FG020326-loaded nanoparticle with PEG and PDLLA improved pharmacodynamics of reversing multidrug resistance in vitro and in vivo1
Introduction
Multidrug resistance (MDR) restricts successful tumor chemotherapy. The overexpression of P-glycoprotein (P-gp) is one of the prominent mechanisms that contribute to the MDR phenotype[1]. In fact, P-gp is a 170 kDa membrane-associated glycoprotein that may actively extrude various substrates such as cytotoxic drugs from the cell cytoplasm to the outside of the plasma membrane, thus lowering the effective drug concentrations within the cells and leading to MDR. To restore the MDR cell sensitivity, a combination of a conventional chemotherapeutic regimen and an MDR modulator is a promising strategy[2]. Recently, drug carriers such as microspheres, liposomes, or nanoparticles were found to improve the intracellular accumulation of anticancer drugs in MDR cells in vitro and in vivo[3]. Barraud et al[4] reported that a higher antitumor efficacy on hepatocellular carcinoma appeared in doxorubicin (Dox)-loaded polyisohexylcyano-acrylate nanoparticles than in free Dox both in vitro and in vivo. The nanoparticle, acting as a drug carrier, could improve solubility for hydrophobic drugs and may increase drug influx into MDR cells through endocytic and/or pinocytic activity, thus avoiding P-gp efflux and increasing intracellular drug accumulation[5].
Polymeric micelles are particles with diameters typically smaller than 100 nm formed by amphiphilic polymers dispersed in aqueous media[6,7]. Polymeric micelles solubilize insoluble drugs by incorporating them into their hydrophobic cores, thus allowing for an increased bioavailability. Meanwhile, their hydrophilic shells can restrict their recognition and uptake by the mononuclear phagocyte system and allow for extended circulation time. On the other hand, the hyperpermeability of tumor vasculature and the lack of an effective lymphatic drainage of tumor tissue lead to an accumulation of particles in tumor tissue. This passive targeting phenomenon was termed enhanced permeability and retention effect[8]. In vitro, IC50 indicated that both cisplatin-loaded polymeric micellar poly(ε-caprolactone)/poly[2-(N,N-dimethylamino)ethyl methacrylate]/Cisplatin (PCL/PDMA/CDDP) and PCL/PEG/CDDP had greatly enhanced cytotoxicity to the tumor cells compared with free cisplatin[9]. Camptothecin (CPT) loaded in polymeric micelles showed enhanced tumor inhibitory activity. The micelles prolonged the drug’s blood circulation time and increased accumulation in tumors compared with free CPT[10].
FG020326, an imidazole derivative, is a highly potent, efficacious MDR modulator in vitro and in vivo. It can inhibit the function of P-gp and increase the accumulation of anticancer drugs in MDR tumor cells[11], but FG020326 is insoluble in water. This work was intended to evaluate the pharmacodynamics of FG020326-loaded nanoparticles with hydrophilic PEG and hydrophobic PDLLA diblock copolymer (PEDLLA) termed as PEDLLA-FG020326 on reversing MDR in vitro and in vivo.
Materials and methods
Materials Dox and vincristine (VCR) were purchased from Hisun Pharmaceutical (Taizhou, Zhejiang, China). Rhodamine 123 (Rh123) and tetrazolium (MTT) were purchased from Sigma Chemical Co (St Louis, MO, USA). RPMI-1640 was purchased from Gibco BRL (Grand Island, NY, USA). FG020326 was synthesized and obtained by chromatography, the isolated powder with a purity of more than 98%. The FG020326 solution was freshly made with 0.1% DMSO which did not exert any cytotoxicity to the cells. PEDLLA-FG020326 was obtained from the School of Chemistry and Chemical Engineering of Sun Yat-Sen University (Guangzhou, China).
Animals Athymic nude mice (BALB/c-nu-nu) were used for the KBv200 cell xenografts. The mice were obtained from and brought up in the Center of Experimental Animals, Sun Yat-Sen University (China). The animals were fed specific pathogen free (SPF)-grade sterilized food and water. Female and male mice used in the experiment were 6–8 weeks old, weighing 18–24 g.
Cell lines and cell culture KB and KBv200 are human epidermoid carcinoma cell lines. KBv200, an MDR cell line with high expression of P-gp, is about 99-fold resistant to VCR compared with its drug-sensitive parental KB cells. The KBv200 cells were cloned from parental drug-sensitive KB cells by stepwise exposure to increasing doses of VCR and ethylmethane sulfonate mutagenesis. The KBv200 cells and parental sensitive KB cells were obtained from Prof Liu YS (Chinese Academy of Medical Sciences, Beijing, China). The KBv200 cells and KB cells were cultured with RPMI-1640 culture medium with 10% fetal blood serum (FBS) at 37 °C in a humidified atmosphere of 5% CO2[12].
MTT cytotoxicity assay The cells were harvested in a logarithmic growth phase and seeded in 96-well plates at 3.0×103 cells each well for KBv200 and KB cells in a final volume of 170 µL. After incubation for 24 h, 20 µL 20 µmol/L FG020326, PEDLLA-FG020326, or drug-unloaded micelle PEDLLA, and 10 µL cytotoxic agent VCR or Dox were added to triplicate wells and incubated for 72 h. Then 10 µL 10 mg/mL MTT solution was added to each well. DMSO (100 µL) was added to each well 4 h later. IC50 was calculated from the cytotoxicity curves (Bliss’s software, Bliss Co, CA, USA). The degree of resistance was calculated by dividing the IC50 for the MDR cells by that for the parental sensitive cells. The reversal fold of MDR was calculated by dividing the IC50 for cells to the anticancer drug in the absence of the modulator by that in the presence of the modulator[13].
