Neurophysiological basis of penile erection1
Introduction
Penile erection is a neurovascular event modulated by psychological and hormonal factors. This brief review will describe the functional response for erection with special emphasis on neural components. The topic of erectile dysfunction will be considered and finally, the recent advances in the treatment of erectile dysfunction will be discussed.
Physiology of penile erection Penile erection involves a complex interaction between the central nervous system and local factors. The penis is innervated by autonomic (sympathetic and parasympathetic nerves) and somatic nerve fibers. Overall, erection is a neurovascular event modulated by psychological and hormonal factors. Upon sexual stimulation, neurotransmitters are released from the cavernous nerve terminals and also vasoactive relaxing factors from the endothelial cells of the penis, which relax arteries and arterioles supplying the erectile tissue, increasing the penile blood flow. Concomitantly, relaxation of the trabecular smooth muscle increases the compliance of the sinusoids, resulting in an engorgement of the penis with blood. Therefore, penile erection takes place when both dilation of the penile arteries and relaxation of the erectile tissue occur. Because the erectile tissue is surrounded by the tunica albuginea, a tissue that does not distend easily, the increased blood flow to the penis increases not only the penile volume but also intrapenile pressure. This distension causes mechanical compression of the emissary veins, which impedes their ability to drain blood and thereby results in penile rigidity. Detumescence is the result of a cessation of neurotransmitter release, the breakdown of second messengers or sympathetic discharge during ejaculation. Contraction of the trabecular smooth muscle restores the venous outflow, the trapped blood is expelled, and flaccidity returns[1].
Peripheral regulation of penile erection The nerves and endothelium of sinusoids and vessels in the penis produce and release transmitters and modulators, which interact in their control of the contractile state of the penile smooth muscles. The different structures of the penis are functionally regulated by efferent sympathetic and parasympathetic nerves, and the major neurotransmitters in postganglionic fibers are norepinephrine and acetyl-choline, respectively. Sympathetic input is antierectile, whereas parasympathetic and somatic input are proerectile. Both sympathetic and parasympathetic fibers reach the pelvic or inferior hypogastric plexus where autonomic input to the penis is integrated; the cavernous nerves originate from this plexus, and innervate the helicine arteries and erectile tissue. Intracavernous nerves are encased in fibrous tissue, which prevents their compression during an erection. The dorsal penile nerves, branches of the pudendal nerves, and the ilioinguinal nerve also innervate the penis. These nerves provide sensory input from the glans penis and skin, and penile root[2].
About a decade ago, several investigators provided evidence for functional roles of nonadrenergic noncholinergic (NANC) inhibitory and excitatory nerves, containing transmitters and transmitter/modulator-generating enzymes, such as nitric oxide synthase (NOS) and heme oxygenases (HO). NANC transmitters/modulators may be found in adrenergic and cholinergic nerves[3], which should make it more meaningful to define nerve populations based on their transmitter content. Although various polypeptides have been regarded as inhibitory neurotransmitters[3-5], the discovery that nitric oxide (NO) functions as a mediator synthesized in and released from the vascular endothelium[6,7] and as a neurotransmitter in inhibitory nerves innervating the penis represented a breakthrough in the comprehension of the neurophysiological basis of erection. Figure 1 demonstrates the experimental protocols for establishing the role of NO as a neurotransmitter in the erectile response of the rat penis.
