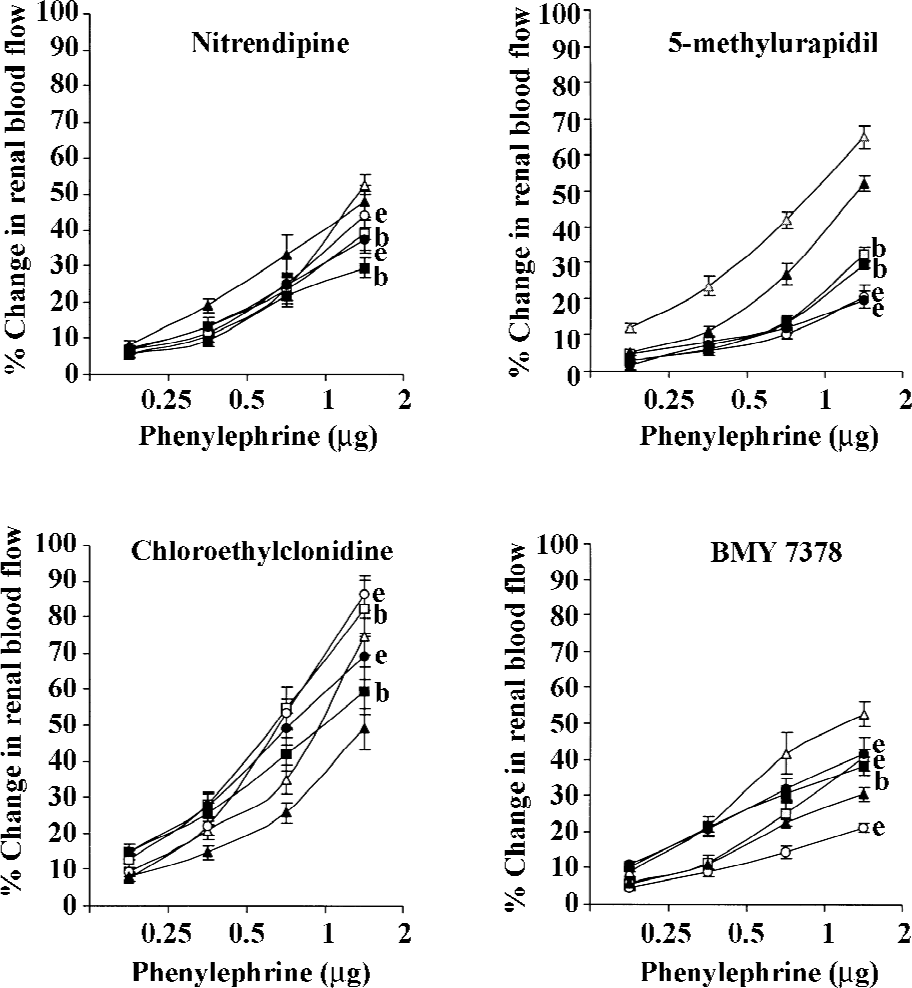
PE PE produced dose-dependent vasoconstrictor responses in both diabetic and non-diabetic 2K1C rats (all P<0.05). Nitrendipine and 5-methylurapidil attenuated the PE-induced renal vasoconstrictor responses in both the non-diabetic and diabetic 2K1C Goldblatt hypertensive rats (P<0.05). BMY 7378 also caused similar attenuating effects of the PE-induced renal vasoconstrictor responses; however, only in the non-diabetic 2K1C Goldblatt hypertensive rats (all P<0.05). In the diabetic 2K1C Goldblatt hypertensive rats, BMY 7378 significantly accentuated the PE-induced renal vasoconstrictor responses (P<0.05). In both the diabetic and non-diabetic 2K1C Goldblatt hypertensive rats, chloroethylclonidine significantly accentuated the PE-induced renal vasoconstrictor responses (all P<0.05; Table 2; Figure 3).