Dox accumulation and efflux The intracellular Dox accumulation was examined as described by Fu et al[13]. In detail, the logarithmically growing KB cells and KBv200 cells were harvested and resuspended at a concentration of 1×106 cells/mL. The cells were treated with FG020326 (1, 2, or 4 µmol/L), PEDLLA-FG020326 (1, 2, or 4 µmol/L), PEDLLA (4 µmol/L), or vehicle at 37 °C for 2 h in the RPMI-1640 medium. Then Dox was added to a final concentration of 10 μmol/L. The cells were incubated for another 3 h at 37 °C, then collected, centrifuged, washed 3 times with cold phosphate buffered solution (PBS), and resuspended in 0.3 mol/L HCl in 60% ethanol. After centrifugation at 13 201×g for 15 min, the supernatant was removed and assayed spectrofluoro-metrically at λex=482 nm and λem=593 nm. PEDLLA-FG020326, FG020326, and PEDLLA did not affect the absorbance or emission spectra of Dox. The accumulation of Dox was calculated by the standard curve of Dox. The fold of the Dox accumulation was calculated by dividing the value in the presence of the modulator by that without the modulator.
To measure drug efflux, the KBv200 or KB cells were incubated in energy-supplied buffer and incubated with 10 µmol/L Dox for 3 h at 37 °C. After that, each dish was washed once with PBS, then FG020326 (4 µmol/L), PEDLLA-FG020326 (4 µmol/L), or PEDLLA (4 µmol/L) was added. The cells were incubated for the indicated times at 37 °C and harvested, then quantified as described earlier[13].
Rh123 accumulation studies The KBv200 cells and KB cells were exposed to FG020326 (1, 2, or 4 µmol/L), PEDLLA-FG020326 (1, 2, or 4 µmol/L), or PEDLLA (4 µmol/L) for 2 h. The cells were collected and washed once and then resuspended in 1 mL RPMI-1640 at the concentration of 2×105 cells/mL. Then the cells were loaded with 5 µg/mL Rh123 for 30 min at 37 °C. After washing with RPMI-1640 once, the cells were allowed to efflux the dye for 10 min in dye-free RPMI-1640 at 37 °C. The cells were then washed and resuspended in 1 mL RPMI-1640 containing 10 ng/mL verapamil, after which fluorescence analysis was carried out on a FACScan flow cytometer (Becton and Dickinson, Mountain View, CA, USA)[13].
Reversal of MDR in the KBv200 cell xenografts The KBv200 cell xenograft model was established as described by Liang et al[14]. Briefly, the KBv200 cells were harvested, resuspended at a concentration of 1.5×107 cells/mL, and implanted subcutaneously with 0.2 mL cell suspension under the right armpits of nude mice. When the tumors had reached a diameter of 5 mm, the animals were divided into 5 groups according to their body weight, and treated with various regimens. The groups included a saline group and groups receiving VCR alone (0.2 mg/kg ip), every 2 d (q2d), PEDLLA-FG020326 alone (50 mg/kg ip) q2d, VCR (0.2 mg/kg) plus FG020326 (50 mg/kg ip) q2d, or VCR (0.2 mg/kg) plus PEDLLA-FG020326 (50 mg/kg ip) q2d. The body weight of the animals was measured every 2 d for the modulation of the drug dosage. The tumor volume was measured in 2 perpendicular diameters (A and B) every 2 d, and the tumor volume (V) was estimated according to the following formula[12,13]:
The curve of tumor growth was drawn according to tumor volulme and time of implantation. The mice were ethically killed when the mean tumor weights were over 1 g in the control group. Tumor tissue was excised from the mice and weighed. The rate of inhibition (IR) was calculated according to the formula[12,13]:
Statistical analysis All data were repeated at least 3 times in independent experiments and differences were determined by Student’s t-test. Significance was determined at P<0.05.
Results
Comparison of PEDLLA-FG020326 and FG020326 in reversing MDR in vitro The KBv200 cells were approximately 99-fold resistant to VCR and 16-fold resistant to Dox in comparison with the KB cells. PEDLLA-FG020326, FG020326, and PEDLLA did not appear to have any cytotoxicity to KBv200 cells and KB cells until 50 µmol/L; PEDLLA did not reverse MDR action. PEDLLA-FG020326 exhibited stronger activity than free FG020326 on reversing MDR in the KBv200 cells. However, both PEDLLA-FG020326 and FG020326 had no effect on the enhancement of drug cytotoxicity in drug sensitive KB cells (Table 1; Figure 1).
