Soluble components of Hericium erinaceum induce NK cell activation via production of interleukin-12 in mice splenocytes1
Introduction
Among the numerous antitumor cellular components in mammals, natural killer (NK) cells play important roles in inhibiting tumor development, growth, and metastasis, as well as having a significant role in tumor treatment[1–3]. Tumor cells are able to evade the adaptive immune system by downregulating the expression of major histocompatibility complex (MHC) molecules; however, NK cells are cytotoxic against MHC class I-deficient tumor cells[4,5]. Besides killing cancer cells directly through cell-mediated cytolysis, NK cells produce cytokines, such as interferon-gamma (IFN-gamma), which stimulate adaptive immunity and restrict tumor angiogenesis[6–8]. Therefore, maintaining or enhancing NK cell-based immune responses is very important for cancer treatment[9]. Accordingly, many cancer immunotherapies that enhance the activities of NK cells have been proven to be effective in both experimental animal models and cancer patients[10,11].
Natural resource-derived compounds such as ginseng extract or echinacea root extract have been investigated as potential immunomodulators for the treatment of cancer patients[12,13]. Many of these studies have focused on the activation of NK cells and demonstrated positive results[14,15]. Furthermore, polysaccharides found in various mushrooms have recently attracted attention due to their NK cell-related immune-activating properties[16–19].
Hericium erinaceum is a widely cultivated edible mushroom and has been used in several Asian countries to treat various human diseases. Recently, H erinaceum was report-ed to have cytotoxic effects on cancer cell lines, as well as nematicidal and antimicrobial activities[20,21]. We previously reported that a water extract of H erinaceum (WEHE) induced the production of NO and interleukin (IL)-1-beta in rat macrophages and a macrophage-like cell line, RAW 264.7[22,23].
In the present study, we examined the immunomodulatory effects of WEHE on the cytolytic function of NK cells against an MHC class I-deficient cell line, Yac-1. Our results indicated that WEHE indirectly facilitated the cytolytic ability of mouse splenocytes by amplifying the NK cell activity induced by IL-12.
Materials and methods
Reagents and chemicals 51Cr-source was obtained from PerkinElmer (Boston, MA, USA), and lipopolysaccharide (LPS, Salmonella typhosa) was obtained from Sigma–Aldrich (St Louis, MO, USA). All RT-PCR reagents were purchased from Bio-Rad (Hercules, CA, USA). Mouse IL-12 and the anti-mouse IL-12 rat antibody were purchased from eBioscience (San Diego, CA, USA). β-D-(1,3)-(1,6)-glucan was obtained from VP GmbH (Hergestellt, Germany).
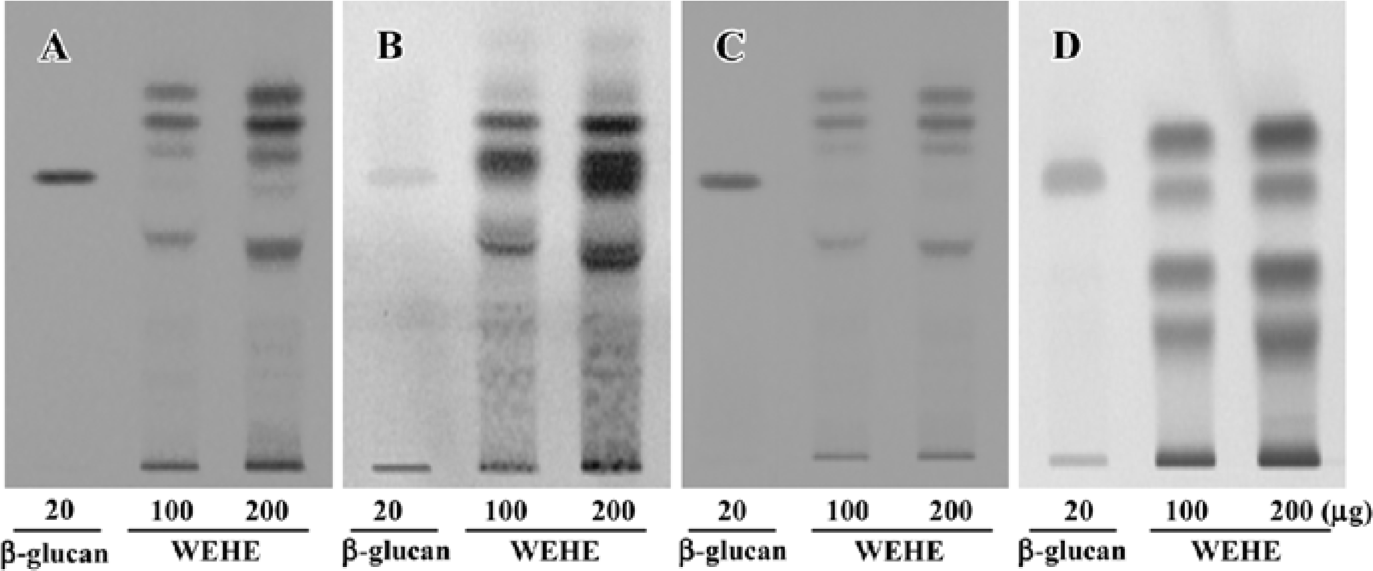