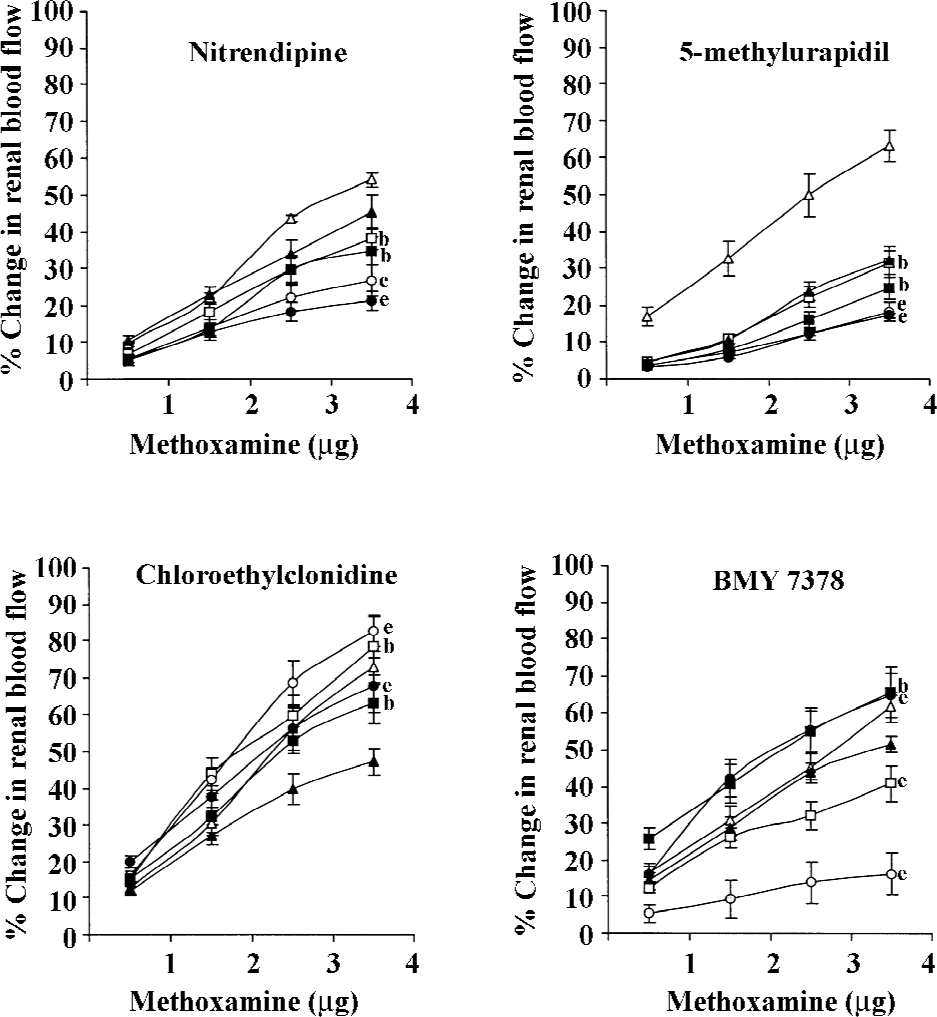
ME Like other adrenergic stimuli, ME also produced dose-dependent changes in the renal blood flow of the non-diabetic and diabetic 2K1C rats (all P<0.05). In both the diabetic and non-diabetic 2K1C Goldblatt hypertensive rats, nitrendipine and 5-methylurapidil significantly attenuated the ME-induced renal vasoconstrictor responses (all P<0.05). A similar significant attenuation of the ME-induced renal vasoconstrictor responses was also caused by BMY 7378, but only in the non-diabetic 2K1C Goldblatt hypertensive rats (P<0.05). Significant accentuation of the ME-induced changes occurred in the diabetic 2K1C Goldblatt hypertensive rats (P<0.05), while in the non-diabetic 2K1C Goldblatt hypertensive rats, it significantly attenuated these responses (all P<0.05). Chloroethylclonidine significantly accentuated the ME-induced renal vasoconstrictor responses in both the diabetic and non-diabetic 2K1C Goldblatt hypertensive rats (all P<0.05; Table 2; Figure 4).
Discussion
Renal vasculature is richly innervated by adrenergic nerves in which a1A-adrenoceptors have consistently been recognized as the predominate subtype over the other two α1-adrenoceptor subtypes in terms of density and functionality[20]. In relation to this observation, adrenergically-induced renal vasoconstriction in normotensive rats, stroke-prone spontaneously hypertensive rats, DOCA-salt, and 2K1C Goldblatt hypertensive rats are found to be mediated by a1A-adrenoceptors[9,10,21–25]. Further studies have, however, described that the renal vasoconstrictor responses are mediated by both α1A-[11] and/or α1D-adrenoceptors[11,12,26,27]. There are reports on the functional co-existence of α1A- and α1D-adrenoceptor subtypes in the renal vasculature of rats with normal[15] and pathophysiological states like in diabetes, hypertensive diabetes, heart failure, and renal failure[5,17–19].