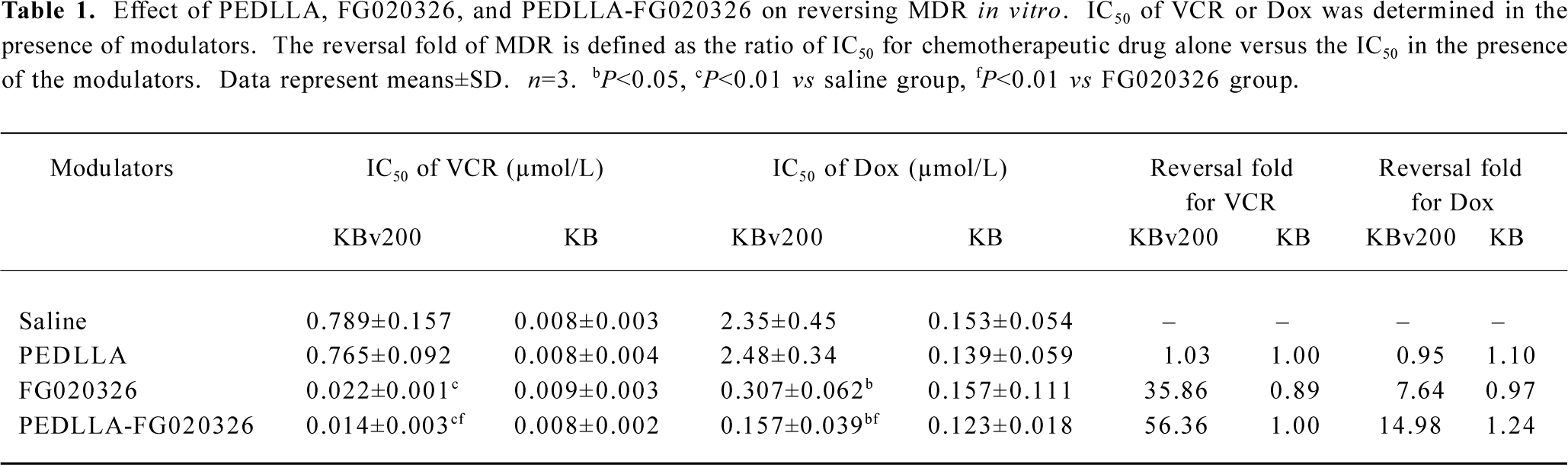
Full table
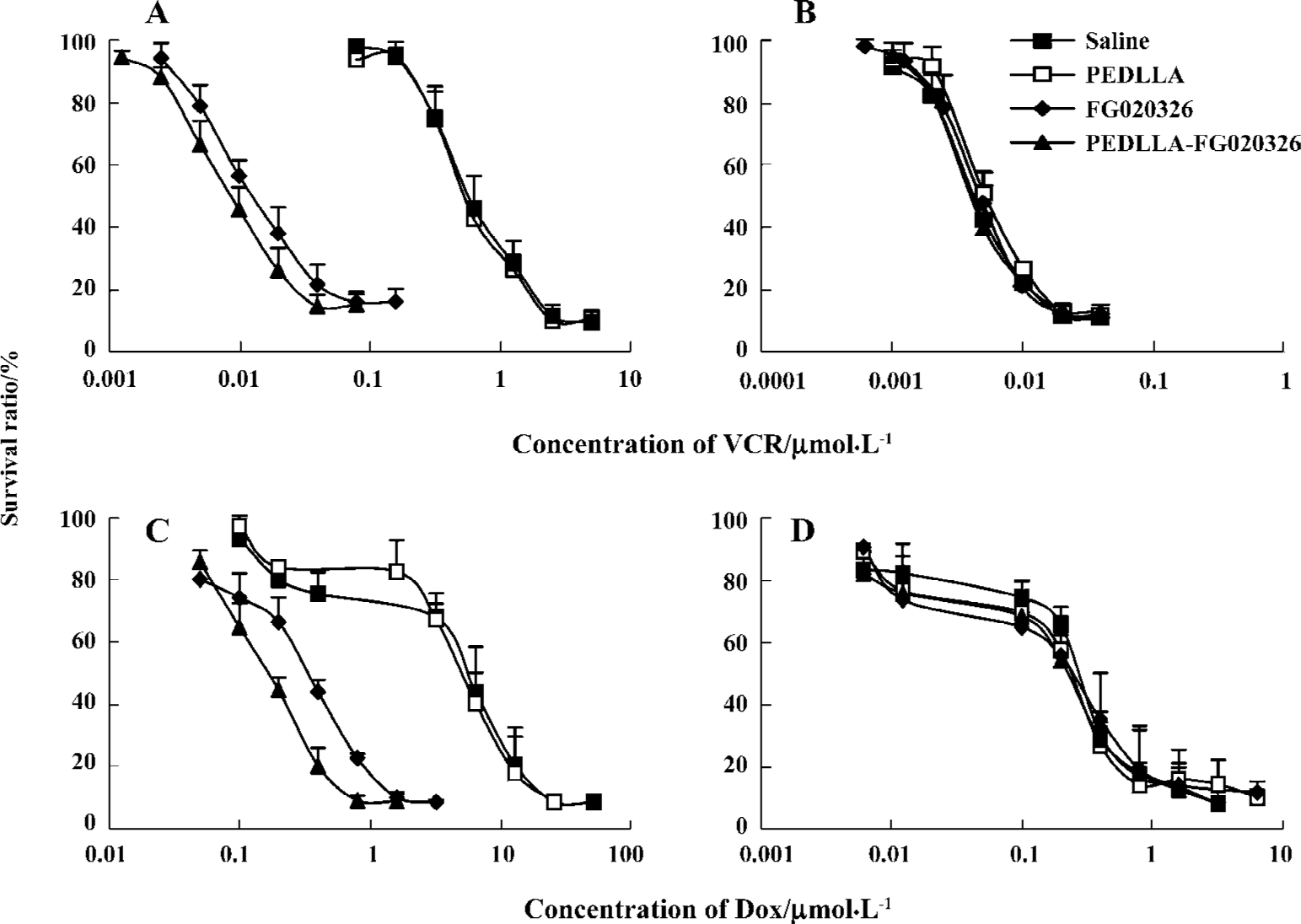
PEDLLA-FG020326 induced more intracellular Dox accumulation and less Dox efflux than FG020326 in KBv200 cells The intracellular accumulation of Dox in the KB cells was 12-fold of that of the KBv200 cells. PEDLLA-FG020326 and FG020326 increased concentration-dependent intracellular Dox accumulation in the KBv200 cells, but not in the KB cells. Importantly, PEDLLA-FG020326 was more effective for the enhancement of intracellular Dox accumulation than FG020326 (Figure 2).
