Artificial pulmonary surfactant as a carrier for intratracheally instilled insulin1
Introduction
There has been a great volume of research on the pulmonary delivery of peptides and proteins[1]. However, bioavailability remains problematic and must be improved as subsequent injury to the lungs still occurs. Natural pulmonary surfactant (PS) is able to disperse on the surface of alveolar cells as a thin film, consisting mainly of phospholipids and surfactant proteins. PS therapy has already been applied to pulmonary diseases such as respiratory distress syndrome (RDS)[2] and asthma[3] with some success. Because of its more homogenous and peripheral lung distribution than other liquids and its inherent therapeutic potential, PS has been proposed to be used as a carrier for antibiotics[4], cortico-steroids, and recombinant adenoviral vectors[5]. From a formulation standpoint, the lipid-based drug delivery system should be compatible with lung tissue, and not readily elicit immunogenic reactions, since they resemble physiological PS in components. It is expected that PS could also be employed as a protein and peptide carrier in pulmonary delivery.
Based on previous studies[6,7], we prepared 4 formulations of artificial pulmonary surfactants (APS) as carriers of insulin (INS) as a model drug. The in vivo bioavailability of INS-APS and the correlation between minimal surface tension and the bioavailability of INS-APS were investigated after intratracheal instillation (IT) in normal rats. Injury to the lungs of normal rats after 7 d of consecutive administration was primarily investigated.
Materials and methods
Materials 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC, Sigma, St Louis, MO, USA); L-α-phosphatidyl-DL- glycerol sodium salt (PG, Sigma, USA); tyloxapol (Tyl, Sigma, USA); 1-hexadecanol (Hex, Acros Organics, Pittsburgh, PA, USA); lecithin (Shanghai Boao Bioscience Co, Shanghai, China); palmitic acid (PA, Sinopharm Group Chemical Reagent Co, Shanghai, China); INS powder (Jiangsu Wan-bang Biochemical & Pharmaceutical Co, Xuzhou, China); enzymatic glucose reagent kit (Shanghai Shenfeng Biochemistry Reagent Factory, Shanghai, China); Radioimmunoassays (RIA) kit (Beijing Furui Bioengineering Co, Beijing, China). Other chemicals were of Analytical reagents (AR). ZX-98 rotavapor (Shanghai Organic Chemical Institute, Shanghai, China); KPS-2 supersonic microniser (Shanghai Kaibo Supersonic Apparatus Factory, Shanghai, China); particle size system (Nicomp 380/ZLS, Santa Barbara, CA, USA); pulsating bubble surface tensiometer (modified based on Enhorning’s method[6,8]); UV-754 ultraviolet spectrophotometer (Shanghai Analytical Instrument General Factory, Shanghai, China); photomicroscope (Olympus BX-50, Tokyo, Japan); and Wistar rats (bred by the laboratory animal department of Fudan University, Shanghai, China). INS-APS and blank DPPC dispersions employed in this experiment were produced within our laboratory.
Preparation of INS-APS The composition of 4 APS dispersions were as follows: DPPC/lecithin/PA (6:3:1, w/w/w), DPPC/Hex/Tyl (13.5:1.5:1, w/w/w), DPPC/PG (3:1, w/w), DPPC/Tyl (13.5:1, w/w). DPPC/lecithin/PA, DPPC/Hex/Tyl and DPPC/PG were prepared by a thin-film sonication method. Various lipids and additives dissolved in ethanol/chloroform were mixed; the organic phase was evaporated by rotavapor and the residual solvent was removed in a vacuum overnight. Lipids and additives formed a thin film on the flask wall and were hydrated in sterile saline at 50 °C for 2 h, then sonicated for 5 min for further dispersing. DPPC/Tyl was prepared by a direct sonication method. DPPC and Tyl were mixed in sterile saline and sonicated for 5 min[9]. The resulting APS dispersions were filtrated through a 0.45 µm filter. INS powder (28 IU/mg) was solubilized with 1 mL of 0.1 mol/L HCl; a sterile saline solution was added to make the final INS concentration of 4 IU/mL.
An aliquot of the INS solution was added to each of the APS dispersions and sonicated for 2 min. Final preparations of INS-APS (INS/DPPC/lecithin/PA, INS/DPPC/Hex/Tyl, INS/DPPC/PG, INS/DPPC/Tyl) dispersed in saline contained 13.5 mg/mL DPPC and 4 IU/mL INS.
Measurement of particle size The particle sizes of all INS-APS were determined with a laser diffraction particle size analysis system.
Measurement of minimal surface tension (γmin) The minimal surface tension of APS and INS-APS was determined by a pulsating bubble surface tensiometer (modified based on Enhorning’s method[6,8]). Each dispersion (5 µL) was added to the gas-liquid interface of the air bubble. According to the shape of the bubble during pulsating cycles at the temperature of 37 °C, the γmin was calculated according to the Bashforth-Adams formulation[10].