As discussed above, although there are number of research that have reported the functional subtypes of α1-adrenoceptor subtypes in the renal vasculature, there is still a large paucity of information on this issue. This is particularly true for the functional involvement of α1-adrenoceptor subtypes in the renal vasculature of rats with pathological states[5,17-19]. It has been reported that some pathophysiological states may change the composition of the α1-adrenoceptors present in different vasculatures[18]. With this background, a hypothesis is formulated that in certain pathological conditions there would be a shift in the functional α1-adrenoceptor subtypes mediating adrenergically-induced renal vasoconstriction. In relation to this, we previously reported that there was a shift in the functional contribution of α1-adrenoceptor subtypes in the renal vasculature of rats with certain disease states like diabetes, a combined state of genetic hypertension and diabetes, heart failure, and a combined state of heart failure and hypertension[17-19]. In the present study, we focused on a unique pathological state that is characterized with diabetes and experimental renovascular hypertension (2K1C Goldblatt hypertension).
The results obtained in this study showed that in some cases, the renal vasoconstrictor responses caused by the neural and adrenergic stimuli were significantly lower in the diabetic 2K1C rats compared to that of the non-diabetic 2K1C Goldblatt hypertensive rats. These observations led us to suggest that in the diabetic 2K1C Goldblatt hypertensive rats, the sympathetic nervous system activity was perhaps enhanced. Such enhancement of the sympathetic nervous system in these rats might have caused a reduction in the sensitivity of the adrenoceptors and/or a potential downregulation of these receptors in order to compromise the impact of higher vascular sympathetic tone on the vasculature. Interestingly, in some cases, it has been further observed that the renal vasoconstrictions are markedly higher in diabetic 2K1C Goldblatt hypertensive rats. This could be due to a possible autonomic dysfunction in these rats, as degenerative changes are observed in the streptozotocin-induced diabetic rats from 3 d of the streptozotocin treatment. The autonomic dysfunction in the diabetic 2K1C Goldblatt hypertensive rats was indicated by their markedly lower blood pressure[28–30].
In the present study, we found that in the non-diabetic 2K1C rats, the adrenergically-induced renal vasoconstrictor responses were attenuated by all the antagonists except chloroethylclonidine. These observations strongly suggested the functional contribution of α1A- and α1D-adrenoceptor subtypes in mediating the adrenergically-induced renal vasoconstrictions in these rats. These observations are supported by several earlier studies reporting the functional involvement of multiple subtypes of α1-adrenoceptors (α1A- and α1D-adrenoceptor subtypes) in the renal vasculature of rats with either normal physiological state or with certain pathophysiological states[5,12,15,17]. Unlike the non-diabetic 2K1C Goldblatt hypertensive rats, in the diabetic 2K1C Goldblatt hypertensive rats, the adrenergically-induced renal vascular responses were attenuated by nitrendipine and 5-methylurapidil, hence, indicating a strong functional contribution of α1A-adrenoceptor subtypes. It is also observed that in these rats, these adrenergic responses were either enhanced or remained unaltered by chloroethylclonidine and BMY 7378. These observations further strengthen the view that in the diabetic 2K1C Goldblatt hypertensive rats, the α1A-adrenoceptor subtypes were to a large extent involved in mediating the adrenergically-induced renal vasoconstriction and that the functional involvement of α1D-adrenoceptor subtypes was minimal. These observations are indeed unique considering the pathological state of the rats (diabetic 2K1C Goldblatt hypertensive rats), and support an earlier view that it is α1A-adrenoceptor subtypes that primarily regulate the renal hemodynamics[5,15,17]. The present study also demonstrated some interesting observations in which chloroethylclonidine caused the accentuation of the adrenergically-induced renal vasoconstrictor responses in both diabetic and non-diabetic 2K1C Goldblatt hypertensive rats. Similar observations have been made in several earlier studies in rats with diabetes, hypertensive diabetes, and in cisplatin-induced renal failure[5,17].