Extraction, composition analysis, and fingerprinting of WEHE Dried H erinaceum was obtained from the Korean Mushroom Corporation (Pochon, Korea). The mushrooms (100 g) were washed 3 times with pyrogen-free water, then soaked in 1.5 L pyrogen-free water for 2 h and boiled for 2 h. Solid particles and aggregates were removed by centrifugation at 3000×g for 30 min, and the supernatant was lyophilized. A total of 26.35 g lyophilized water extract was obtained for use in this experiment. According to the Association of Analytical Communities[24], the general chemical composition of WEHE is crude protein (44.82%), carbohydrate (27.63%), crude ash (16.84%), moisture (9.05%), crude fiber (0.94%), and crude fat (0.72%). For additional quality control of the tested samples, high-performance-thin layer chromatography (HPTLC)-based fingerprinting was performed using the CAMAG Application System (Muttenz, Switzerland) as follows. WEHE and β-D-(1,3)-(1,6)-glucan were dissolved in 90% HPLC-grade methanol and applied to a pre-washed silica gel 60 F254 HPTLC plate (10×10 cm, 0.2 mm thick silica gel, Merck, Darmstadt, Germany) with an automated applicator (Linomat IV, CAMAG, Muttenz, Switzerland). The samples were then separated (migration distance: 60 mm) using HPLC-grade n-butanol/methanol/water (50:25:20). Thereafter, glucose-specific staining with aniline-diphenylamine-phosphoric acid or protein-specific staining with ninhydrin reagent was separately performed. Because the β-D-(1,3)-(1,6)-glucan preparation used in our experiment was likely to have proteins, bands with the same Rf value in β-D-(1,3)-(1,6)-glucan were stained with both reagents. The developed plate was visualized at 254 nm using a Reprostar 3 Digital Camera System (CAMAG; Figure 1A–1D).
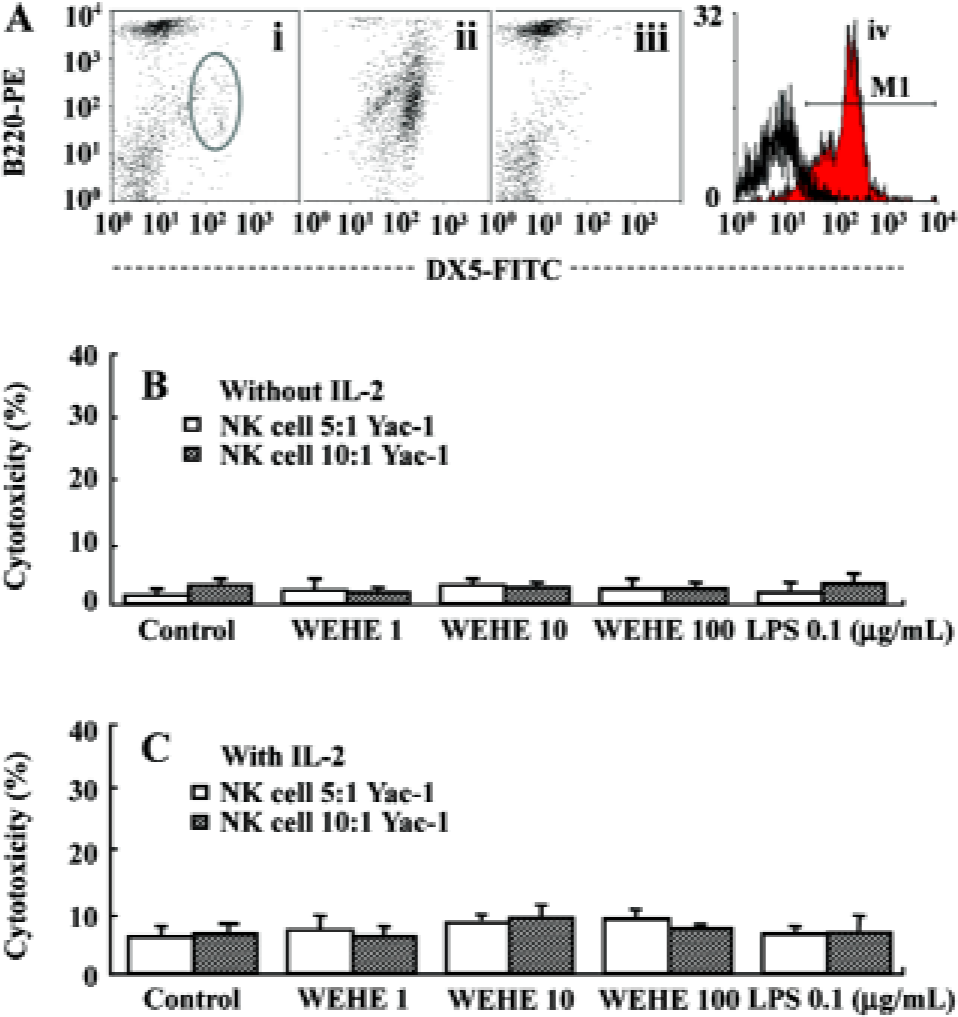
Preparation of splenocytes and purified NK cells, and fluorescence-activated cell sorter analysis The spleens were removed aseptically from BALB/c mice and homogenized with an iron mesh and syringe plunge. The red blood cells were lysed by adding lysis buffer (0.15 mol/L ammomium chloride) to the cell pellet and washed with phosphate buffered saline (PBS). The single-cell suspension was used for NK cell isolation or cultured in RPMI-1640 medium (JBI, Daegu, Korea) containing 10% fetal bovine serum (FBS, JBI, Korea) without antibiotics for use in other assays. NK cell isolation was performed by magnetic cell separation (MACS) following the manufacturer’s instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, the splenocytes were labeled with CD49B (DX5) microbeads and then loaded onto a MACS column to capture the NK cells. The magnetically-retained NK cells were eluted as a positively-selected cell fraction. The NK cells and NK cell-depleted splenocytes were then used for 51Cr release assays. To evaluate the efficacy of the NK cell selection, fluorescence-activated cell sorter (FACS) analysis was performed with anti-CD3-PE, B220-PE, and anti-DX5 FITC antibody labeling (BD Pharmingen, San Diego, CA, USA).