Pharmacodynamic and pharmacokinetic experiments
INS preparations administration In all of the in vivo experiments, healthy Wistar rats [body weight (BW) 200±30 g] had free access to food and water. Before the experiment, the animals fasted overnight (14 h). Forty-two male rats were divided into 7 groups randomly as follows: (1) subcutaneous injection (sc) INS solution (1 IU/kg); (2) IT INS solution (4 IU/kg); (3) IT INS/DPPC/lecithin/PA (4 IU/kg); (4) IT INS/DPPC/Hex/Tyl (4 IU/kg); (5) IT INS/DPPC/PG (4 IU/kg); (6) IT INS/DPPC/Tyl (4 IU/kg); and (7) IT blank DPPC dispersion. The dose of PA was about 0.2 mL INS-APS per rat and the dose of sc was about 0.2 mL INS solution per rat.
All of the rats were anaesthetized with aether and attached to a board with an elevation of 80° to the horizontal plane. Rat tongues were drawn out and each INS formulation was instilled intratracheally into the lungs through a syringe while the rats were inspiring. The rats were maintained for 30 s on the board after drug delivery and then the board was rotated to a 30° horizontal elevation for 1 min. 0.4 mL of blood was taken from the tail vein shortly before drug administration for a baseline sample and subsequently 15, 30, 60, 90, 120, 180, 240, 300, and 360 min after dosing to determine respective serum glucose and INS levels. The experimental design of the animal study in this paper was approved by the appropriate ethical committee on animal studies at Fudan University (China).
Calculation of the area above the curve (AAC) and the area under the curve (AUC) of INS formulations Blood samples were centrifuged at 3000 r/min for 10 min at 4 °C, and the serum glucose level was determined immediately according to the glucose oxidase method. The serum INS level was quantitated by a double-antibody radioimmunoassay using a commercial RIA kit. The percentage of serum glucose change and the serum INS level were plotted as a function of time. The area above the percentage serum glucose change versus time curve (AAC) and the area under the serum INS level versus time curve (AUC) were calculated by the linear trapezoidal method. The relative pharmacological bioavailability (f)[11] and relative bioavailability (F)[12] were calculated by the following formulations:
Pilot study on lung injury in rats In lung injury experi-ments, healthy Wistar rats (BW 200±30 g) had free access to food and water. Thirty-six male rats were divided into 6 groups randomly as follows: (1) IT INS solution (4 IU/kg); (2) IT INS/DPPC/lecithin/PA (4 IU/kg); (3) IT INS/DPPC/Hex/Tyl (4 IU/kg); (4) IT INS/DPPC/PG (4 IU/kg); (5) IT INS/DPPC/Tyl (4 IU/kg); and (6) control group (normal rats).
Groups 1, 2, 3, 4, and 5 were consecutively administered INS preparations for 7 d (about 0.2 mL INS-APS per rat) and once a day as per the above mentioned method. The control group (normal rats) was fed with lab chow and water without any administration for 7 d. All of the rats were sacrificed on d 7. The changes of the pulmonary edema index and histopathology of lungs were investigated to evaluate the severity of injury to the lungs.
Pulmonary edema index All of the rats were sacrificed on d 7. The whole lungs were removed and dissected. The right-sided lungs were weighed both before drying (WW) and after drying (WD) at 60 °C. The pulmonary edema index was calculated by the following equation: Index=WW/WD [12].
Histopathology observation The left-sided lungs were fixed in 10% formalin, dehydrated, embedded in paraffin, sectioned using a microtome, and stained using hematoxylin-eosin. The lung slices were observed under a photomicroscope and photographic records were taken.
Statistical analysis Data were expressed as mean±SD. Statistical significant differences were evaluated with an analysis of ANOVA in the groups and Bonferroni (Dunnett) t-test between 2 groups.
Results
Appearance and particle size of INS-APS All APS dispersions and INS-APS were dispersed in sterile saline homogeneously and had emulsive lights. The appearance of APS and INS-APS particles were circular and smooth. The particle sizes of INS-APS (INS/DPPC/lecithin/PA, INS/DPPC/Hex/Tyl, and INS/DPPC/PG, INS/DPPC/Tyl) were 395.55±17.14, 439.78±46.84, 460.63±45.18, and 390.73±35.53 nm, respectively. There was no significant difference (P>0.05) between them.
γmin of various dispersions The γmin of APS and INS-APS is shown in Table 1. The γmin value of APS and INS-APS decreased to 10 mN/m compared to the surface tension of H2O (72 mN/m) and the γmin of DPPC (32 mN/m) and INS solution (64.48 mN/m). There were significant differences (P<0.05) in the γmin of 4 APS dispersions and in the γmin of 4 INS-APS, but no significant difference (P>0.05) in the γmin between all APS and corresponding INS-APS. The lowest γmin of APS and INS-APS were obtained by DPPC/Tyl and INS/DPPC/Tyl. The relative potency of the γmin of 4 INS-APS was γINS/DPPC/Tyl<γINS/DPPC/PG<γINS/DPPC/Hex/Tyl<γINS/DPPC/lecithin/PA. The same trend of the γmin was observed in APS dispersions.
