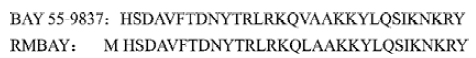A novel recombinant, VPAC2-selective agonist enhancing insulin release and glucose disposal1
Introduction
Pituitary adenylate cyclase activating polypeptide (PACAP) has a specific receptor, PAC1, and shares 2 receptors, VPAC1 and VPAC2, with vasoactive intestinal peptide (VIP)[1]. The activation of multiple receptors by PACAP or VIP has broad physiological effects on nervous, endocrine, cardiovascular, reproductive, muscular, and immune systems[2]. Different effects are mediated by different receptors; for example, VPAC2 activation enhances glucose disposal by stimulating insulin secretion, while VPAC1 activation elevates hepatic glucose output[3–6]. It is recognized that a VPAC2-selective agonist can enhance glucose disposal by stimulating insulin secretion without causing increased hepatic glucose production, thus VPAC2 selectivity has been identified as a prerequisite for potential type II diabetes treatment[4,7].
rMBAY was designed to imitate BAY 55-9837, which is a VPAC2-selective agonist developed by Bayer Corporation (USA) as a potential therapy for type II diabetes[4]. BAY 55-9837, consisting of 31 amino acid residues, is produced by chemical synthesis that is time-consuming and costly. To achieve rapid and efficient purification of the recombinant VPAC2 receptor agonist as potential treatment for type II diabetes, we fused rMBAY as a target protein to the N-terminus (Cys1) of a modified intein from Saccharomyces cerevisiae (Sce VMA intein, 454 amino acids), which in turn links to the chitin-binding domain (CBD)[8,9]. The CBD allows the binding of the fusion protein to a chitin column and the intein is capable of undergoing peptide bond cleavage at its terminus. The C-terminal residue (Asn454) of the intein was mutated to an alanine, which blocked the splicing reaction but still allowed an N-S acyl rearrangement at the intein N-terminus (Cys1) leading to the formation of a thioester linkage between the target protein and the intein. Cleavage of the thioester bond can be induced by thiol reagents, such as 1,4-dithiothreitol (DTT), β-mercaptoethanol, or cysteine. The use of DTT or β-mercaptoethanol results in the formation of a thioester bond between the thiol compound and the C-terminal residue of the target protein. This thioester is not stable and hydrolyzes to yield a free C-terminus. As a result, rMBAY is released from its affinity tag without the use of a protease and separated from its fusion partner on the same column.
BAY 55-9837 not only has an extra N-terminal methionine coded by initiator codon ATG, but also a displacement of Val17 with Leu17 (Figure 1). The preliminary bioactivity assay showed that rMBAY had an effect on insulin release and reducing the plasma glucose after intraperitoneal injection with high concentrations of glucose to the NIH mice. A novel recombinant peptide was produced effectively for it further development as a new treatment to type II diabetes.
Materials and methods
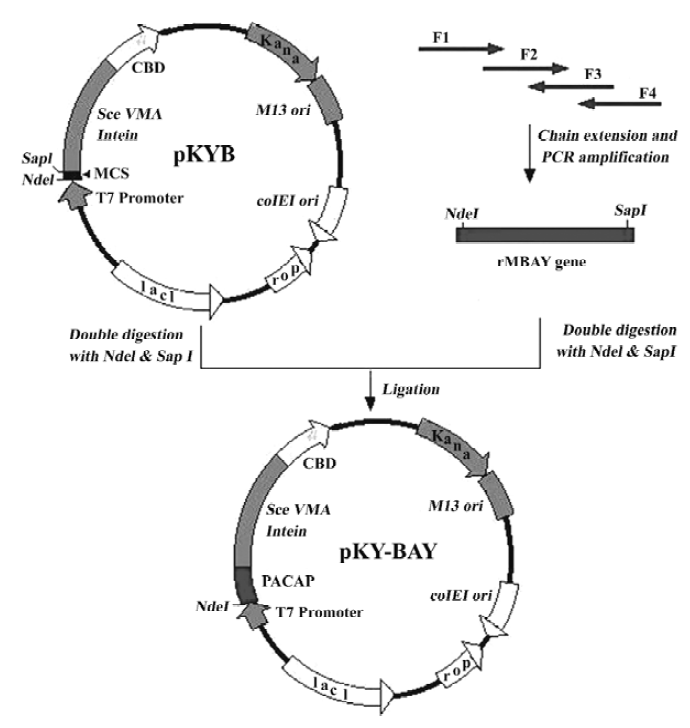
Synthesis of rMBAY gene The rMBAY gene was designed according to the bias of E coli for the codons to ensure the high expression in E coli. The gene was synthesized and amplified in 2 steps using 4 oligonucleotides primers as described[10](Figure 2).