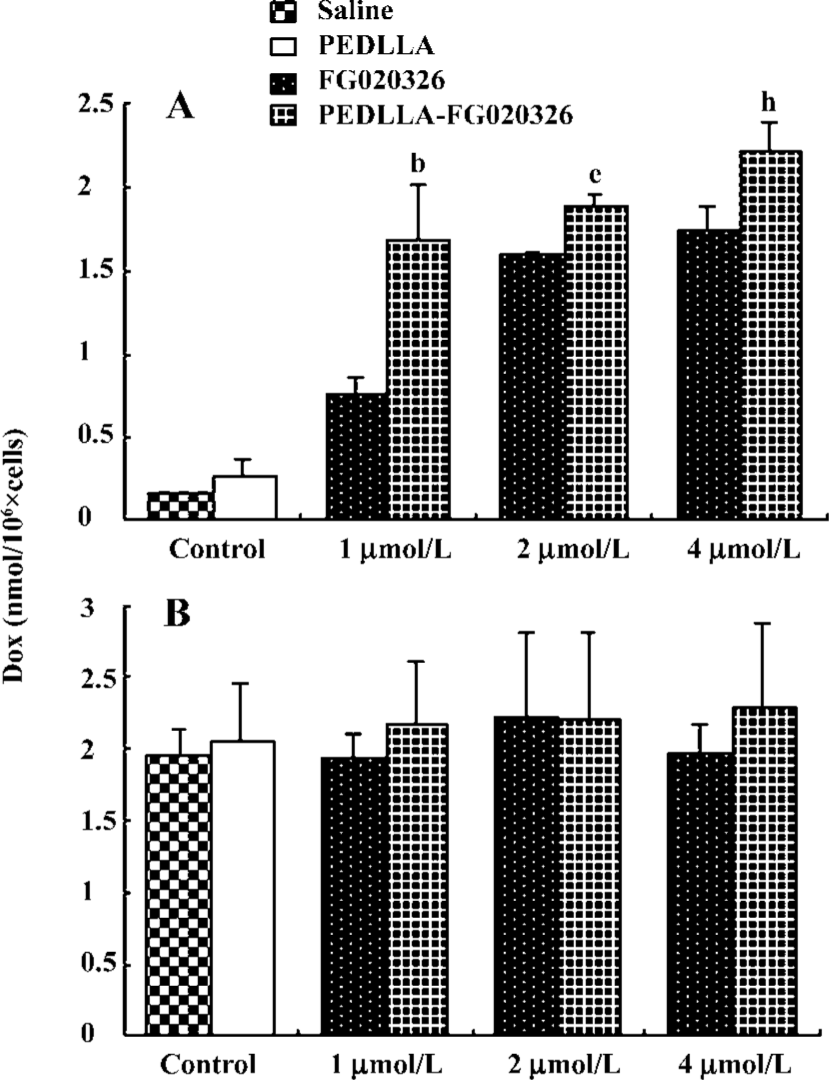
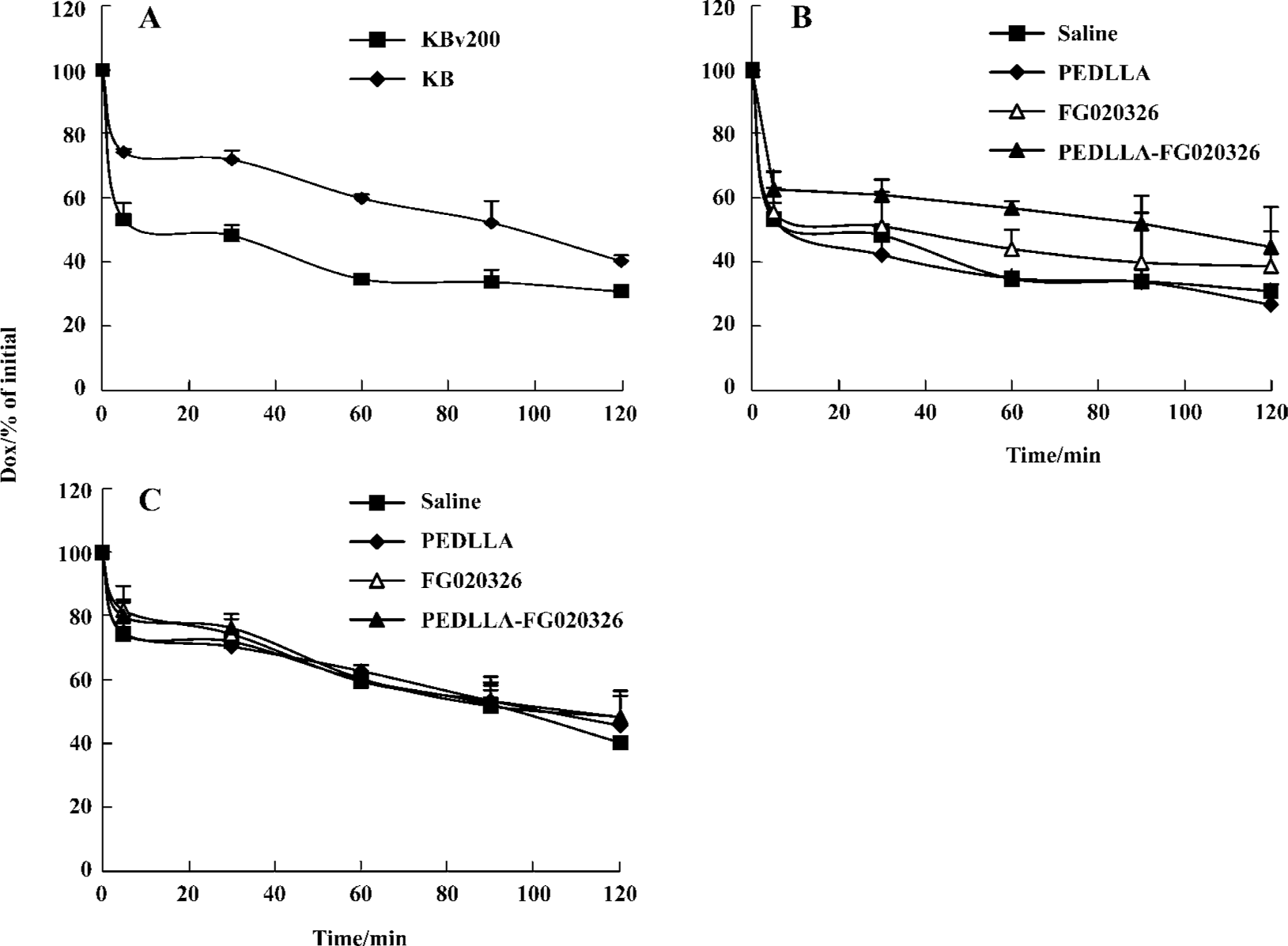
A higher percentage of Dox extrusion appeared in the KBv200 cells than that in the KB cells. At 60 min, more than 70% of the accumulated Dox was extruded from the KBv200 cells, while only 40% was extruded from the KB cells. PEDLLA did not affect the Dox efflux in the KBv200 and KB cells. PEDLLA-FG020326 was stronger in inhibiting the efflux of Dox than FG020326 in the KBv200 cells, but both had no effect on the KB cells (Figure 3).
PEDLLA-FG020326 was stronger in the inhibition of P-gp function than FG020326 Rh123 is a substrate of P-gp so the intracellular Rh123 accumulation acts as an index of P-gp function. PEDLLA-FG020326 and FG020326 increased concentration-dependently intracellular Rh123 accumulation in the KBv200 cells, but not in the KB cells. Importantly, the Rh123 accumulation in the KBv200 cells treated with PEDLLA-FG020326 was much more than that of the KBv200 cells treated with FG020326 at the same concentration (Figure 4).
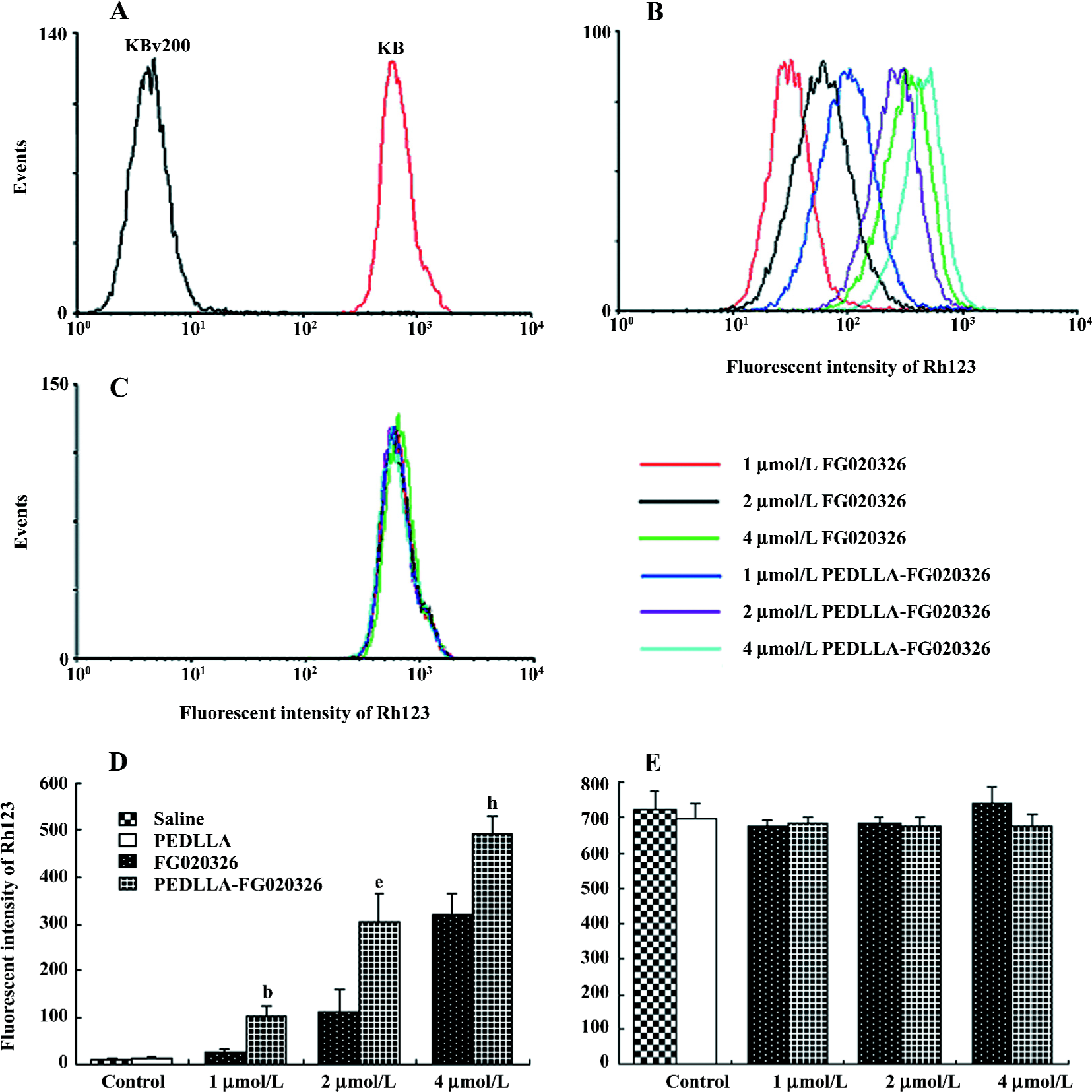
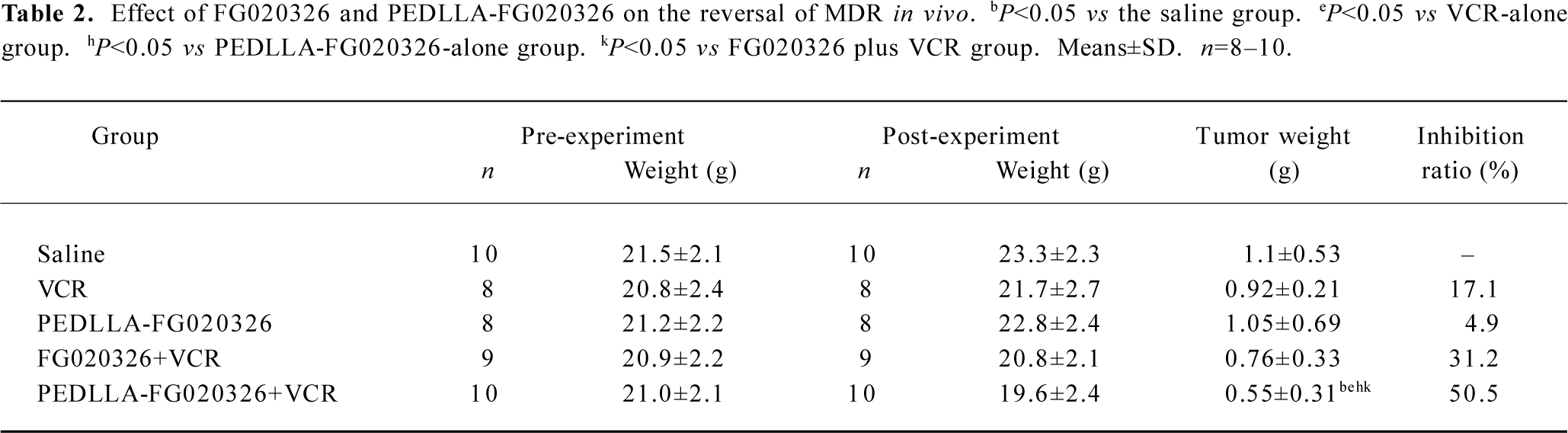
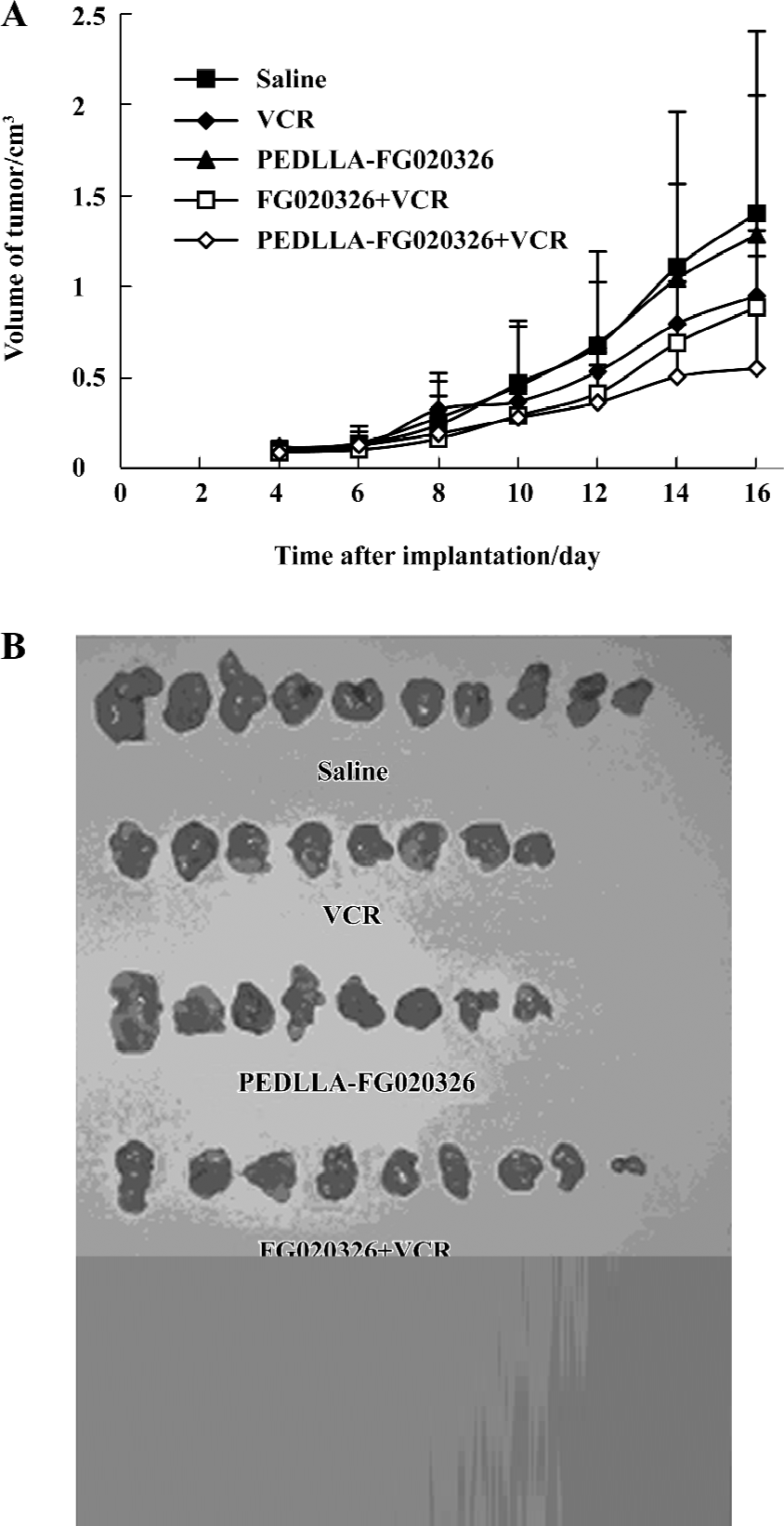
PEDLLA-FG020326 improved the pharmacodynamics of FG020326 in reversing MDR in vivo PEDLLA-FG020326 had stronger activity in reversing MDR in vitro than FG020326. To determine whether PEDLLA-FG020326 would improve the pharmacodynamics of FG020326 in reversing MDR in vivo, a tumor growth