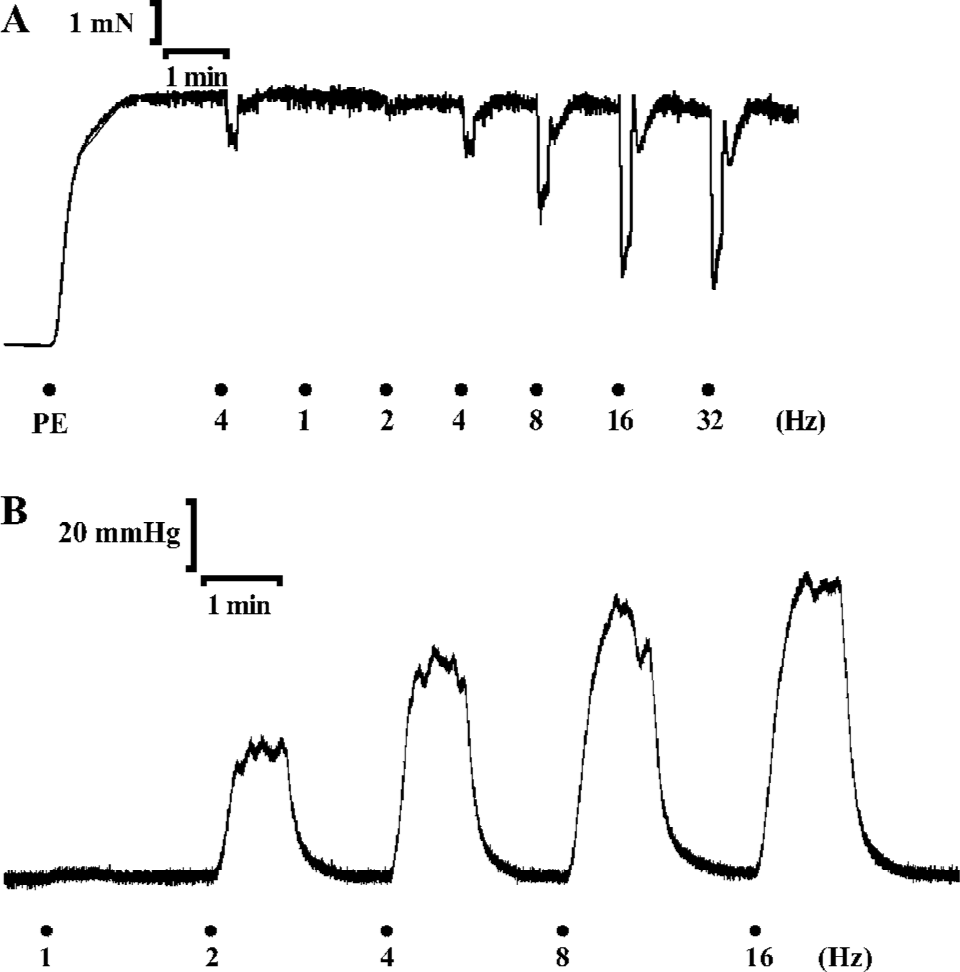
Erectile function and nitric oxide
Synthesis of NO and the consequences of NO binding to soluble guanylyl cyclase is essential for the erectile process. Identification of NO to be a neurotransmitter has been achieved by the use of NOS inhibitors in the corpus cavernosum of the penis[8]. NO, an inorganic and labile molecule, is liberated immediately upon synthesis by neuronal NOS (nNOS) from substrate L-arginine. To date, it is widely accepted that NO is the main neurotransmitter mediating penile erection, which is released during NANC neuro-transmission. Upon its release, NO diffuses locally into adjacent smooth muscle cells of the corpus cavernosum and binds with its physiologic receptor, soluble guanylyl cyclase[9]. The enzyme becomes activated following this interaction whereupon the enzyme catalyzes the conversion of guanosine triphosphate (GTP) to 3',5'-cyclic guanosine monophosphate (cGMP). This cyclic nucleotide then serves as a second-messenger function by activating protein kinase G, alternatively known as cGMP-dependent protein kinase I (cGKI), which in turn exerts actions involving ion channels and contractile regulatory proteins that regulate the contractile state of corporal smooth muscle. The consequence is the decay in cytosolic calcium concentration and relaxation of the smooth muscle, resulting in dilation of arterial vessels and increased blood flow into the sinuses of the corpora cavernosa[1,10]. Thus, at the onset of sexual stimulation, neuronal NO induced by neuronal depolarization and endothelial NO largely generated in response to shear forces brought on by increased blood flow in the penis serve, respectively, as a neurotransmitter initiating the erectile process and as a paracrine factor sustaining the full physiologic response. On the other hand, phosphodiesterase-5 (PDE5) operates in this signal transduction pathway to restrain erectile effects. This enzyme is predominantly expressed in the corpus cavernosum[11] and functions as a cGMP-specific phosphodiesterase, which catalyzes the hydrolysis of cGMP to GMP[12,13]. Accordingly, in the penis, the enzyme controls cGMP accumulation caused by NO signaling and consequently limits its relaxant actions.