Four primers for the synthesis of rMBAY were F1: 5'-NNNNNN CATATG CAT AGC GAT GCG GTG TTT ACC GAT AAC TAT ACC CGT CTG CGT AAA CAG -3', containing a NdeI site (underlined); F2: 5'-CTG GCG GCG AAA AAA TAT CTG CAG AGC ATT AAA AAC AAA CGT TAT-3'; F3: 5'-CGC CAG CTG TTT ACG CAG ACG GGT ATA GTT ATC GGT AAA CAC CGC ATC GCT ATG CA-3'; and F4: 5'-NNNNNN GCTCTTC C GCA ATA ACG TTT GTT TTT AAT GCT CTG CAG ATA TTT TTT CGC 3', containing a SapI site (underlined). “N” represented the protecting base.
The PCR product containing the synthesized gene was purified on a Qiagen QIAquick column (Qiagen, USA). After double digestion with NdeI and SapI (NEB, USA), the purified DNA fragment was directly ligated to a gel-purified NdeI-SapI double digested pKYB vector (NEB) to yield pKY-BAY (Figure 2). Insertion of the rMBAY gene was verified by DNA sequencing using the T7 promoter as the sequencing primer.
Fermentation of engineering strain pKY-BAY-ER2566 The pKY-BAY plasmid was transformed to the E coli strain ER2566 [fhuA2(lon) ompT lacZ::T7 gene1 galsulA11 D (mcrC-mrr) 114::IS10 R (mcr-73::miniTn10–TetS) 2R (zgb-210::Tn10–TetS) endA1 (dcm), NEB], which allowed for IPTG-regulated expression of the T7 RNA polymerase. The fermentation was carried on in a CF-5L fermenter (Biotop, Taiwan). Three hundred mL cultures were grown in an air shaker (250 rpm) at 37 oC in LB medium containing 50 mg/L of kanamycin overnight, and then inoculated into 3 L LB containing 1% glycerin in 5 L fermenter. At A600=8–8.5, IPTG was added to a final concentration of 0.4 mmol/L to induce T7 RNA polymerase-based expression. The culture was cooled to 30 oC and allowed to continue expression for 4 h. SDS-PAGE was used to identify the expression of the fusion protein.
Purification of target peptide Five grams of induced cells (wet weight) were resuspended in 60 mL of column buffer (20 mmol/L Tris-HCl, 0.5 mol/L NaCl, 1 mmol/L EDTA, pH 8.0) and then disrupted by pulse sonication. The supernatant (soluble fraction) was separated from cell debris by centrifugation at 20 000×g for 30 min and passed through a column (2.5×10 cm) packed with 20 mL chitin beads at a flow rate of 0.5 mL/min. After the supernatant were loaded on the column, the flow rate was raised to 2 mL/min and the column was thoroughly washed with more than 10 bed volume of the column buffer until the protein content of the eluate reached a minimum (A595<0.05 as measured by Bradford assay). Column buffer (60 mL) containing 100 mmol/L β-mercaptoethanol was then quickly passed through the column in order to distribute β-mercaptoethanol evenly throughout the resin and the column flow was stopped. The column was incubated at 16 oC for 24 h. Fractions containing rMBAY were obtained by eluting the column with the column buffer. Part of the fusion protein eluted with the target peptide was removed by ultrafiltration (retention molecular of 50 kDa). Protein concentrations were estimated by the Bradford method. The rMBAY was further purified by dialysis overnight at 4 oC to remove β-mercaptoethanol. SDS-PAGE and Tricine-SDS-PAGE (according to Yang et al[11]) were both used to identify the purification of the target peptide. Laser Flying Mass Spectrometry analysis of the purified peptide was performed in the Test Center at the Military Physic Academy of Science (Beijing, China).