suppression experiment was conducted in nude mice bearing a KBv200 cell solid tumor. In vivo experiments showed that neither PEDLLA-FG020326 alone nor VCR alone resulted in the inhibition of tumor growth. However, the combination of VCR and FG020326 or PEDLLA-FG020326 elicited marked inhibition of tumor growth. The tumors were much smaller in the group of VCR plus PEDLLA-FG020326 than VCR plus FG020326; the inhibition ratio of tumor growth was 50.5% and 31.2%, respectively (Table 2; Figure 5).
Full table
Discussion
MDR is the main obstacle to successful chemotherapy. To deal with this issue, a number of MDR reversal agents with P-gp inhibitory activity, also known as “modulators”, were identified[1]. FG020326 is a recently developed modulator[11]. In comparison to the early generation of modulators (eg cyclosporin-A, verapamil) that are not optimized for MDR reversal[1,2], FG020326 has a higher potency for P-gp and lower cellular toxicity[11].
Drug delivery systems such as microspheres, liposomes, or polymeric nanoparticles may improve the effectiveness and decrease the side effects of cancer chemotherapeutics. Several in vivo studies have shown that polymeric micelles are able to improve the efficiency of anticancer drugs against leukemia[19,20]and solid tumors[21,22]. There are some mechanisms for the increased pharmaceutical activity of drug-loaded polymeric micelles such as specific targeting in tumor tissues, increased permeability of the drug through the biological membrane, and prolonged circulation time due to slow drug release[7].