Erectile dysfunction
Erectile dysfunction (ED) is defined as the persistent inability to achieve or maintain an erection sufficient for satisfactory sexual performance[14]. ED is highly prevalent and by current estimates, 30 million men in the US and 150 million men worldwide are affected[15] and occurs in 19%−64% of men aged 40–80 years, both in developing and industrialized countries. Emotional, physical, and medical factors contribute to ED, and this condition may also be a symptom of various chronic diseases. ED may affect total health, rela-tionships, and overall quality of life[16,17]. Organic causes are now understood to constitute more than 80% of clinical presentations. Associations include diabetes mellitus, cardiovascular disease, hyperlipidemia, cigarette smoking and obesity, indicating their significance as a public health problem. Furthermore, the disorder is correlated with anxiety, depression, interpersonal relationship difficulties and even violence[18].
The exact etiological mechanisms responsible for abnormal erectile response have yet to be determined. However, organic and psychogenic factors may cause alterations in the NO/cGMP pathway and impair smooth muscle relaxation and/or increase smooth muscle contraction, thereby resulting in ED. Ultimately, the treatment of ED has been revolutionized from only surgical options (penile prostheses or revascularization) to intracavernosal and intraurethral administered agents [eg, prostaglandin E1 (PGE1), papaverine, phentolamine] that paved the way to an effective oral therapy such as PDE5 inhibitors. The clinical efficacy of oral agents such as apomorphine, phentolamine, sildenafil, tadalafil, and vardenafil represent the beginnings of noninvasive pharmacological treatment for ED.
It is widely known that ED is associated with diseases reported to be related with decreased NO bioavailability such as arterial hypertension, hypercholesterolemia and diabetes. However, a recent study showed that ED appears in spontaneously hypertensive rats before they become hypertensive, suggesting that ED might be a marker for hypertension[19]. Also, it was recently demonstrated an impairment in both endothelium-dependent and -independent dilation in patients with ED, who did not present diseases such as coronary artery disease or diabetes mellitus. It was suggested that ED is associated with an abnormal function in the NO-cGMP pathway even in the absence of any apparent cardiovascular or metabolic disease[20]. Besides its direct effects in the cavernosal smooth muscle, NO also contributes to the maintenance of the erectile state by inhibiting contractile mechanisms involving noradrenaline release, reactive oxygen species (ROS) formation and Rho-kinase activity, thus favoring the corpus cavernosum relaxation.
Treatment of erectile dysfunction
In the last decade, even with the introduction of orally-administrated PDE5 inhibitors, the search for new drugs for the treatment of erectile dysfunction has been extensive. Since the discovery of NO as the main neurotransmitter mediating penile erection, several studies regarding the role of NO/cGMP pathway in the erectile function have been performed. Both the endothelium and the NANC nerves of the corpus cavernosum serve as the source of NO, and thus, more than one isoform of NOS is involved. Several investigators have demonstrated the presence of nNOS in the cavernous nerves and their terminal endings within the corpora cavernosa, as well as in the branches of the dorsal penile nerves and nerve plexuses in the adventitia of the deep cavernous arteries[9,21–26]. In human corpus cavernosum, nNOS is present in nerve fibers innervating the cavernous body and cavernosal arteries whereas eNOS is largely found in the endothelial cells covering the cavernous spaces and helicine arteries but not in the trabecular smooth muscle cells[27]. Given the importance of NOS in the generation of NO, NOS activity and expression represents an important factor to be investigated. It is known that the activity of constitutive NOS is completely dependent on calcium, calmodulin and NADPH and addition of tetrahydrobiopterin (BH4) increases NOS activity by approximately 30%[28]. Furthermore, it was recently demonstrated that the BH4, applied systemi-cally, improves erectile function[29], suggesting that cofactors for NOS also might be important targets in the treatment of erectile dysfunction.