Chloroethylclonidine is found to cause the accentuation of RNS (both in diabetic and non-diabetic 2K1C Goldblatt hypertensive rats) and NA (both diabetic and non-diabetic 2K1C Goldblatt hypertensive rats)-induced renal vasoconstrictor responses in the experimental animal groups used in this study. These observations can be explained in light of the findings that in some vasculatures, the NA, which is released due to the stimulation of renal nerve, caused α1A-adrenoceptor subtype-mediated vasoconstriction[31,32]. Moreover, like endogenously-released NA, exogenously-administered NA has also been reported to exert its action through α1A-adrenoceptor subtypes with little or no evidence for the involvement of α1B-, α1D-, and even α2-adrenoceptors[32]. However, as asserted in some earlier findings, in the presence of chloroethylclonidine and enhanced sympathetic activity, any such involvement of α1B- and α1D-adrenoceptors in mediating renal vasoconstriction will be abolished or impeded[33-35]. It has been stated that chloroethylclonidine has selectivity for α1-adrenoceptors in the order of α1B>α1D>>α1A, and the α1A-adrenoceptor is insensitive to chloroethylclonidine. Hence, it can be suggested that α1B- and α1D-adrenoceptor subtypes will be preferentially inactivated by chloroethyl-clonidine[33-36] leaving α1A-adrenoceptors to be acted upon by the adrenergic stimuli leading to vasoconstrictions.
With this background, it can be suggested that chloroethylclonidine blocks α1B-adrenoceptor subtypes and causes α1A-adrenoceptor subtype-mediated renal vasoconstrictor responses to be accentuated by the endogenous or exogenously-administered NA. It can also be suggested that in these rats, there could be an upregulation of α1A-adrenoceptors or involvement of presynaptic α1B-adrenoceptors in determining renal vasoconstriction. In the later case, it can be suggested that when these receptors are blocked by chloroethylclonidine, the presynaptic auto-inhibitory feedback is removed and allows more NA to be released, which consequently results in a larger post-synaptic response[37]. Another possible explanation of these observations could be the downregulation of certain α1-adrenoceptors in the renal vasculature. In support of this view, it was reported that in diabetes and hypertension, the α1B-adrenoceptors could be downregulated by the adrenergic nerve leaving post-synaptic receptors like the α1A-adrenoceptor (the predominant type in the renal vasculature) to be enhanced in order to maintain the effectiveness of the α1-adrenergic nervous system[38]. In the diabetic and non-diabetic 2K1C rats, chloroethylclonidine showed a similar accentuating effect on the vasoconstrictions caused by PE and ME-mediated responses. These accentuations of the renal vasoconstriction caused by PE (selective to α1-adrenoceptor subtypes) and ME (selective to α1A-adrenoceptor subtypes) can be explained in light of our discussion on the accentuation of NA and RNS-mediated responses in the presence of chloroethylclonidine. This study also reported that in the diabetic 2K1C rats, the α1D-adrenoceptor subtype selective antagonist BMY 7378 caused the accentuation of some of the adrenergically (NA, PE, and ME)-induced renal vasoconstrictor responses. This observation could be explained based on the notion that this compound also has low-affinity, presynaptic α1B-adrenoceptors, hence, enhancing the effect of the post-synaptic α1-adrenoceptor like the α1A-subtypes[38-40].
The present study has clearly shown a difference in the functional population of α1-adrenoceptor subtypes in the renal vasculature of 2K1C Goldblatt hypertensive rats with and without diabetes. In non-diabetic 2K1C rats, the adrenergically-induced renal vasoconstrictor responses were largely mediated by multiple subtypes of α1-adrenoceptors (α1A- and α1D-adrenoceptors), while in the diabetic 2K1C rats, these responses were largely mediated by a single subtype (α1A-adrenoceptor subtype). A minor involvement of presynaptic α1-adrenoceptors along with a complex interaction between some of the subtypes of α1-adrenoceptors (particularly between the α1A- and α1B-subtypes) was also apparent in the rats of either experimental group. Although some of these observations in terms of the functional involvement of α1-adrenoceptors in the renal vasculature are already stated in several earlier studies, the data pertaining to diabetic and non-diabetic 2K1C Goldblatt hypertensive rats are unique.
References
- Hieble JP, Bylund DB, Clark DE, Eikenburg DC, Langer SZ, Lefkowitz RJ, et al. International Union of Pharmacology X. Recommendation for nomenclature of α1-adrenoceptors: consensus update. Pharmacol Rev 1995;47:267-70.
- Bylund DB, Regan JW, Faber JE, Hieble JP, Triggle CR, Rufollo RR. Vascular α-adrenoceptors: from the gene to the human. Can J Physiol Pharmacol 1995;73:533-43.
- Vargas HM, Gorman AJ. Vascular α1- adrenergic receptor subtypes in the regulation of arterial pressure. Life Sci 1995;57:2291-308.