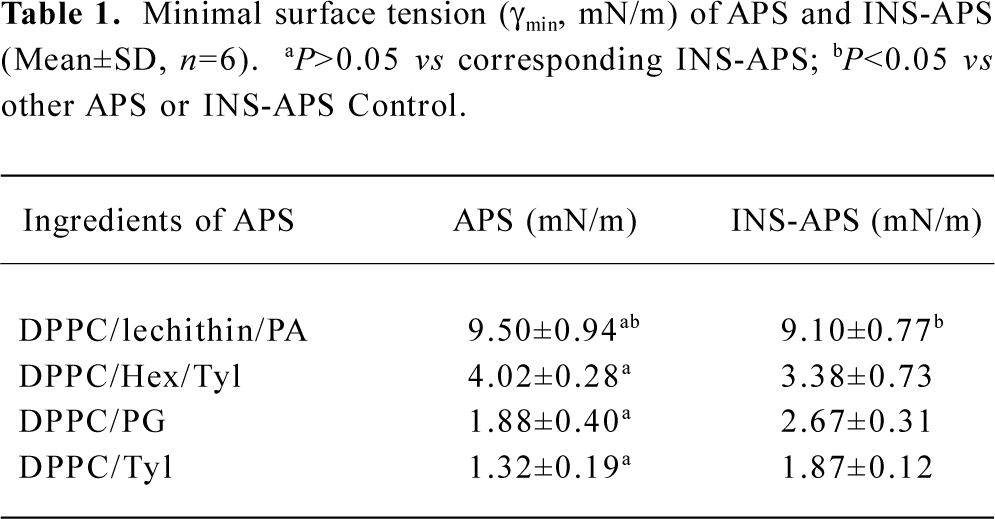
Full table
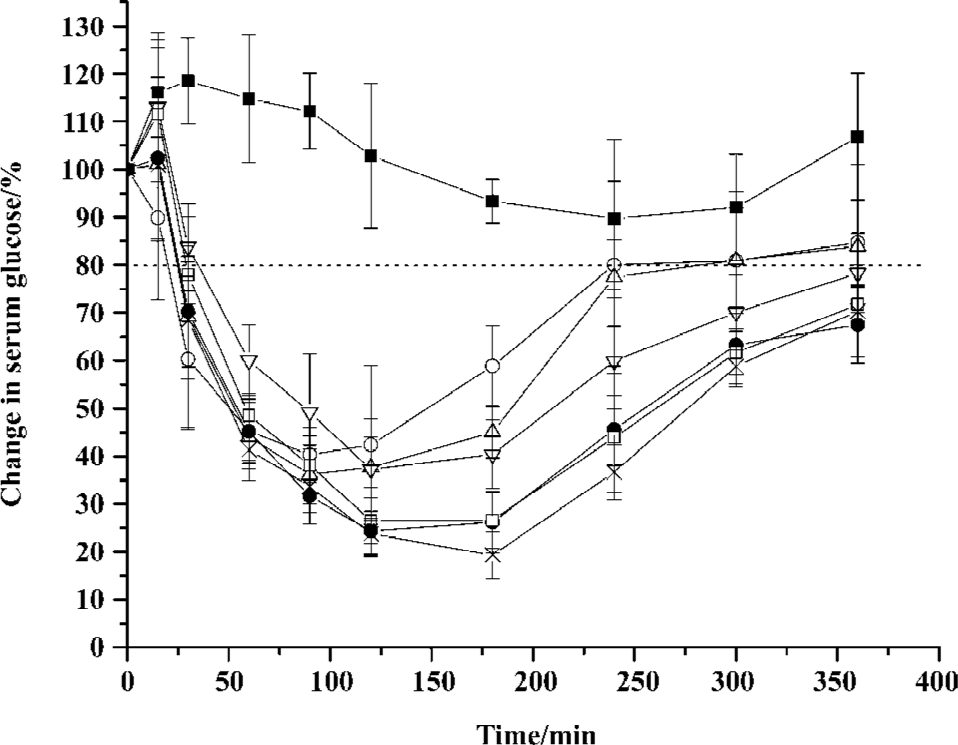
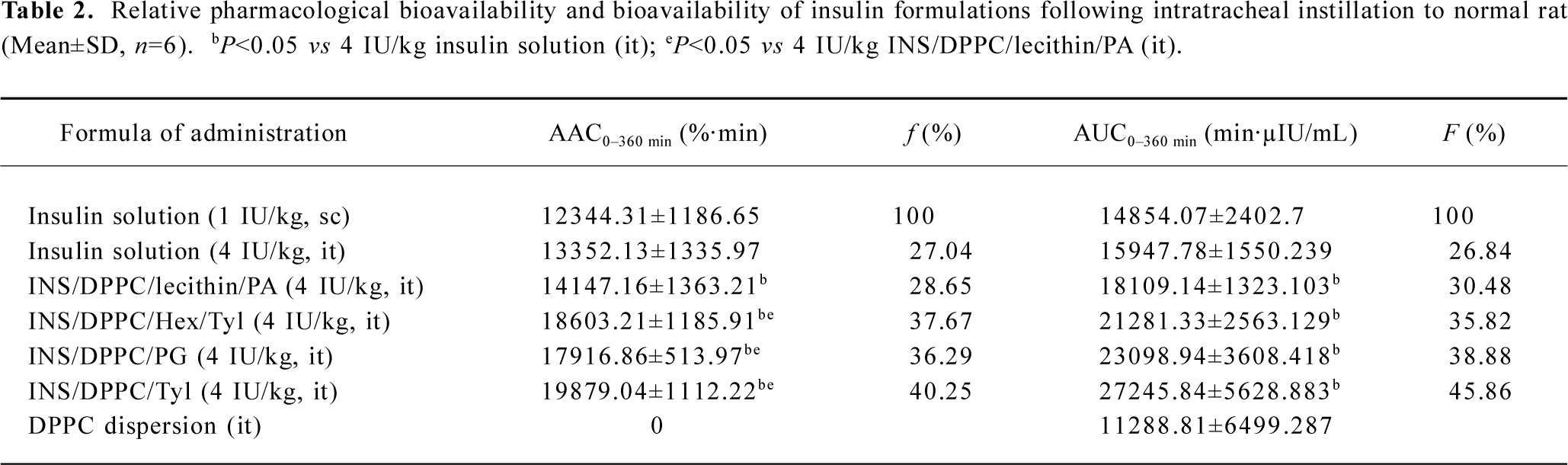
Hypoglycemic effects and plasma INS levels after pulmonary delivery of INS preparations As illustrated in Figure 1, the maximum blood glucose reduction after IT of INS preparations at a dose of 4 IU/kg was more than 60%. The values of the AAC of the 4 INS-APS (14147.16~19876.04) were significantly higher (P<0.05) than the value of the INS solution (13351.13). The optimal hypoglycemic effect of 4 INS-APS was obtained with INS/DPPC/Tyl, which generated a maximum glucose reduction of 80% and a maximum AAC of 19876.04. The maximum glucose reduction of the remaining 3 INS-APS (INS/DPPC/lecithin/PA, INS/DPPC/Hex/Tyl, and INS/DPPC/PG) were 63%, 76%, and 74%, respectively. The AAC and relative pharmacological bioavailability (f) of the INS formulations are listed in Table 2. The AAC of INS/DPPC/Hex/Tyl, INS/DPPC/PG, and INS/DPPC/Tyl were significantly higher (P<0.05) than that of