Cell lines and membrane preparation The cells clones used were referred to as PAC1-CHO (receptor density of 3.3±0.3 pmol/mg protein), VPAC1-CHO (receptor density of 1.3±0.2 pmol/mg protein), and VPAC2-CHO (receptor density of 1.1±0.2 pmol/mg protein), as earlier described[10]. The cells were maintained in DMEM medium supplemented with 10% bovine serum albumin (BSA) and 0.8 mg/mL G418 with an atmosphere of 95% air, 5% CO2 at 37 oC. Membrane preparation and competition binding assay were performed following the protocol described by Gourlet et al[15]. The membranes were prepared from PAC1-CHO, VPAC1-CHO, and VPAC2-CHO. The cells were harvested with a rubber policeman and pelleted by low speed centrifugation. The supernatant was discarded and the cells were lysed in 1 mmol/L NaHCO3 solution and immediately frozen in liquid nitrogen. After thawing, the lysate was first centrifuged at 4 oC for 10 min at 400×g, and the supernatant was further centrifuged at 20 000×g for 10 min. The pellet resuspended in 1 mmol/L NaHCO3 was used immediately as a crude membrane prepara-tion.
Competition binding assay and cAMP accumulation assay To measure the receptor binding of rMBAY, 10 µg membrane was incubated with 0.1 nmol/[125I]PACAP38 (1813.14 Ci/mmol, Phoenix Pharmaceuticals, Mountain View, CA, USA) in the presence of increasing concentrations of rMBAY peptide, in a total volume of 100 µL of 20 mmol/L HEPES (pH 7.4), 150 mmol/L NaCl, 0.5% BSA, 2 mmol/L MgCl2, and 0.1 mg/mL bacitracin. After incubating at 37 oC for 20 min, the bound ligand was collected on GF/C filters pretreated with 0.1% polyethylenimine. The filters were washed with cold 25 mmol/L NaPO4 containing 1% BSA and counted onto a gamma counter. Nonspecific binding was defined as the residual binding in the presence of 1 mmol/L RMPACAP (a recombinant analogue of PACAP38[12]) and was always below 20% of the total binding. Each assay was performed at least 3 times.
The PAC-CHO, VPAC1-CHO, and VPAC2-CHO cells cultured in the DMEM nutrient at 37 oC were scraped off with rubber policeman and washed with PBS twice; the density of the cells was adjusted to 2×106/mL. rMBAY was added to 500 µL cell suspension, and the working concentrations of the peptide were changed from 1×10-12 mol/L to 1×10-5 mol/L. The reactions were incubated at 37 oC for 5 min and then were incubated at room temperature for 20 min after 2 volume 0.2 mol/L HCl was added. The mixture was dissociated by pipetting up and down until the suspension was homogeneous. The precipitate was removed by centrifugation at 1000×g for 10 min, and the supernatant was collected to the test tube and assayed for cAMP quantities using the enzyme immunoassay kit for cyclic AMP (Cayman Chemical Co, Ann Arbor, MI, USA) following the operating instructions.
Bioactivity assay of reducing plasma glucose in vivo Masculine NIH mice (25–30 g) fasted overnight (17 h) and were randomly divided into groups according to the weight; each group contained 10 mice. Glucose (1.8 mmol/kg) with or without peptide was intraperitoneally injected to the NIH mice. Blood was collected from the tail vein before injection and at 10 min after the injection. The plasma glucose levels were determined using One Touch Ultra Meter (Johnson, USA) and the blood sera were sent to Dongshan Hospital (Guangzhou, China) for the plasma insulin assay.
Protein analysis The concentration of proteins in the clear lysate was determined by the Bradford method using BSA as a standard. The percentage of the purity of the target proteins were estimated by analyzing Tricine-SDS-PAGE gel stained with Coomassie blue using Chemilnager V5.5 software (USA) for image collecting and analyzing. The cleavage efficiency and the binding efficiency were calculated as described[12].