Liu et al[17] and Li et al[18] have reported that the co-encapsulation of anticancer drugs and modulators by polymeric particles can enhance MDR reversal by the simultaneous delivery approach. Moreover, lower normal tissue drug toxicity and fewer drug-drug interactions have generally been observed in the combinative treatments. However, so far, there has not been a consensus as to which strategy provides the optimal treatment outcome.
In this study, we compared the reversal activity of PEDLLA-FG020326 with FG020326 in vitro and in vivo. The in vitro results showed that PEDLLA-FG020326 was more effective than FG020326 in reversing MDR in KBv200 cells. P-gp-positive MDR cells demonstrated that the emergence of MDR in these cells was linked to a marked decrease in the intracellular accumulation of the various cytotoxic drugs. Most MDR modulators could restore drug accumulation in MDR tumor cells[15]. In this study, PEDLLA-FG020326 and FG020326 were tested for their effect on intracellular drug accumulation. At the same concentration, PEDLLA-FG020326 caused a marked increase of Dox accumulation compared with FG020326 in the KBv200 cells but not in the sensitive KB cells. Rh123 is a specific fluorescent substrate of P-gp which functions as a drug transporter and mediates drug efflux[16]. Intracellular Rh123 accumulation could be a good indicator of P-gp function. Chen et al[11] found that FG020326 can inhibit the function of P-gp and increase Rh123 accumulation in MDR cells. To evaluate whether the increased accumulation of Dox in the KBv200 cells was due to the inhibition of Dox efflux, the extrusion of Dox and P-gp function were examined. PEDLLA-FG020326 caused a slower extrusion of Dox and more increased Rh123 accumulation in the KBv200 cells than FG020326. These results suggest that PEDLLA-FG020326 can inhibit the function of P-gp more effectively than FG020326. Moreover, PEDLLA-FG020326 can increase intracellular drug accumulation compared with FG020326 in MDR KBv200 cells.
The KBv200 cell xenografts in nude mice were established and proved to be extremely resistant to VCR, and retained the characteristics of the MDR phenotype[14]. The combination of PEDLLA-FG020326 and VCR exhibited a more potent inhibitory effect on the growth of the tumors than the co-administration of FG020326 and VCR. Neither PEDLLA-FG020326 alone nor VCR alone resulted in the inhibition of tumor growth. The results demonstrate that PEDLLA-FG020326 has stronger reversal activity than FG020326 in vivo. More importantly, the difference of body weight between the PEDLLA-FG020326 plus VCR-treated group and the control or VCR-treated groups is not significant, so co-administration with PEDLLA-FG020326 does not increase the toxicity of VCR obviously.