Culture of Yac-1 cells and radio-labeling Yac-1 cells were cultured in RPMI-1640 medium supplemented with 10% FBS without antibiotics at 37 oC in a humidified incubator with 5% CO2. To test for NK cell-derived cytolytic activity, 4×106 Yac-1 cells were labeled with 100 µCi 51Cr by incubation for 2 h at 37 oC in a humidified incubator set with 5% CO2. After washing twice with RPMI-1640 medium containing 10% FBS, the cells were resuspended in 10 mL RPMI- 1640 medium supplemented with 10% FBS for use as target cells.
51Cr release assay with splenocytes or NK cells The 51Cr release assay was performed as previously described with slight modifications[25]. Briefly, the total splenocytes, isolated NK cells, or NK cell-depleted splenocytes were prepared in RPMI-1640 medium as effector cells. Aliquots (100 µL) of each cell suspension were plated onto round-bottom 96-well plates (3 wells per group), with 50 µL WEHE at various concentrations (0, 1, 10, or 100 mg/L) or LPS (0.1 mg/L), with or without IL-2 (300 U/mL), and incubated for 20 h at 37 oC in a humidified incubator with 5% CO2. Thereafter, 50 µL target cells (1×104 cells) were mixed with the effector cells (5×105 or 1×106 spleen cells or NK cell-depleted splenocytes, and 5×104 or 1×105 isolated NK cells) and incubated for an additional 4 h. The maximum-release groups were induced by adding 50 μL 2% NP-40, and the spontaneous-release groups were induced by adding 150 μL complete medium. Gamma irradiation from each well was then assessed using a scintillation counter (Packard Instruments, Meriden, CT, USA). Cytotoxic activity was defined as the percentage of specific 51Cr released using the following equation:
Specific lysis (%)=
(RAexperimental-RAspontaneous)/(RAmaximal-RAspontaneous)×100.
RA =radioactivity
In addition to the above procedure, 2000 pg/mL anti-IL-12 (p40) neutralizing antibody was added to the splenocytes to confirm the effect of WEHE on IL-12-mediated NK cell activation.
RT-PCR for the analysis of IL-12 The total RNA was extracted from splenocytes treated for 12 h with various concentrations of WEHE (0, 1, 10, or 100 mg/L) or LPS (0.1 mg/L) using Trizol (Invitrogen, Carlsbad, CA, USA) and RNeasy columns (Qiagen, Valencia, CA, USA). cDNA was synthesized using 10 pmol oligo dT and 10 pmol random hexamer (Bioneer, Daejeon, Korea). After the cDNA synthesis, RT-PCR was performed using the following primers (forward and reverse, respectively). β-actin: 5'-GTGGGGCGCCCCAGGCACCA-3' and 5'-CTCCTTAATGTCACGCACGATTTC-3'; IL-12 (p40): 5'-TCA GGG GAA CTG CTA CTG CT-3' and 5'-TGA CAC GCC TGA AGA AGATG-3'. The reactions were performed with 0.2 μL Go Taq DNA polymerase (5 U/mL; Promera, Madison, Wisconsin, USA), 1 μL 10 pmol primer pairs, 3 μL 10 mmol dNTP, 6 μL 5x reaction buffer, 19.8 μL distilled water, and 1 μL cDNA. PCR was performed for 27 and 40 amplification cycles for β-actin and IL-12 (p40), respectively, under the following conditions: initial denaturation at 95 oC for 5 min, denaturation for each cycle at 95 oC for 1 min, annealing at 58 oC for 40 s, and elongation at 72 oC for 40 s.