Results
Construction of the recombinant plasmid pKY-BAY The synthesized human rMBAY gene was inserted into the plasmid vector pKYB by NdeI and SapI, and the site recognized by SapI disappeared in the recombinant plasmid pKY-ROM. The recombinant clone identified by PCR and DNA sequencing (Figure 3) was transformed into E coli strain, ER2566, and the engineering strain pKY-BAY-ER2566 was constru-cted. The sequencing of pKY-BAY is shown in Figure 3.
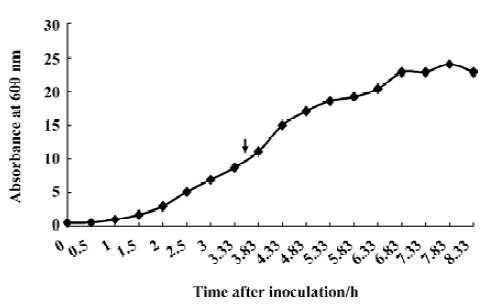
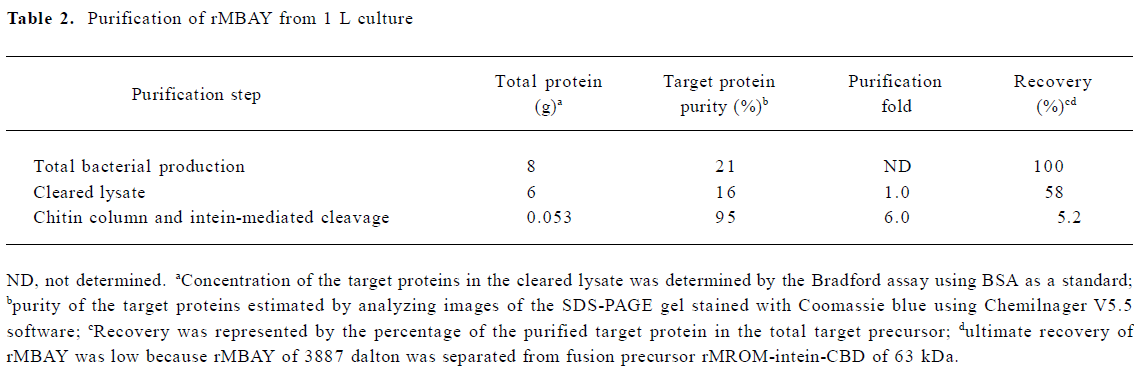
Fermentation of engineering strain pKY-BAY-ER2566 When A600 reached 8.2, IPTG was added to a final concentration of 0.4 mmol/L, and the culture was cooled to 30 oC. The maximal expression of the fusion protein reached 21% of the total bacteria protein at 4 h after inoculation and did not rise much more for a longer induction time (Figure 4, Figure 5 lanes 6 and 7). Thirty grams of cells (wet weight) was collected from 1 L culture. Approximately 58% of the total precursor accumulated as soluble form which reached 16% of the total soluble proteins in the clarified lysate (Figure 5, lane 8). The growth curve of the engineering strain pKY-BAY-ER2566 fermented in 5 L fermenter is shown in Figure 6.
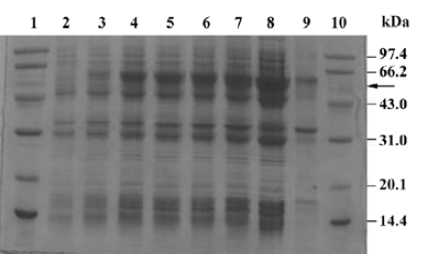
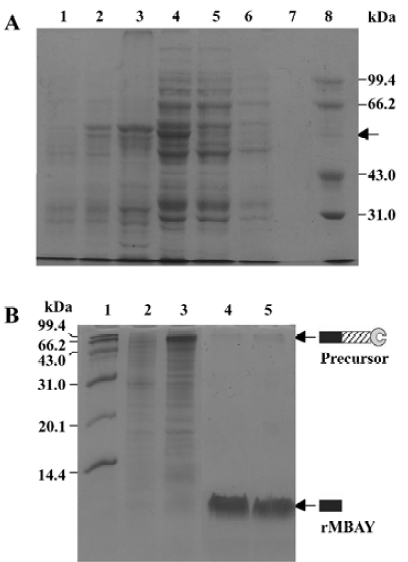
Affinity purification and cleavage of fusion protein After IPTG induction, the rMBAYintein-CBD fusion precursor accumulated as a soluble product observed in the clarified lysate and reached approximately 16% of the total soluble proteins (Figure 6A, lane 4), while a part of the fusion precursor accumulated as inclusion bodies existing in the deposit (Figure 6A, lane 3). After passing the supernatant through the chitin column, more than 98% of the fusion precursor was bound to the resin due to extremely high affinity of the CBD for chitin (Figure 6A, lanes 4 and 5). In addition, since the CBD could not be eluted from the chitin resin under no denaturing conditions, extensive washing was performed to remove most non-specifically bound contaminating proteins (Figure 6, lanes 6 and 7).