INS/DPPC/lecithin/PA. The relative pharmacological bioavailability followed the sequence: fINS/DPPC/Tyl>fINS/DPPC/Hex/Tyl>fINS/DPPC/PG>fINS/DPPC/lecithin/PA. The control group showed that DPPC dispersion alone did not induce any decrease in the glucose level.
Full table
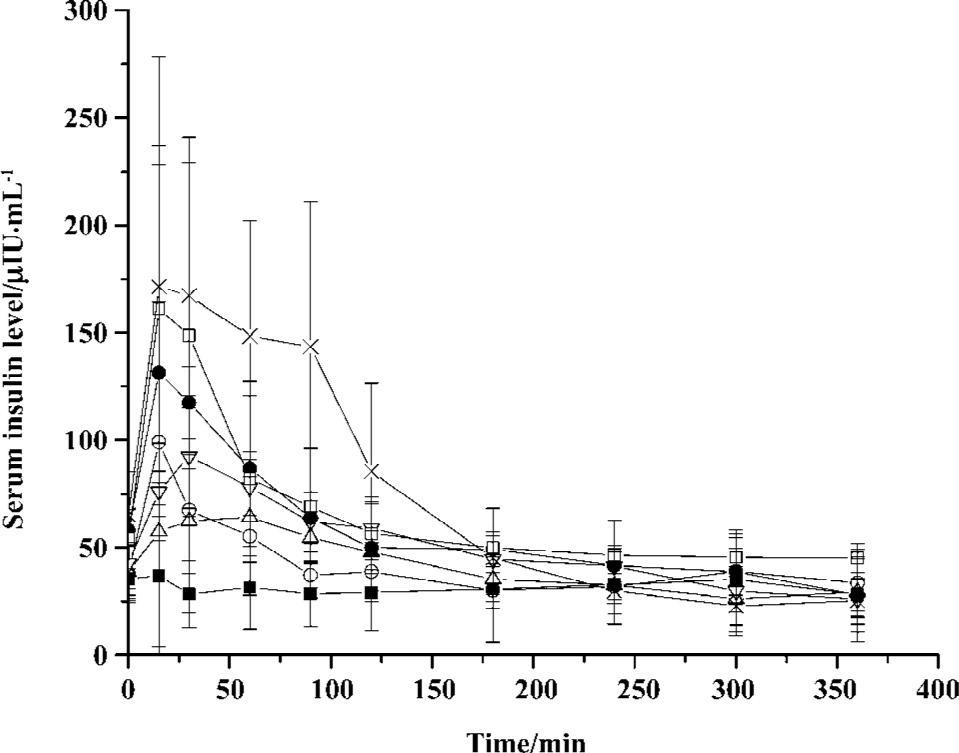
Figure 2 shows the serum INS level after pulmonary administration. The serum INS level was enhanced after administration of INS-APS. The highest serum INS level was obtained by 172 µIU/mL of INS/DPPC/Tyl at 15 min, which was slightly higher than the level of INS/DPPC/PG (161 µIU/mL), 1.3 times that of INS/DPPC/Hex/Tyl (131 µIU/mL), 1.8 times that of INS/DPPC/lecithin/PA (93 µIU/mL), and 2.7 times that of the INS solution (64 µIU/mL) after IT. The difference in the AUC of the INS formulations was similar to that of pharmacological bioavailability (Table 2). The value of the AUC of INS/DPPC/Tyl (27243.84±5628.883) was significantly higher (P<0.05) than the values of the other 3 INS-APS (15947.78~23097.94). The