- Bishop MJ. Recent advances in the discovery of α1-adrenoceptor agonists. Curr Top Med Chem 2007;7:135-45.
- Khan AH, Sattar MA, Abdullah NA, Johns EJ. Influence of cisplatin-induced renal failure on the alpha (1)-adrenoceptor subtype causing vasoconstriction in the kidney of the rat. Eur J Pharmacol 2007;569:110-8.
- Hye Khan MA, Sattar MA, Abdullah NA, Johns EJ. Cisplatin cause altered renal hemodynamics in rats: role of augmented α-adrenergic responsiveness. Exp Toxicol Pathol 2007;53:253-60.
- Rosini M, Bolognesi ML, Giarrdina D, Minarini A, Tumitatti V, Melchiorre C. Recent advances in α1-adrenoceptor antagonists as pharmacological tools and therapeutic agents. Curr Top Med Chem 2007;7:147-62.
- Moriyama N, Kurooka Y, Nasu K, Akiyama K, Takeuchi T, Nishimatsu H, et al. Distribution of α1-adrenoceptor subtype mRNA and identification of subtype responsible for renovascular contraction in human renal artery. Life Sci 2000;66:915-26.
- Abdul Sattar M, Johns EJ. Alpha 1-adrenoceptor subtypes mediating adrenergic vasoconstriction in kidney, one-clip Goldblatt and deoxycorticosterone acetate-salt hypertensive rats. J Cardiovasc Pharmacol 1994;24:420-8.
- Sattar MA, Johns EJ. Evidence for an α1-adrenoceptor subtype mediating adrenergic vasoconstriction in Wistar normotensive and SPSHR kidney. J Cardiovasc Pharmacol 1994;23:232-9.
- Villalobos-Molina R, Ibarra M. α1-adrenoceptor mediating contraction in arteries of normotensive and SH rats are of α1D- or α1A-subtypes. Eur J Pharmacol 1996;298:257-63.
- Villalobos-Molina R, Lopez-Guerrero JJ, Ibarra M. α1D- and α1A-adrenoceptors mediate contraction in rat renal artery. Eur J Pharmacol 1997;322:225-7.
- Eltze M, Konig H, Ullrich B, Grebe T. Buspirone functionally discriminates tissues endowed with α1-adrenoceptor subtypes A, B, D and L. Eur J Pharmacol 1999;378:69-83.
- Satoh M, Enomoto K, Takayanagi I, Koike K. Analysis of α1-adrenoceptor subtypes in rabbit aorta and arteries: regional difference and co-existence. Eur J Pharmacol 1999;374:229-40.
- Salomonsson M, Brannstrom K, Arendshorst WJ. Alpha (1)-adrenoceptor subtypes in rat renal resistance vessels: in vivo and in vitro studies. Am J Physiol 2000;278:F138-47.
- Porte D, Schwartz MW. Diabetes complications. Why is glucose potentially toxic? Science 1996;272:699-700.
- Armenia A, Munavvar AS, Abdullah NA, Helmi A, Johns EJ. The contribution of adrenoceptor subtypes in the renal vasculature of diabetic spontaneously hypertensive rats. Br J Pharmacol 2004;142:719-26.
- Abbas SA, Munavvar AS, Abdullah NA, Johns EJ. Involvement of α1-adrenoceptor subtypes in the cardiac failure in spontaneously hypertensive rats. J Basic Appl Sci 2006;2:59-69.
- Abbas SA, Munavvar AS, Abdullah NA, Johns EJ. Role of alpha1-adrenoceptor subtypes in renal haemodynamics in heart failure and diabetic SD rats. Can J Pure Appl Sci 2007;1:21-34.
- Stassen FR, Maas R, Schiffers PMH, Janssen GM, De Mey JG. A positive and Reversible relationship between adrenergic nerves and alpha1A-adrenoceptors in rat arteries. J Pharmacol Exp Ther 1998;284:399-405.
- Elhawary AM, Pettinger WA, Wolff DW. Subtype-selective α1-adrenoceptor alkylation in the rat kidney and its effect on the vascular pressor response. J Pharmacol Exp Ther 1992;260:709-13.
- Villalobos-Molina R, Lopez-Guerrero JJ, Ibarra M. Functional evidence of α1D -adrenoceptors in vasculature of young and adults spontaneously hypertensive rats. Br J Pharmacol 1999;126:1534-6.