ELISA analysis for IL-12 and IFN-gamma The splenocytes (3×107 cells) were plated on 24-well plates and pre-cultured for 4 h before being incubated with various concentrations of WEHE (0, 1, 10, or 100 mg/L) or LPS (0.1 mg/L) for 12 or 24 h in RPMI-1640 medium containing 10% FBS. The cell-free supernatant was collected and used for measuring the concentration of released IL-12 (p40) and IFN-gamma using an ELISA assay kit according to the manufacturer’s instructions (BD Biosciences, San Jose, CA, USA). Briefly, a 96-well microplate was coated with a capture antibody by incubating overnight at room temperature, then washed, blocked, and rewashed. The samples and standards were added to the plate and incubated for 2 h at room temperature. After adding streptavidin-horseradish peroxidase and mixing the substrate solution, the plate was read at 450 nm and 560 nm (ie, the reference wavelengths) using a microplate reader (Molecular Devices, Union City, CA, USA).
Results
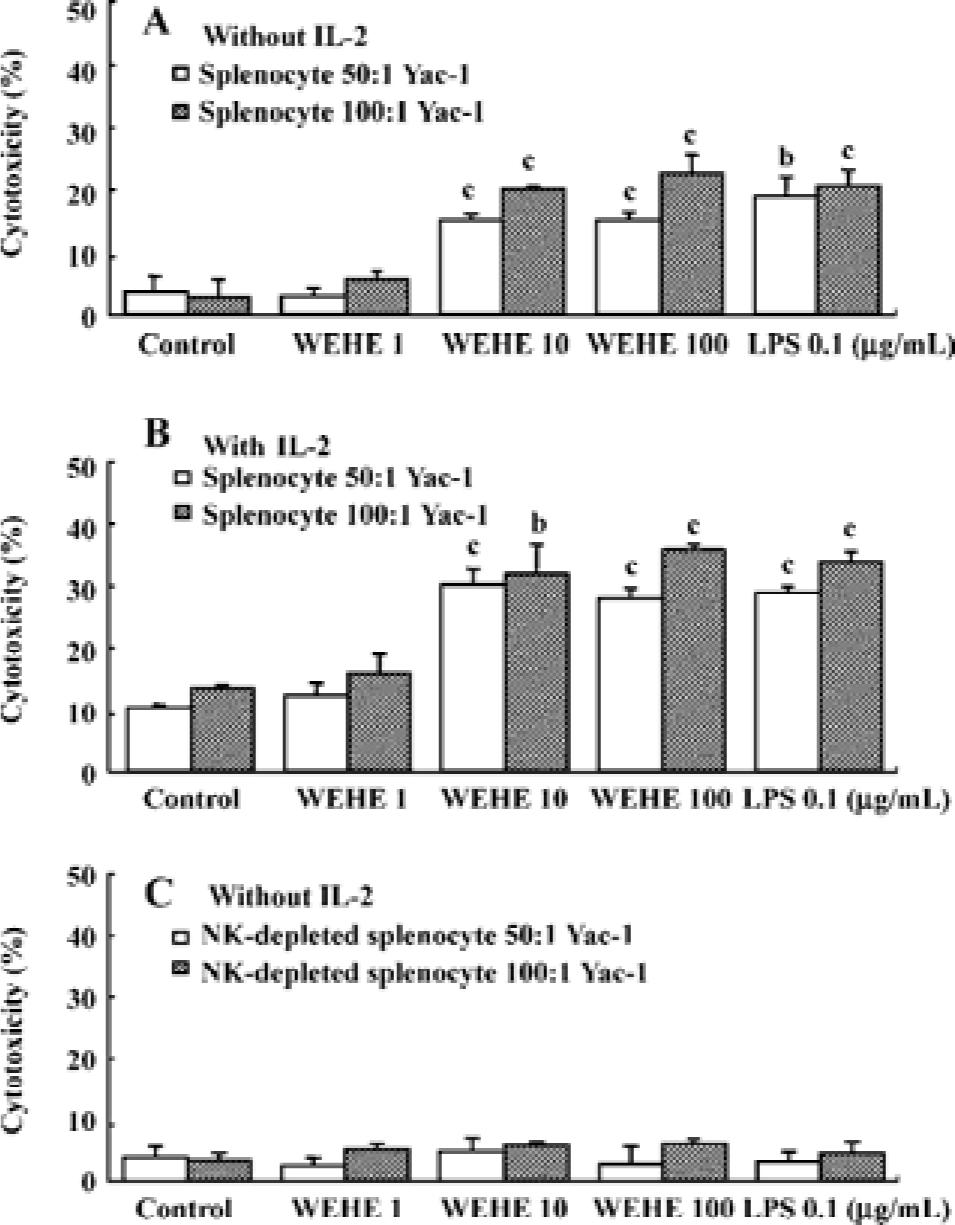
WEHE activates NK cells among splenocytes to lyse Yac-1 cells Yac-1, a NK cell-sensitive target cell line[26], was labeled with 51Cr to examine the effect of WEHE on NK cell cytotoxicity using BALB/c splenocytes. The splenocytes treated for 20 h with 1, 10, or 100 mg/L WEHE or 0.1 mg/L LPS as a positive control were co-cultured with 51Cr-labeled Yac-1 cells for 4 h. Upon exposure of the cells to WEHE, the cytolytic activity of the splenocytes was significantly increased in a dose-dependent manner (P<0.01, Figure 2A). A synergic effect was observed following co-treatment with WEHE and IL-2 as expected (P<0.05 or 0.01, Figure 2B). We then investigated whether the WEHE-driven cytolytic effect was caused by NK cells and not by other cellular components within the spleen. Thus, we repeated the assay with NK cell-depleted splenocytes, and found no enhancement of cytotoxicity following treatment with WEHE (Figure 2C). Those results showed that WEHE-activated NK cells among the splenocytes were responsible for lysis of the Yac-1 cells.