AUC and the relative bioavailability (F) of INS/DPPC/lecithin/PA were lowest of the 4 INS-APS. The relative bioavailability followed the sequence: FINS/DPPC/Tyl>FINS/DPPC/PG>FINS/DPPC/Hex/Tyl>FINS/DPPC/lecithin/PA. DPPC dispersion alone seemed to have no effect on the serum INS level.
The duration of glucose levels maintained at a level below 80% after pulmonary delivery of INS were summarized. This was provided as a means of evaluating the prolonged hypoglycemic effects of the INS preparations[13]. As displayed in Figure 1, the duration of IT of INS-APS were all above 6 h, which was longer than the hypoglycemic effect of an INS solution sc injection (4 h) and IT (5 h).
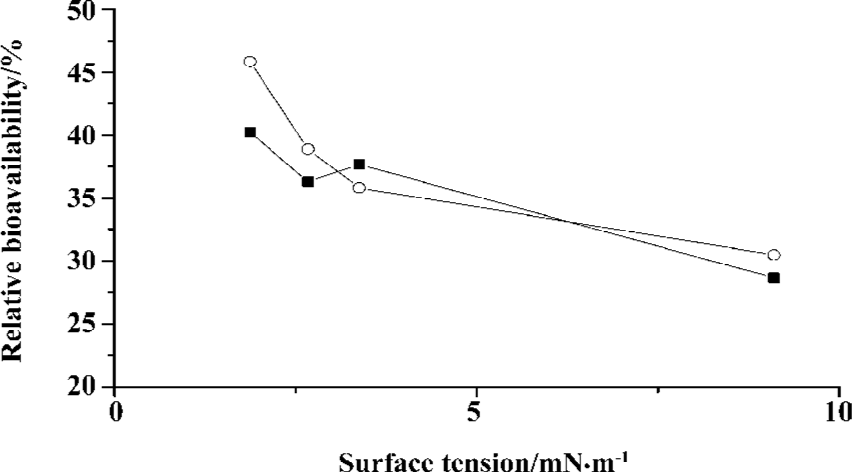
Correlation between γmin and bioavailability of INS-APS The correlation coefficients between the f, F, and γmin values of the INS preparation were -0.7421 and -0.7245, whereas the correlation coefficients between the f, F, and γmin of INS-APS were -0.9688 and -0.8632, respectively (Figure 3).