- Blue DR, Vimont RL, Clarke DE. Evidence for a noradrenergic innervation to α1A-adrenoceptors in rat kidney. Br J Pharmacol 1992;107:414-7.
- Blue DR, Bonchaus DW, Ford APDW, Pfister JR, Syarif NA, Shieh IA, et al. Functional evidence equating the pharmacologically-defined α1A- and cloned α1C-adrenoceptor: studies in isolated perfused kidney of rat. Br J Pharmacol 1995;115:283-94.
- Perez DM, Piascik MT, Malik N, Gaivin R, Graham RM. Cloning, expression, and tissue distribution of the rat homolog of the bovine α1C-adrenergic receptor provide evidence for its classification as the α1A-subtype. Pharmacology 1994;46:823-31.
- Zhu W, Zhang Y, Han C. Characterization of subtype α1-adrenoceptor mediating vasoconstriction in perfused rat hind limb. Eur J Pharmacol 1997;329:55-61.
- Hrometz SL, Edelmann SE, McCune DF, Olges JR, Hadley RW, Perez DM, et al. Expression of multiple α1-adrenoceptors on vascular smooth muscle: correlation with the regulation of contraction. J Pharmacol Exp Ther 1999;290:452-63.
- Maeda CY, Fernandes TG, Timm HB, Irigoyen MC. Autonomic dysfunction in short-term experimental diabetes. Hypertension 1995;26:1100-4.
- Schaan BD, Dall’Ago P, Maeda CY, Ferlin E, Fernandes TG, Schmid H, et al. Relationship between cardiovascular dysfunction and hyperglycemia in streptozotocin-induced diabetes in rats. Braz J Med Biol Res 2004;37:1895-902.
- Maeda CY, Fernandes TG, Lulhier F, Irigoyen MC. Streptozotocin diabetes modifies arterial pressure and baroreflex sensitivity in rats. Braz J Med Biol Res 1995;28:497-501.
- Jarajapu YPR, Hillier C, MacDonald A. The α1A-adrenoceptor subtypes mediate contraction in rat femoral resistance arteries. Eur J Pharmacol 2000;422:127-35.
- Zacharia J, Hillar C, MacDonald A. α1-adrenoceptor subtypes involved in vasoconstrictor responses to exogenous and neurally released in rat femoral resistance arteries. Br J Pharmacol 2004;141:915-24.
- Hirasawa A, Sugawara T, Awaji T, Tsumaya K, Ito H, Tsujimoto G. Subtype-species differences in sub-cellular localization of α1-adrenoceptors: chloroethylclonidine preferentially alkylates the accessible cell surface α1-adrenoceptors irrespective of the subtype. Mol Pharmacol 1997;52:764-70.
- Yang M, Reese J, Cotecchia S, Michel MC. Murine alpha1-adrenoceptor subtypes. I. Radioligand binding studies. J Pharmacol Exp Ther 1998;286:841-7.
- Gómez-Zamudio J, Lázaro-Suárez ML, Villalobos-Molina R, Urquiza-Marín H. Evidence for the use of agonists to characterize α1- adrenoceptors in isolated arteries of the rat. Proc West Pharmacol Soc 2002;45:159-60.
- Arévalo-León LE, Gallardo-Ortíz IA, Urquiza-Marín H, Villalobos-Molina R. Evidence for the role of alpha1D- and α1A-adrenoceptors in contraction of rat mesenteric artery. Vascul Pharmacol 2003;40:9196.
- Li Z, Silver WP, Koman LA, Strandhoy JW, Rosencrance E, Gordon S, et al. Role of α1A-adrenoceptor subtypes mediating constriction of the rabbit ear thermoregulatory microvasculature. J Orthop Res 2000;18:156-63.
- Stassen FR, Willemsen MJ, Janssen GM, De Mey JG. Alpha 1-adrenoceptor subtypes in rat aorta and mesenteric small arteries are preserved during left ventricular dysfunction post-myocardial infarction. Cardiovasc Res 1997;33:706-13.
- Goetz AS, King HK, Ward SD, True TA, Rimele TJ, Saussy DL. BMY 7378 is a selective antagonist of the D subtype of α1-adrenoceptors. Eur J Pharmacol 1995;272:R5-R6.
- Zhou L, Varga S. Vascular α1D-adrenoceptors have a role in the pressor response to phenylephrine in pithed rat. Eur J Pharmacol 1996;305:173-6.