WEHE indirectly activates NK cells to kill Yac-1 cells Since WEHE stimulated splenocyte-derived NK cells to lyse Yac-1 cells, we next examined whether WEHE could directly activate NK cells using NK cells purified from splenocytes by MACS (Figure 3A). Interestingly, regardless of whether the NK cells were treated with WEHE alone or in combination with IL-2, no enhancement of NK cell cytolytic activity was observed (Figure 3B, 3C). This result indicated that WEHE activated NK cells indirectly via the induction of other immuno-mediators or cellular components.
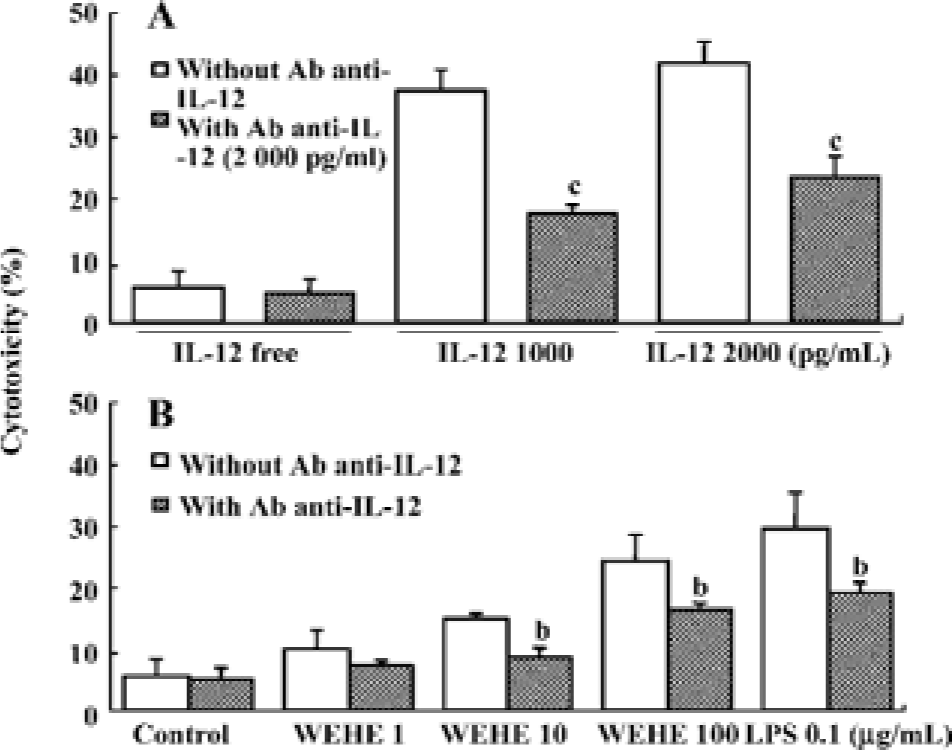
Treatment with anti-IL-12 antibody reduces WEHE-induced cytolytic activity towards Yac-1 cells The above results led us to hypothesize that WEHE generates the cytolytic activity of NK cells by inducing IL-12 production, which is the main activator of NK cells[27]. We found that IL-12-treated splenocytes were strongly activated to lyse Yac-1 cells, but this activity was abolished by co-treatment with a neutralizing antibody against IL-12 (P<0.01, Figure 4A). We also observed a significant decrease of WEHE-derived cytolytic activity in splenocytes by treatment with an anti-IL-12 antibody as expected (P<0.05, Figure 4B). These results strongly suggest the involvement of IL-12 in the WEHE-derived augmentation of NK cell activity.