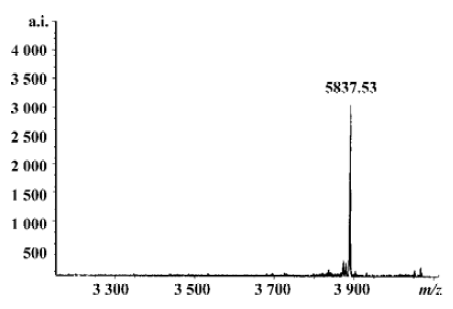
Flushing the column with β-mercaptoethanol triggered the intein-mediated cleavage reaction, which released rMBAY, whereas the intein-CBD fusion partner remained bound to the resin. After the induction of the cleavage reaction by β-mercaptoethanol at 16 oC for 24 h, the target peptide was released from the intein-CBD tag and was eluted from the column using the column buffer (Figure 6B, lanes 4 and 5). After ultrafiltration, rMBAY with purity over 95% was obtained. β-mercaptoethanol was removed by dialysis, and laser flying mass spectrometry showed the precise molecular weight of rMBAY with purity over 95% as 3887.03 (Figure 7), which was consistent with the theoretical molecular weight.
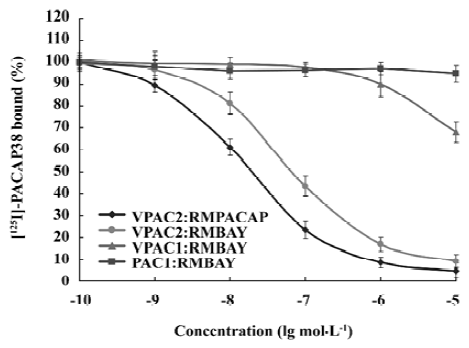
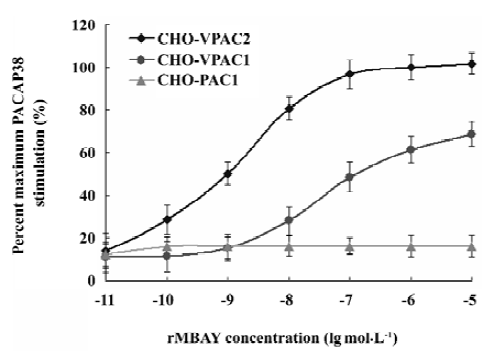
Competition binding assay of rMBAY to PACAP receptors Competition binding of [125I]PACAP38 on membranes purified from CHO cells expressing each of the 3 subtypes of human PACAP receptors identified rMBAY as a VPAC2-selective peptide (Figure 8). rMBAY competitively displaced [125I]PACAP38 from VPAC2, with a half-maximal inhibitory concentration (IC50) of 53±8 nmol/L, whereas a recombinant PACAP38 analogue[12], RMPACAP, had an IC50 of 18±5 nmol/L. The IC50 for rMBAY at human VPAC1 and was over 10 µmol/L, whereas no competition was observed up to 50 µmol/L at PAC1.
PACAP receptor activation by rMBAY The accumulation of cAMP in human PACAP receptor-transfected cells was used as an index of the agonist activity. rMBAY was a potent, full agonist of the human VPAC2, with a half-maximal stimulatory concentration (EC50) of 0.8 nmol/L. It was 250-fold less potent at human VPAC1 (EC50 of 200 nmol/L, Figure 9) and had no activity toward the human PAC1.