In summary, we observed an improvement in MDR reversal activity in vitro and in vivo when FG020326 or PEDLLA-FG020326 was co-administered with a conventional anticancer drug. Importantly, PEDLLA-FG020326 was more effective in the reversal of MDR than FG020326 in vitro and in vivo, which was associated with inhibiting the function of P-gp and enhancing chemotherapeutic drug accumulation in MDR cells. These results suggest that the pharmacodynamics of FG020326 is improved by incorporating into micellar nanoparticles formed with PEG-block-PDLLA diblock copolymers.
Acknowledgements
We would like to thank Prof Y S LIU (Chinese Academy of Medical Sciences, Beijng, China) for providing us with the KB and KBv200 cell lines.
References
- Endicott JA, Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem 1989;58:137-71.
- Kellen JA. The reversal of multidrug resistance in cancer. Anticancer Res 1993;13:959-61.
- Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev 2002;54:631-51.
- Barraud L, Merle P, Soma E, Lefrancois L, Guerret S, Chevallier M, et al. Increase of doxorubicin sensitivity by doxorubicin-loading into nanoparticles for hepatocellular carcinoma cells in vitro and in vivo. J Hepatol 2005;42:736-43.
- Koziara JM, Whisman TR, Tseng MT, Mumper RJ. In-vivo efficacy of novel paclitaxel nanoparticles in paclitaxel-resistant human colorectal tumors. J Control Release 2006;112:312-9.
- Kwon GS, Kataoka K. Block copolymer micelles as longcircu-lating drug vehicles. Adv Drug Deliv 1995;16:295-309.
- Jones M, Leroux J. Polymeric micelles – a new generation of colloidal drug carriers. Eur J Pharm Biopharm 1999;48:101-11.
- Maeda H, Fang J, Inutsuka T, Kitamoto Y. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. Int Immunopharmacol 2003;3:319-28.
- Xu P, Van Kirk EA, Li S, Murdoch WJ, Ren J, Hussain MD, et al. Highly stable core-surface-crosslinked nanoparticles as cisplatin carriers for cancer chemotherapy. Colloids Surf B Biointerfaces 2006;48:50-7.
- Kawano K, Watanabe M, Yamamoto T, Yokoyama M, Opanasopit P, Okano T, et al. Enhanced antitumor effect of camptothecin loaded in long-circulating polymeric micelles. J Control Release 2006;112:329-32.
- Chen LM, Wu XP, Ruan JW, Liang YJ, Ding Y, Shi Z, et al. Screening novel, potent multidrug-resistant modulators from imidazole derivatives. Oncol Res 2004;14:355-62.
- Fu LW, He LR, Liang YJ, Chen LM, Xiong HY, Yang XP, et al. Experimental chemotherapy against xenografts derived from multidrug resistant KBv200 cells and parental drug-sensitive KB cells in nude mice by annonaceous acetogenin 89-2. Acta Pharm Sin 2003;38:565-70.
- Fu LW, Zhang YM, Liang YJ, Yang XP, Pan QC. The multidrug resistance of tumour cells was reversed by tetrandrine in vitro and in xenografts derived from human breast adenocarcinoma MCF-7/adr cells. Eur J Cancer 2002;38:418-26.
- Liang YJ, Fu LW, Feng HL, He LR, Feng GK, Yang XP, et al. Establishment of the model of KBv200 nude mice xenograft and studies on its characterization of multidrug resistance. Chin Pharmacol Bull 2000;16:705-9.
- Yokoyama M, Miyauchi M, Yamada N, Okano T, Sakurai Y, Kataoka K, et al. Characterization and anticancer activity of the micelle-forming polymeric anticancer drug adriamycin-conjugated poly(ethylene glycol)-poly(aspartic acid) block copolymer. Cancer Res 1990;50:1693-700.
- Zhang X, Burt HM, Von Hoff D, Dexter D, Mangold G, Degen D, et al. An investigation of the antitumour activity and biodistribu-tion of polymeric micellar paclitaxel. Cancer Chemother Pharmacol 1997;40:81-6.
- Zhang X, Burt HM, Mangold G, Dexter D, Von Hoff D, Mayer L, et al. Anti-tumor efficacy and biodistribution of intravenous polymeric micellar paclitaxel. Anticancer Drugs 1997;8:696-701.
- Yokoyama M, Okano T, Sakurai Y, Ekimoto H, Shibazaki C, Kataoka K. Toxicity and antitumor activity against solid tumors of micelle-forming polymeric anticancer drug and its extremely long circulation in blood. Cancer Res 1991;51:3229-36.
- Liu Z, Bendayan R, Wu XY. Triton-X-100-modified polymer and microspheres for reversal of multidrug resistance. J Pharm Pharmacol 2001;53:1-12.
- Li X, Ruan GR, Lu WL, Hong HY, Liang GW, Zhang YT, et al. A novel stealth liposomal topotecan with amlodipine: apoptotic effect is associated with deletion of intracellular Ca2+ by amlodipine thus leading to an enhanced antitumor activity in leukemia. J Control Release 2006;112:186-98.
- Tsuruo T, Naito M, Tomida A, Fujita N, Mashima T, Sakamoto H, et al. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci 2003;94:15-21.
- Croce AC, Supino R, Lanza KS, Locatelli D, Baglioni P, Bottiroli G. Photosensitizer accumulation in spontaneous multidrug resistant cells: a comparative study with rhodamine 123, rose bengal acetate and photofrine. Photochem Photobiol Sci 2002;1:71-8.