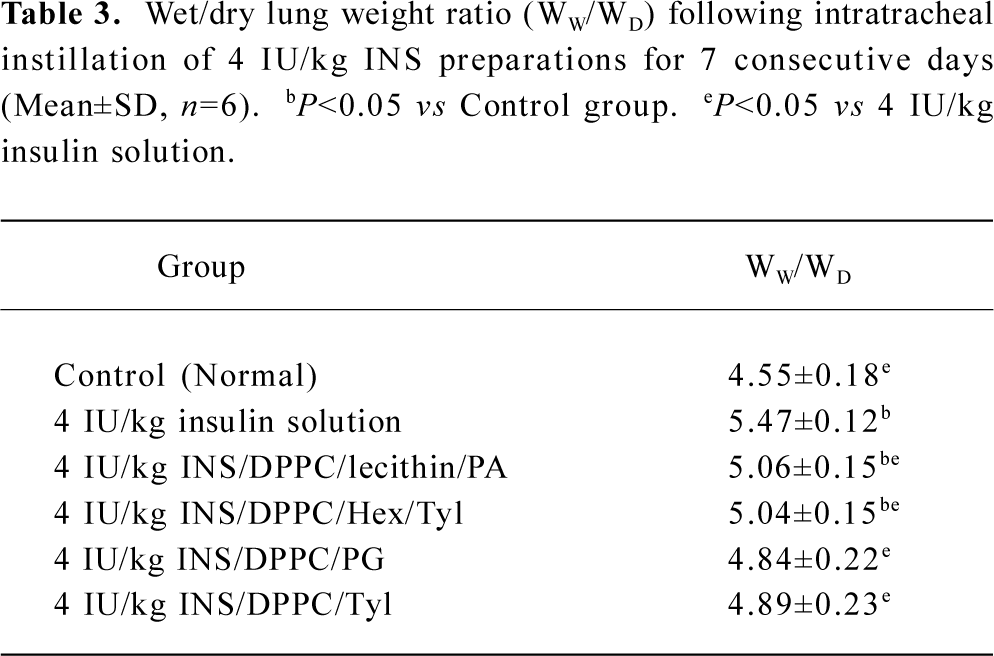
Pulmonary edema index After 7 d of consecutive administration, a significant difference (P<0.05) in the pulmonary edema index of the 6 groups was observed (Table 3). The index for the INS solution group was significantly higher (P<0.05) than that of the control group, and the indices of the INS-APS groups were significantly lower than that of the INS solution group. This indicated that the INS-APS groups could alleviate lung injury while the INS solution conferred marked injury to the lung tissue following 7 d of consecutive administration. The pulmonary edema indices of INS/DPPC/Tyl and INS/DPPC/PG showed no significant difference compared with the control group (P>0.05).
Full table
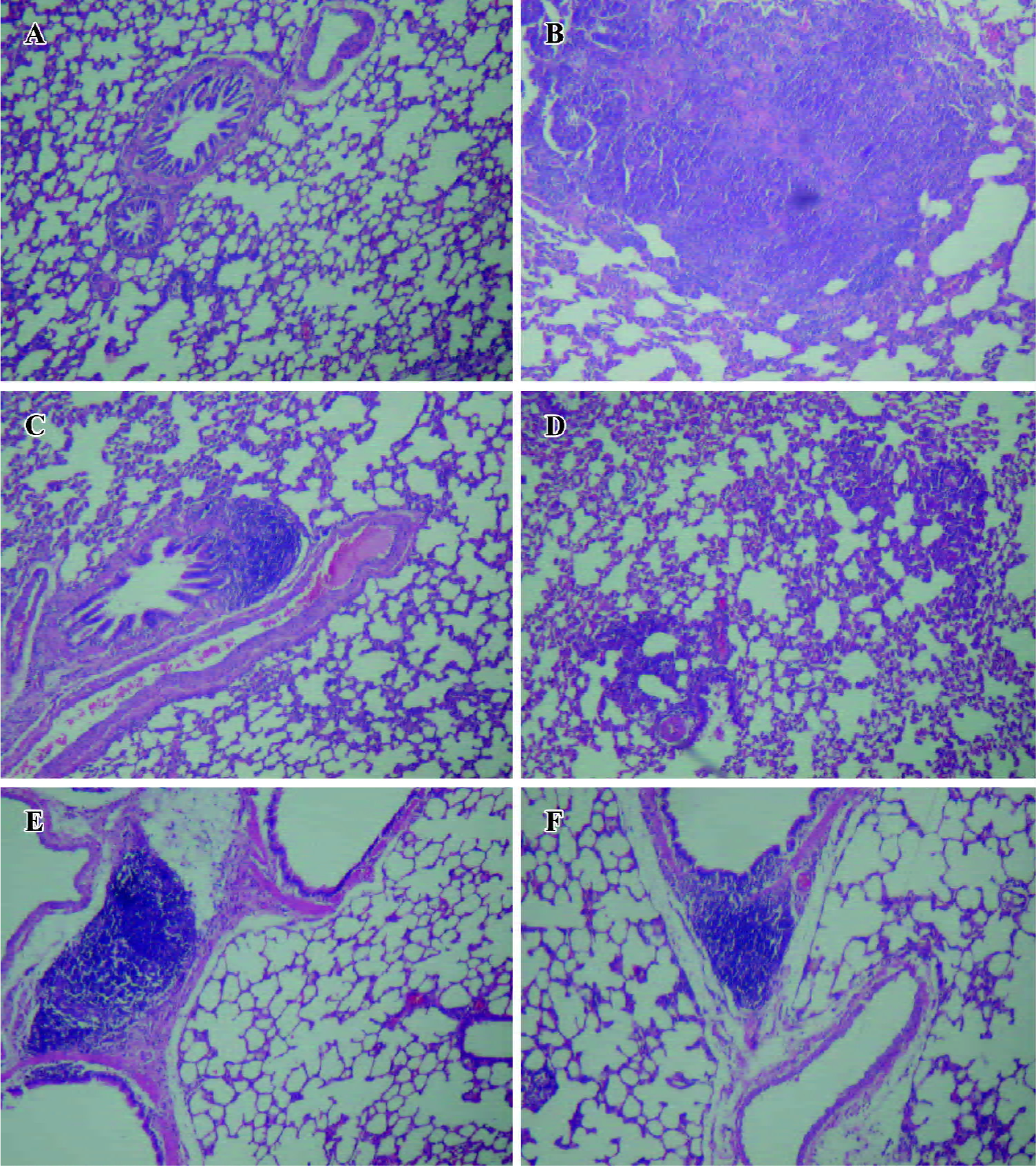
Histopathological examination of lungs As shown in Figure 3, following IT for 7 consecutive days, thickened alveolar septa were clearly observed in the lung slices in rats administrated with INS solution (Figure 4B), as compared to the control group (Figure 4A). Diffused disruption of alveolar capillaries with leakage of red blood cells into the alveolar lumina and lung interstitium was generally noted in the INS solution group. Prominent inflammatory cell infiltration and lung vascular epithelial degeneration were also observed in this group. The pathological changes became less severe in the slices after INS-APS administration (Figure 4C–4F). The width of alveolar septa was slighter broader than the control group. Disruption of the alveolar wall and leakage of red blood cells were not evident. There was only very slight visible epithelial degeneration and no other alveolar structural changes observed in the INS-APS groups, as compared with the control groups. Inflammatory cell infiltration was still found in some samples although this was only negligible.