In vivo effects of rMBAY on insulin release and glucose disposal in fasted mice rMBAY (50 ng/kg) dramatically promoted the insulin release and decreased the level of plasma glucose after intraperitoneal injection with high concentrations of glucose (1.8 mmol/kg) in NIH mice using vehicle as control (Table 1).
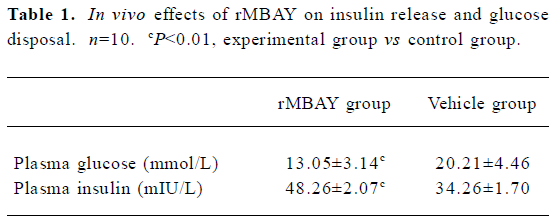
Full table
Discussion
Few VPAC2 agonists have been discovered or developed until now. Ro 25-1553 is a synthetic cyclic VIP derivative with the potency of bronchodilator first identified as a VPAC2 agonist[13–16]. The hexanoyl-VIP (C6-VIP) exhibits the high selectivity and potency for VPAC2, but recognizes different receptor domains from those recognized by Ro 25-1553[17]. BAY 55-9837 is a potent and highly-selective agonist for VPAC2 generated through site-directed mutagenesis based on sequence alignments of PACAP, VIP, and related analogs. Continuous intravenous or subcutaneous infusion of BAY 55-9837 reduces the glucose area under the curve following an intraperitoneal glucose tolerance test. BAY 55-9837 is considered as a novel therapy for type II diabetes[4].
The recombinant peptide, rMBAY, designed to imitate BAY 55-9837, was a potential VPAC2-selective agonist with corresponding activity of reducing plasma glucose. Compared with BAY 55-9837, rMBAY had an extra N-terminal Met and an amino acid residue substitute (Val17/Leu17). The preliminary bioactivity assay showed that rMBAY enhanced insulin release and glucose disposal. Although it is reported that the proper modification of the N-terminus of peptides such as acylation[16] and hexanoylation[18] helps to increase its selectivity and potency for VPAC2, it still needs to be further assayed and determined how the changes in rMBAY contribute to the selectivity and potency of rMBAY to the VPAC2 receptor and bioactivity.
A recombinant expression system had been used to produce recombinant, PACAP–derived, mutant peptide, in which the target peptide was purified as a fusion to the glutathione S-transferase and was separated from its purification tag by the cleavage of Factor Xa[7]. In this paper, we used an intein-mediated purification with an affinity chitin-binding tag (IMPACT) system from New England BioLabs (Beverly, MA, USA.) to achieve rapid purification of a VPAC2-selective agonist using affinity chromatography, but avoiding protease treatment and extra purification steps.
The expression of the fusion protein reached 21% of the total bacteria proteins and 16% of the total soluble proteins (Table 2). A part of the fusion precursor formed insoluble inclusion bodies resulting in low recovery after sonication and centrifugation. The final yield of purified rMBAY was mainly affected by the efficiency of the intein-mediated cleavage reaction. It had been demonstrated that the higher temperature and longer time of the incubation helped to improve the cleavage efficiency. We at last obtained 53 mg rMBAY from 1 L induced culture when the column was incubated at 25 oC for 24 h, and the cleavage efficiency was approximately 80% (data not shown). Because the intact fusion precursor protein and the intein-CBD tag remained bound to the chitin resin during the intein-mediated cleavage and the target peptide rMBAY elution. Furthermore, no proteases or extra protein splitting agents were needed. The rMBAY yielded with high purity (over 95% was obtained by just one-step purification in a single column).
Full table
An efficient production method for a potential VPAC2-selective agonist with the activity of decreasing the plasma glucose was established using intein-mediated purification with an affinity chitin-binding tag. Our work may pave the way for the exploitation of the recombinant peptide rMBAY as a novel therapy for type II diabetes.
References
- Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, et al. International union of pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev 1998;50:265-70.
- Gozes I, Fridkinb M, Hill JM, Brenneman DE. Pharmaceutical VIP: prospects and problems. Curr Med Chem 1999;6:1019-34.