Similarly, several studies have shown that ROS generation decreases NO bioavailability, impairing the erectile function[30]. However, in the corpus cavernosum of streptozoto-cin-induced diabetic mice, vitamin E and sodium selenate partially reversed the endothelial dysfunction and the impairment of neurogenic relaxation[31]. Similarly, ascorbic acid also prevented the impaired relaxation of acetylcholine observed in middle aged non-diabetic and diabetic rats[32], suggesting that decreased oxidative stress increases NO bio-availability improving the erectile function. Also, physical training is shown to reduce ROS generation and to raise NOS gene expression and activity. Indeed, neurogenic relaxation elicited by electrical field stimulation as well as the relaxant response evoked by exogenous NO were increased in rats submitted to endurance training, demonstrating that physical training improves the NO/cGMP signaling pathway, and thus the erectile response[33,34]. Taken together, these data show an improvement of erectile function likely related to a decreased ROS generation.
Since the primary synthesis of cGMP, driven by NO production and release during sexual arousal, is a key to the mechanism for erection, other means to achieve an enhancement of NO responses is represented by the use of PDE5 inhibitors, such as sildenafil, vardenafil and tadalafil. These drugs target PDE5 and inhibit the hydrolysis of cGMP, thus preserving cGMP and permitting the cyclic nucleotide to activate cGKI to a greater extent than at baseline conditions such that corporal smooth muscle relaxation is enhanced. Their precise mode of action is to bind to the catalyticdomain of PDE5 blocking substrate degradation. However, the efficacy of the PDE5 inhibitor, sildenafil, in the relaxa-tion of the corpus cavernosum is decreased when NOS is blocked[35]. Recently, a novel NO-donating derivative of sildenafil, NCX 911, was developed and showed to improve the relaxation induced by carbachol and decrease the superoxide formation compared to sildenafil citrate, in the corpus cavernosum of hypercholesterolemic rabbits[36]. Also, it was demonstrated that the potency of this compound in the corpus cavernosum is not altered when the synthesis of NO is inhibited by L-NAME[35], suggesting that a combination of a NO-releasing compound with a PDE5 inhibitor, might be a more interesting tool for the treatment of ED.
Another pathway that has been associated to ED and extensively investigated is the RhoA/Rho-kinase pathway, which mediates Ca2+ sensitization in the penile circulation and maintains the penis in the flaccid state. Indeed, it was recently demonstrated that intraperitoneal administration of H-1152, a Rho-kinase inhibitor, enhanced the erectile response produced by stimulation of the cavernous nerve[37]. Futhermore, the ED observed in aged rats or in the vasculo-genic model of ED is likely associated to an increased RhoA/Rho-kinase pathway activity[38–40]. Further, it was shown that the chronic treatment with fasudil, a Rho-kinase inhibitor administrated orally, prevented the impaired erectile function by reversing the increased RhoA/Rho-kinase activity seen in vasculogenic model of ED[40]. Similarly, the impaired corpus cavernosum pressure observed in castrate model of ED, was restored by inhibiting this pathway[41]. Taken together, these data show that the RhoA/Rho-kinase pathway interferes with the NO/cGMP pathway and also might represent an important target in the treatment of the erectile dysfunction.
All of these observations indicate that, although several studies have been searching new approaches for the treatment of ED, it seems that increase in NO bioavailability and consequent improvement of the corpus cavernosum relaxation, still represents the main target for the treatment of ED.
Concluding remarks
There is a broad range of evidence indicating that NANC neurotransmission has a vital role in mediating penile erection via a NO/cGMP mechanism. Continuous advances in our understanding of the physiology of penile erection should help to elucidate further the mechanisms involved in the pathophysiology of ED, and ultimately define alternate therapeutic strategies to preserve this signaling pathway.
References
- Lue TF. Erectile dysfunction. N Engl J Med 2000;342:1802-13.