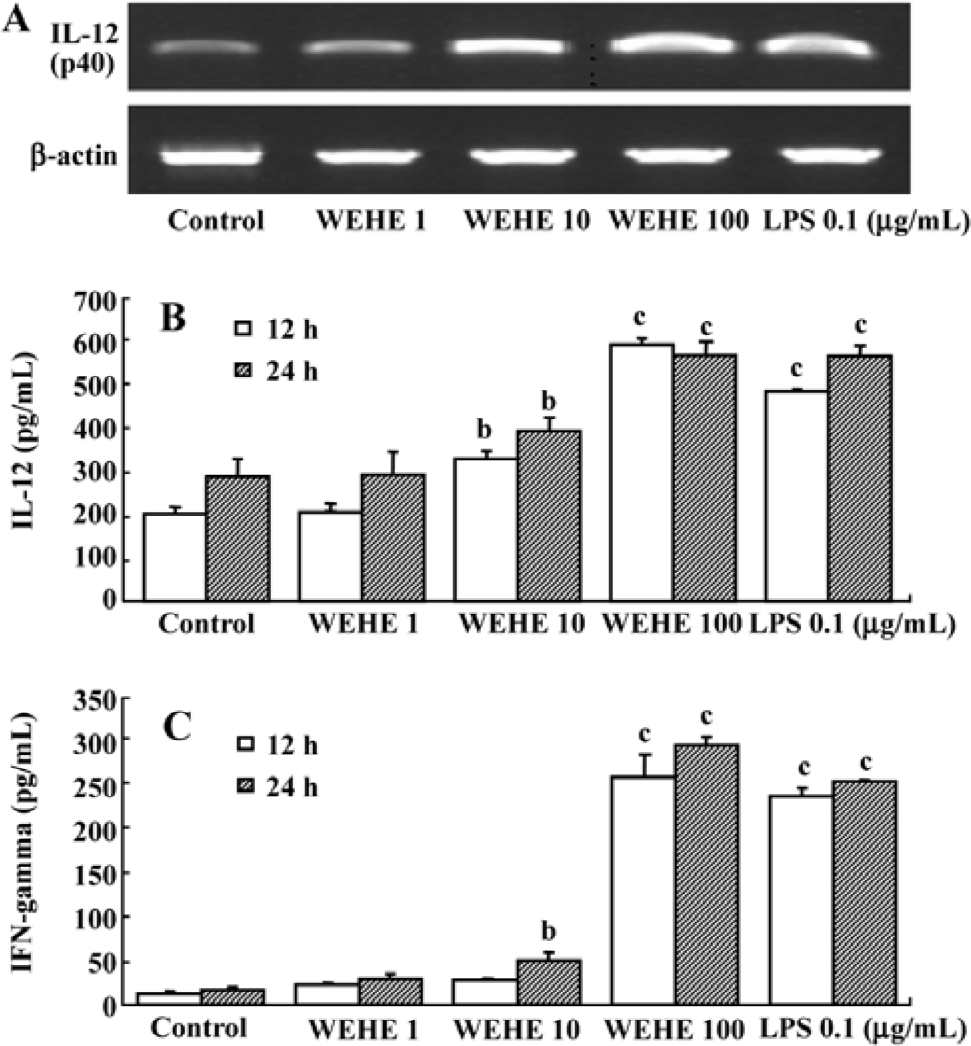
WEHE induces the transcription and translation of IL-12 and IFN-gamma in splenocytes Based on the above results indicating that IL-12 mediates the activation of NK cells by WEHE, we examined the changes in the expression of IL-12 (p40) and protein production in splenocytes treated with WEHE for 12 h. In addition, we measured the production of IFN-gamma in WEHE-treated splenocytes. WEHE treatment increased the expression of the IL-12 (p40) in splenocytes in a dose-dependent manner (Figure 5A). In addition, the production of the IL-12 (p40) and IFN-gamma proteins increased in a dose-dependent manner at 12 and 24 h (P<0.05 and 0.01, respectively, Figure 5B, 5C). No increase in IL-12 p70 production was observed under the same experimental condition (data not shown).
Discussion
NK cells are an important part of the innate immune system and efficiently eliminate cancer cells in the body. Thus, the development of NK cell-based immunomodulating agents with minimal side effects could be very important for treating cancer patients[28–30]. Here, we report that WEHE enhances NK cell activity in mouse splenocytes. In addition, WEHE induces the expression of IL-12, which efficiently activates the cytolytic ability of NK cells.
The increase in NK cell cytolytic activity appears to be an indirect effect of WEHE. Indeed, isolated NK cells treated with WEHE do not exhibit increased cytolytic activities towards Yac-1 cells, although a significant increase in NK cell activity by WEHE was observed in splenocytes containing mixed immune cells. It is possible that mediators positively influence NK cells. For example, the cytokine IL-12 may mediate the WEHE-derived activation of NK cells, since IL-12 remarkably increased the cytotoxic activities of NK cells. In addition, when WEHE plus an anti-IL-12 neutralizing antibody were co-administered to splenocytes, Yac-1 cell lysis was significantly attenuated. Moreover, IFN-gamma, whose expression is strongly induced by IL-12, was highly expressed in splenocytes cultured with WEHE. These results support previous data showing that IL-12 and IFN-gamma are important anticancer cytokines with anti-angiogenic and antimetastatic effects[31–34].