- Inagaki N, Yoshida H, Mizuta M, Mizuno N, Fujii Y, Gonoi T, et al. Cloning and functional characterization of a third pituitary adenylate cyclase-activating polypeptide receptor subtype expressed in insulin-secreting cells. Proc Natl Acad Sci USA 1994;91:2679-83.
- Tsutsumi M, Claus TH, Liang Y, Li Y, Yang L, Zhu J, et al. A potent and highly selective VPAC2 agonist enhances glucose-induced insulin release and glucose disposal: a potential therapy for type 2 diabetes. Diabetes 2002;51:1453-60.
- Wei Y, Mojsov S. Multiple human receptors for pituitary adenylyl cyclase-activating polypeptide and vasoactive intestinal peptide are expressed in a tissue-specific manner. Ann N Y Acad Sci 1996;805:624-27.
- Yokota C, Kawai K, Ohashi S, Watanabe Y, Yamashita K. PACAP stimulates glucose output from the perfused rat liver. Peptides 1995;16:55-60.
- Yung SL, Cruz FD, Hamren S, Zhu J, Tsutsumi M, Bloom JW, et al. Generation of highly selective VPAC2 receptor agonists by high throughput mutagenesis of vasoactive intestinal peptide and pituitary adenylate cyclase-activating peptide. J Biol Chem 2003;278:10273-81.
- Chong S, Mersha FB, Comb DG, Scott ME, Landry D, Vence LM, et al. Single-column purification of free recombinant proteins using a self-cleavable affinity tag derived from a protein splicing element. Gene 1997;192:277-81.
- Chong S, Shao Y, Paulus H, Benner J, Perler FB, Xu MQ. Protein splicing involving the Saccharomyces cerevisiae VMA intein: the steps in the splicing pathway, side reactions leading to protein cleavage, and establishment of an in vitro splicing system. J Biol Chem 1996;271:22159-68.
- Yu RJ, Xie QL, Dai Y, Gao Y, Zhou TH, Hong A. Intein-mediated rapid purification and characterization of a novel recombinant agonist for VPAC2. Peptides 2006;27:1357-66.
- Yang LP, Kong XP, Yi XR. Analyzing peptides of low weights molecular with SDS-polyacrylamide gel electrophoresis. Prog Biotechnol 1998;18:19-21. Chinese.
- Yu RJ, Hong A, Dai Y, Gao Y. Intein-mediated rapid purification of recombinant human pituitary adenylate cyclase activating polypeptide. Acta Biochim Biophys Sin 2004;36:759-66.
- O’Donnell M, Garippa RJ, Rinaldi N, Selig WM, Simko B, Renzetti L, et al. Ro 25-1553: a novel, long-acting vasoactive intestinal peptide agonist, part I: in vitro and in vivo bronchodilator studies. J Pharmacol Exp Ther 1994;270:1282-8.
- O’Donnell M, Garippa RJ, Rinaldi N, Selig WM, Tocker JE, Tannu SA, et al. Ro 25-1553: a novel, long-acting vasoactive intestinal peptide agonist, part II: effect on in vitro and in vivo models of pulmonary anaphylaxis. J Pharmacol Exp Ther 1994;270:1289-94.
- Gourlet P, Vertongen P, Vandermeers A, Vandermeers-Piret MC, Rathe J, De Neef P, et al. The long-acting vasoactive intestinal polypeptide agonist RO 25-1553 is highly selective of the VIP2 receptor subclass. Peptides 1997;18:403-8.
- Moreno D, Gourlet P, De Neef P, Cnudde J, Waelbroeck M, Robberecht P. Development of selective agonists and antagonists for the human vasoactive intestinal polypeptide VPAC2 receptor. Peptides 2000;21:1543-49.
- Juarranz MG, Rampelbergh JV, Gourlet P, De Neef P, Cnudde J, Robberecht P, et al. Different vasoactive intestinal polypeptide receptor domains are involved in the selective recognition of two VPAC2-selective ligands. Mol Pharmacol 1999;56:1280-7.
- Langer I, Gregoire F, Nachtergael I, De Neef P, Vertongen P, Robberecht P. Hexanoylation of a VPAC2 receptor-preferring ligand markedly increased its selectivity and potency. Peptides 2004;25:275-8.