- Shabsigh R, Anastasiadis AG. Erectile dysfunction. Annu Rev Med 2003;54:153-68.
- Lundberg JM. Pharmacology of cotransmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol Rev 1996;48:113-78.
- Burnstock G. Comparative studies of purinergic nerves. J Exp Zool 1975;194:103-33.
- Owman C. Peptidergic vasodilator nerves in the peripheral circulation and in the vascular beds of the heart and brain. Blood Vessels 1990;27:73-93.
- Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 1987;84:9265-9.
- Furchgott RF. Studies on relaxation of rabbit aorta by sodium nitrite: the basis for the proposal that the acid-activatable inhibitory factor from retractor penis is inorganic nitrite and the endotheliumderived relaxing factor is nitric oxide. In: Vanhoutte PM, editor. Vasodilatation: vascular smooth muscle, peptides, autonomic nerve and endothelium. New York: Raven Press; 1988. p401–14
- Ignarro LJ, Bush PA, Buga GM, Wood KS, Fukuto JM, Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun 1990;170:843-50.
- Burnett AL. Nitric oxide in the penis: physiology and pathology. J Urol 1997;157:320-4.
- Rajfer J, Aronson WJ, Bush PA, Dorey FJ, Ignarro LJ. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med 1992;326:90-4.
- Francis SH, Turko IV, Corbin JD. Cyclic nucleotide phospho-diesterases: relating structure and function. Prog Nucleic Acid Res Mol Biol 2001;65:1-52.
- Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res 1996;8:47-52.
- Turko IV, Ballard SA, Francis SH, Corbin JD. Inhibition of cyclic GMP-binding cyclic GMP-specific phosphodiesterase (Type 5) by sildenafil and related compounds. Mol Pharmacol 1999;56:124-30.
- NIH Consensus Conference. Impotence: NIH consensus development panel on impotence. JAMA 1993;270:83-9.
- Benet AE, Melman A. The epidemiology of erectile dysfunction. Urol Clin North Am 1995;22:699-709.
- Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537-44.
- Jonler M, Moon T, Brannan W, Stone NN, Heisey D, Bruskewitz RC. The effect of age, ethnicity and geographical location on impotence and quality of life. Br J Urol 1995;75:651-5.
- Lewis RW. Definitions, classification, and epidemiology of sexual dysfunction. In: Lue TF, Basson R, Rosen R, Giuliano F, Khoury S, Montorsi F, editors. Sexual Medicine: Sexual Dysfunction in Men and Women. Health Publications: Paris; 2004. p 39–72
- Behr-Roussel D, Gorny D, Mevel K, Compagnie S, Kern P, Sivan V, Bernabe J, Bedigian MP, Alexandre L, Giuliano F. Erectile dysfunction: an early marker for hypertension? A longitudinal study in spontaneously hypertensive rats. Am J Physiol 2005;288:R276-83.
- Kaya C, Uslu Z, Karaman I. Is endothelial function impaired in erectile dysfunction patients? Int J Impot Res 2006;18:55-60.
- Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science 1992;257:401-3.
- Burnett AL, Tillman SL, Chang TS, Epstein JI, Lowenstein CJ, Bredt DS, et al. Immunohistochemical localization of nitric oxide synthase in the autonomic innervation of the human penis. J Urol 1993;150:73-6.
- Burnett AL, Nelson RJ, Calvin DC, Liu JX, Demas GE, Klein SL, et al. Nitric oxide-dependent penile erection in mice lacking neuronal nitric oxide synthase. Mol Med 1996;2:288-96.
- Alm P, Larsson B, Ekblad E, Sundler F, Andersson KE. Immunohistochemical localization of peripheral nitric oxide synthase-containing nerves using antibodies raised against synthesized C- and N-terminal fragments of a cloned enzyme from rat brain. Acta Physiol Scand 1993;148:421-9.
- Dail WG, Barba V, Leyba L, Galindo R. Neural and endothelial nitric oxide synthase activity in rat penile erectile tissue. Cell Tissue Res 1995;282:109-16.