Discussion
The natural PS is a complex mixture produced by alveolar type II cells in lung. It can stabilize lungs for efficient respiration and prevent or treat RDS by reducing alveolar surface tension and increasing lung compliance. Due to the small diameter of peripheral airways, fluid with a high surface tension requires high pressure delivery for distribution. Studies have shown that PS is superior to saline in distributing a radioactive colloid within healthy lungs, and is more homogenous and peripheral in lungs than saline[14]. PS carry out this function by forming a film at the air-liquid interface. This film mainly consists of lipids and surfactant proteins. The hydrophilic polar heads of amphiphilic phospholipid molecules remain in water while the fatty acids turn towards air. The phospholipids molecules interluding in water affect the affinity in molecules. Due to this film, surface tension is lowered and undergoes considerable changes during the respiratory cycle[15].
Of the many components of natural PS, DPPC appears to have the necessary thermodynamic properties to reduce surface tension effectively. However the high cohesive energy of compressed monolayers of disaturated long-chain fatty acids, combined with significant hydration of the headgroups, inhibits the rapid spreading of DPPC molecules in an air/liquid interface. Therefore, the synergistic effect of other lipids are required to reduce the cohesion of DPPC in lipid mixture, such as PG, cholesterol and free PA, and of several lung specific proteins[16]. Most research on APS have documented a potential for antigenicity due to the presence of a foreign protein[17,18]. In this paper, DPPC was used as the key ingredient and other components were employed to prepare APS dispersions. It was shown that APS could decrease the surface tension at the air/liquid interface significantly and also enhance INS absorption with resulting alleviation of lung injury.
This experiment showed that the γmin value of the 4 INS-APS were all lower than DPPC dispersion alone. DPPC and PG are generally considered as components of PS; in this study they were employed as 1 formulation of APS. Lecithin contains unsaturated fatty acid residues which probably enhance the fluidity of the DPPC films[16]; furthermore, the addition of PA often makes the dispersion more homogeneous[19]. DPPC/lecithin/PA was designed and added to INS. In the DPPC/Hex/Tyl dispersion, Hex acts as a spreading agent and Tyl as a dispersion agent[20]. So DPPC/Hex/Tyl and DPPC/Tyl were prepared as formulations of APS. The difference of the γmin between DPPC/Hex/Tyl and DPPC/Tyl requires further research. With intratracheally instilled INS preparation, the serum glucose level of the INS-APS groups was clearly lower than that of the INS solution group. In addition, the duration of the hypoglycemic effect of the INS-APS groups was significantly longer than that of the INS solution group. The lowest γmin value and the largest hypoglycemic effect were obtained by the INS/DPPC/Tyl preparation. The relative pharmacological bioavailabilities of INS/DPPC/Hex/Tyl, INS/DPPC/PG, and INS/DPPC/Tyl were higher than INS/DPPC/lecithin/PA, and similar trends were noted for the relative bioavailability of INS-APS. The correlation between γmin and f, and F suggested that the decrease of the γmin values of APS and INS-APS directly affected f and F. It was also revealed that the in vivo hypoglycemic effect could be predicted with the γmin values of INS-APS. The mechanism whereby INS absorption was enhanced is believed to be via an APS-mediated decrease in the γmin of the gas-liquid interface in alveolar tissue. This property appears to increase the facility and affect spreading, which in return increases the absorption area and produces greater INS absorption with APS.