Although we found that IL-12 was involved in the activation of NK cells, we did not identify which cell types among the splenocytes preferentially respond to WEHE. Spleno-cytes include various cell types, including B cells, T cells, macrophages, NK cells, and dendritic cells. Previous studies have shown that IL-12 is largely produced by activated antigen presenting cells, such as dendritic cells, B cells, and macrophages[35]. Thus, future studies are needed to identify which cells respond to WEHE, leading to the production of cytokines, including IL-12, that subsequently enhance NK cell activity. It remains to be clarified how WEHE controls the expression of IL-12. Even though IL-12 is comprised with two distinct subunits, p35 and p40, we here verified the induction of IL-12 as only p40 at the gene and protein levels. So, further study should be performed for the expression of p35 and p70).
Despite these unresolved matters, the enhanced NK cell cytotoxicity and production of IL-12 and IFN-gamma suggest that WEHE may be a valuable immunomodulating agent for cancer patients. We previously reported that approximately 28% (w/w) of WEHE was composed of polysac-charides, mainly β-glucan, that participate in the induction of NO and IL-1-beta expression via increased NF-κB binding activity in rat macrophages and RAW 264.7 cells[22,23]. Similarly, several studies have shown that mushroom-derived fractions or polysaccharides activate NK cell function and cytokine production[36,37]. One group reported an increase in the activity and number of NK cells, including a high IFN-gamma plasma concentration following a 12-week treatment with Gano-derma lucidum polysaccharide extract in advanced cancer patients[38].
Taken together, the current data indicate that WEHE has an inductive effect on splenocyte-derived NK cell activation leading to cytolysis of Yac-1 cells. We also verified that the underlying mechanism of the immunomodulatory effect of WEHE on splenocytes involves the stimulation of IL-12 production. This study enhances our understanding of the cancer-related biological properties of H erinaceum and supports its clinical applications as an immunomodulator.
References
- Hayakawa Y, Smyth MJ. Innate immune recognition and suppression of tumors. Adv Cancer Res 2006;95:293-322.
- Dewan MZ, Terunuma H, Ahmed S, Ohba K, Takada M, Tanaka Y, et al. Natural killer cells in breast cancer cell growth and metastasis in SCID mice. Biomed Pharmacother 2005;59:S375-9.
- Waller EK. Cellular immunotherapy and cancer. Semin Oncol 2004;31:87-90.
- Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer 2002;2:850-61.
- Takaki R, Hayakawa Y, Nelson A, Sivakumar PV, Hughes S, Smyth MJ, et al. IL-21 enhances tumor rejection through a NKG2D-dependent mechanism. J Immunol 2005;175:2167-73.
- Liebau C, Merk H, Schmidt S, Roesel C, Karreman C, Prisack JB, et al. Interleukin-12 and interleukin-18 change ICAM-I expres-sion, and enhance natural killer cell mediated cytolysis of human osteosarcoma cells. Cytokines Cell Mol Ther 2002;7:135-42.
- Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol 2001;19:197-223.
- Hayakawa Y, Takeda K, Yagita H, Smyth MJ, Van Kaer L, Okumura K, et al. IFN-gamma-mediated inhibition of tumor angiogenesis by natural killer T-cell ligand, alpha-galactosylceramide. Blood 2002;100:1728-33.
- Boyiadzis M, Foon KA. Natural killer cells: from bench to cancer therapy. Expert Opin Biol Ther 2006;6:967-70.
- Sentman CL, Barber MA, Barber A, Zhang T. NK cell receptors as tools in cancer immunotherapy. Adv Cancer Res 2006;95:249-52.
- Guven H, Gilljam M, Chambers BJ, Ljunggren HG, Christensson B, Kimby E, et al. Expansion of natural killer (NK) and natural killer-like T (NKT)-cell populations derived from patients with B-chronic lymphocytic leukemia (B-CLL): a potential source for cellular immunotherapy. Leukemia 2003;17:1973-80.
- Liou CJ, Huang WC, Tseng J. Short-term oral administration of ginseng extract induces type-1 cytokine production. Immuno-pharmacol Immunotoxicol 2006;28:227-40.
- Brousseau M, Miller SC. Enhancement of natural killer cells and increased survival of aging mice fed daily Echinacea root extract from youth. Biogerontology 2005;6:157-63.
- Li Q, Nakadai A, Matsushima H, Miyazaki Y, Krensky AM, Kawada T, et al. Phytoncides (wood essential oils) induce human natural killer cell activity. Immunopharmacol Immunotoxicol 2006;28:319-33.
- Ishikawa H, Saeki T, Otani T, Suzuki T, Shimozuma K, Nishino H, et al. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J Nutr 2006;136:S816-20.
- Kodama N, Asakawa A, Inui A, Masuda Y, Nanba H. Enhancement of cytotoxicity of NK cells by D-Fraction, a polysaccharide from Grifola frondosa. Oncol Rep 2005;13:497-502.