- Hedlund P, Ny L, Alm P, Andersson KE. Cholinergic nerves in human corpus cavernosum and spongiosum contain nitric oxide synthase and heme oxygenase. J Urol 2000;164:868-75.
- Stanarius A, Uckert S, Machtens SA, Stief CG, Wolf G, Jonas U. Immunocytochemical distribution of nitric oxide synthase in the human corpus cavernosum: an electron microscopical study using the tyramide signal amplification technique. Urol Res 2001;29:168-72.
- Bush PA, Gonzalez NE, Ignarro LJ. Biosynthesis of nitric oxide and citrulline from L-arginine by constitutive nitric oxide synthase present in rabbit corpus cavernosum. Biochem Biophys Res Commun 1992;186:308-14.
- Sommer F, Klotz T, Steinritz D, Bloch W. Evaluation of tetra-hydrobiopterin (BH4) as a potential therapeutic agent to treat erectile dysfunction. Asian J Androl 2006;8:159-67.
- Jones RW, Rees RW, Minhas S, Ralph D, Persad RA, Jeremy JY. Oxygen free radicals and the penis. Expert Opin Pharmacother 2002;3:889-97.
- Gocmen C, Secilmis A, Kumcu EK, Ertug PU, Onder S, Dikmen A, et al. Effects of vitamin E and sodium selenate on neurogenic and endothelial relaxation of corpus cavernosum in the diabetic mouse. Eur J Pharmacol 2000;398:93-8.
- Gur S, Karahan ST, Ozturk B, Badilli M. Effect of ascorbic acid treatment on endothelium-dependent and neurogenic relaxation of corpus cavernosum from middle-aged non-insulin dependent diabetic rats. Int J Urol 2005;12:821-8.
- Claudino MA, Priviero FB, Teixeira CE, De Nucci G, Antunes E, Zanesco A. Improvement in relaxation response in corpus cavernosum from trained rats. Urology 2004;63:1004-8.
- Claudino MA, Priviero FB, Camargo EA, Teixeira CE, De Nucci G, Antunes E, Zanesco A. Protective effect of prior physical conditioning on relaxing response of corpus cavernosum from rats made hypertensive by nitric oxide inhibition. Int J Impot Res 2007;19:189-95.
- Kalsi JS, Ralph DJ, Thomas P, Bellringer J, Minhas S, Kell PD, Cellek S. A nitric oxide-releasing PDE5 inhibitor relaxes human corpus cavernosum in the absence of endogenous nitric oxide. J Sex Med 2005;2:53-7.
- Shukla N, Jones R, Persad R, Angelini GD, Jeremy JY. Effect of sildenafil citrate and a nitric oxide donating sildenafil derivative, NCX 911, on cavernosal relaxation and superoxide formation in hypercholesterolaemic rabbits. Eur J Pharmacol 2005;517:224-31.
- Teixeira CE, Ying Z, Webb RC. Proerectile effects of the Rho-kinase inhibitor (S)-(+)-2-methyl-1-[(4-methyl-5-isoquinolinyl)sulfonyl]homopiperazine (H-1152) in the rat penis. J Pharmacol Exp Ther 2005;315:155-62.
- Rajasekaran M, White S, Baquir A, Wilkes N. Rho-kinase inhibition improves erectile function in aging male Brown-Norway rats. J Androl 2005;26:182-8.
- Jin L, Liu T, Lagoda GA, Champion HC, Bivalacqua TJ, Burnett AL. Elevated RhoA/Rho-kinase activity in the aged rat penis: mechanism for age-associated erectile dysfunction. FASEB J 2006;20:536-8.
- Park K, Kim SW, Rhu KS, Paick JS. Chronic administration of an oral rho kinase inhibitor prevents the development of vasculogenic erectile dysfunction in a rat model. J Sex Med 2006;3:996-1003.
- Wingard CJ, Johnson JA, Holmes A, Prikosh A. Improved erectile function after Rho-kinase inhibition in a rat castrate model of erectile dysfunction. Am J Physiol 2003;284:R1572-9.