In comparison to the pulmonary edema index, it was concluded that INS-APS could decrease lung injury compared with the INS solution following 7 d of consecutive IT. The lower index was obtained by INS/DPPC/PG and INS/DPPC/Tyl, which might contribute to the lower γmin value of the 2 INS-APS. Combining the results of the pulmonary edema index and histopathology examination, it was suggested that the pulmonary delivery of INS-APS might reduce the severity of any lung injury during administration and would thus be more appropriate for pulmonary delivery than the INS solution.
In summary, DPPC/Hex/Tyl, DPPC/PG, and DPPC/Tyl, with their low γmin, appeared to be effective absorption enhancers and significantly decreased lung injury. INS/DPPC/Tyl and INS/DPPC/PG in this study were shown to be efficient INS pulmonary delivery carriers with several advantages over drug preparations current utilized clinically.
Acknowledgements
We thank Dr Zhi-qiang JIANG and Prof Yuan-ming MA for their suggestions.
References
- Agu RU, Ugwoke MI, Armand M, Kinget R, Verbeke N. The lung as a route for systemic delivery of therapeutic proteins and peptides. Respir Res 2001;2:198-209.
- Merrill JD, Ballard RA. Pulmonary surfactant for neonatal respiratory disorders. Curr Opin Pediatr 2003;15:149-54.
- Erpenbeck VJ, Hagenberg A, Dulkys Y, Elsner D, Balder R, Krentel H, et al. Natural porcine surfactant augments airway inflammation after allergen challenge in patients with asthma. Am J Respir Crit Care Med 2004;169:578-86.
- Van’t Veen A, Gommers D, Mouton JW, Kluytmans JA, Krijt EJ, Lachmann B. Exogenous pulmonary surfactant as a drug delivering agent: influence of antibiotics on surfactant activity. Br J Pharmacol 1996;118:593-8.
- Katkin JP, Husser RC, Langston C, Welty SE. Exogenous surfactant enhances the delivery of recombinant adenoviral vectors to the lung. Hum Gene Ther 1997;8:171-6.
- Jiang ZQ, Sun GM, Ma YM. Artificial reconstituted pulmonary surfactant in prevention and treatment of respiratory distress syndrome in neonates. Acta Pharmacol Sin 1997;18:182-4.
- Mitra R, Pezron I, Li Y, Mitra AK. Enhanced pulmonary delivery of insulin by lung lavage fluid and phospholipids. Int J Pharm 2001;217:25-31.
- Enhorning G. Pulsating bubble technique for evaluating pulmonary surfactant. J Appl Physiol 1977;43:198-203.
- Ji Y, Pei YY. Primary study of artificial pulmonary surfactant as carrier of insulin pulmonary delivery. Chin Pharm J 2006;41:766-8.
- Paddy JF. Surface tension. Part III. Tables relating the size and shape of liquid drops to the surface tension. In Matijevic E, editor. Surface and colloid science. New York: Wiley-Interscience; 1969. p151–97.
- Pan Y, Li YJ, Zhao HY, Zheng JM, Xu H, Wei G, et al. Bioadhesive polysaccharide in protein delivery system: chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int J Pharm 2002;249:139-47.
- Lu J, Ji Y, Jiang ZQ. Study on the bioavailability and initial investigation on the rat following pulmonary delivery of insulin lipid suspension. Chin J Biochem Pharm 2002;23:271-4.
- Zhang Q, Shen ZC, Tsuneji N. Prolonged hypoglycemic effect of insulin-loaded polybutylcyanoacrylate nanoparticles after pulmonary administration to normal rats. Int J Pharm 2001;218:75-80.
- Kharasch VS, Sweeney TD, Fredberg J, Lehr J, Damokosh AI, Avery ME, et al. Pulmonary surfactant as a vehicle for intratracheal delivery of technetium sulfur colloid and pentamidine in hamster lungs. Am Rev Respir Dis 1991;144:909-13.
- Enhorning G. Pulmonary surfactant function studied with the pulsating bubble surfactometer (PBS) and the capillary surfacto-meter (CS). Comp Biochem Physiol A Mol Integr Physiol 2001;129:221-6.
- Tanaka Y, Takei T, Aiba T, Masuda K, Kiuchi A, Fujiwara T. Development of synthetic lung surfactants. J Lipid Res 1986;27:475-85.
- Latallo JFK, Chen CL, Eichman J, Bielinska AU, Baker JR. Enhancement of endrimer-mediated transfection using synthetic lung surfactant exosurf neonatal in vitro. Biochem Biophys Res Commun 1999;264:253-61.
- Lewis JF, Brackenbury A. Role of exogenous surfactant in acute lung damage. Crit Care Med 2003;31:S324-8.
- Palmblad M, Gustafsson M, Curstedt T, Johansson J, Schurch S. Surface activity and film formation from the surface associated material of artificial surfactant preparations. Biochim Biophys Acta 2001;1510:106-17.
- Park SY, Hannemann RE, Franses EI. Dynamic tension and adsorption behavior of aqueous lung surfactants. Colloids Surf B Biointerfaces 1999;15:325-38.