- Sarangi I, Ghosh D, Bhutia SK, Mallick SK, Maiti TK. Anti-tumor and immunomodulating effects of Pleurotus ostreatus mycelia-derived proteoglycans. Int Immunopharmacol 2006;6:1287-97.
- Kim GY, Lee JY, Lee JO, Ryu CH, Choi BT, Jeong YK, et al. Partial characterization and immunostimulatory effect of a novel polysaccharide-protein complex extracted from Phellinus linteus. Biosci Biotechnol Biochem 2006;70:1218-26.
- Jia LM, Liu L, Dong Q, Fang JN. Structural investigation of a novel rhamnoglucogalactan isolated from the fruiting bodies of the fungus Hericium erinaceus. Carbohydr Res 2004;339:2667-71.
- Mizuno T, Wasa T, Ito H, Suzuki C, Ukai N. Antitumor-active polysaccharides isolated from the fruiting body of Hericium erinaceum, an edible and medicinal mushroom called yamabushitake or houtou. Biosci Biotechnol Biochem 1992;56:347-8.
- Stadler M, Mayer A, Anke H, Sterner O. Fatty acids and other compounds with nematicidal activity from cultures of Basidiomycetes. Planta Med 1994;60:128-32.
- Son CG, Shin JW, Cho JH, Cho CK, Yun CH, Chung W, et al. Macrophage activation and nitric oxide production by water soluble components of Hericium erinaceum. Int Immunopharmacol 2006;6:1363-9.
- Son CG, Shin JW, Cho JH, Cho CK, Yun CH, Han SH. Induction of murine interleukin-1 beta expression by water-soluble components from Hericium erinaceum. Acta Pharmacol Sin 2006;27:1058-64.
- Association of Analytical Communities (AOAC). Official methods of analysis of the Association of Official Analytical Chemists. 15th ed. Washington DC: Association of Official Analytical Chemists; 1990.
- Langhans B, Ahrendt M, Nattermann J, Sauerbruch T, Spengler U. Comparative study of NK cell-mediated cytotoxicity using radioactive and flow cytometric cytotoxicity assays. J Immunol Methods 2005;306:161-8.
- Piontek GE, Taniguchi K, Ljunggren HG, Gronberg A, Kiessling R, Klein G, et al. YAC-1 MHC class I variants reveal an association between decreased NK sensitivity and increased H-2 expression after interferon treatment or in vivo passage. J Immunol 1985;135:4281-8.
- Miller G, Lahrs S, Dematteo RP. Overexpression of interleukin-12 enables dendritic cells to activate NK cells and confer systemic antitumor immunity. FASEB J 2003;17:728-30.
- Ahn WS, Kim DJ, Chae GT, Lee JM, Bae SM, Sin JI, et al. Natural killer cell activity and quality of life were improved by consumption of a mushroom extract, Agaricus blazei Murill Kyowa, in gynecological cancer patients undergoing chemo-therapy. Int J Gynecol Cancer 2004;14:589-94.
- Jensen GS, Hart AN. Immunomodulation by SanPharma fungal metabolic products. J Altern Complement Med 2006;12:409-16.
- Farag SS, VanDeusen JB, Fehniger TA, Caligiuri MA. Biology and clinical impact of human natural killer cells. Int J Hematol 2003;78:7-17.
- Siddiqui F, Ehrhart EJ, Charles B, Chubb L, Li CY, Zhang X, et al. Anti-angiogenic effects of interleukin-12 delivered by a novel hyperthermia induced gene construct. Int J Hyperthermia 2006;22:587-606.
- Shao X, Liu C. Influence of IFN-alpha and IFN-gamma on lymphangiogenesis. J Interferon Cytokine Res 2006;26:568-74.
- Pulaski BA, Smyth MJ, Ostrand-Rosenberg S. Interferon-gamma-dependent phagocytic cells are a critical component of innate immunity against metastatic mammary carcinoma. Cancer Res 2002;62:4406-12.
- Rodrigues EG, Garofalo AS, Travassos LR. Endogenous accumulation of IFN-gamma in IFN-gamma-R(-/-) mice increases resistance to B16F10-Nex2 murine melanoma: a model for direct IFN-gamma anti-tumor cytotoxicity in vitro and in vivo. Cytokines Cell Mol Ther 2002;7:107-16.
- Carra G, Gerosa F, Trinchieri G. Biosynthesis and posttranslational regulation of human IL-12. J Immunol 2000;164:4752-61.
- Zhong M, Tai A, Yamamoto I. In vitro augmentation of natural killer activity and interferon-gamma production in murine spleen cells with Agaricus blazei fruiting body fractions. Biosci Biotechnol Biochem 2005;69:2466-9.
- Kodama N, Harada N, Nanba H. A polysaccharide, extract from Grifola frondosa, induces Th-1 dominant responses in carcinoma-bearing BALB/c mice. Jpn J Pharmacol 2002;90:357-60.
- Gao Y, Zhou S, Jiang W, Huang M, Dai X. Effects of ganopoly (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol Inves 2003;32:201-15.
