Slack and Slick KNa channels are required for the depolarizing afterpotential of acutely isolated, medium diameter rat dorsal root ganglion neurons1
Introduction
Na+-activated K+ (KNa) channels were originally identified in cardiomyocytes and may provide protection against ischemia[1]. KNa channels are accompanied by an increase in intracellular Na+ and may be involved in action potential shortening during ischemia[2]. They have been described to have many different functions in various neurons[3–7]. KNa channels are activated at resting states in quail trigeminal ganglion neuron[5]. The accumulation of intracellular Na+ during a train of action potentials may result in the activation of KNa channels in the soma of rat motor neurons[7]. It has also been proposed that Na+ influx through voltage-gated Na+ channels during a single action potential produces a transient activation of KNa channels, resulting in action potential repolarization[8,9].
The molecular identity of native KNa channels is considered as Slo2, originally called Slack (also termed Slo2.2)[10]. The second KNa channel gene is called Slick (also termed Slo2.1), which is homologous to Slack[3,10]. The Slick KNa channel is activated rapidly in response to depolarization and cytoplasmic Cl–. There is an ATP-binding site in the N-terminal regions of Slick. Slack and Slick KNa channels could be activated by both Na+ and Cl–. However, the Slick channel has low sensitivity to Na+, but high sensitivity to elevating the internal Cl– concentration[3].
In rat dorsal root ganglion (DRG) neurons, the largest cell bodies Aα- and Aβ-type DRG neurons usually transmit proprioceptive and tactile information, while smaller cell bodies Aδ- and C-type DRG neurons usually transmit pain and thermal information[11]. Scroggs and Fox reported that T-type Ca2+ currents were lower in small (20-27 μm) diameter DRG cell bodies (100 pA-1 nA) than observed in medium diameter (33-38 μm) DRG cell bodies (1-6 nA), and were not observed in large (45-51 μm) diameter DRG cell bodies[12]. Their results suggest the different distribution of Ca2+ channels in different size DRG neurons. Bischoff et al reported that KNa channels set and stabilize the resting potential, but do not participate in single action potentials in rat small (20-25 μm) DRG neurons[4]. However, whether KNa channels play the same role in medium diameter DRG neurons is still unknown.
In the present study, rat medium DRG neurons (25-35 μm), which were acutely isolated from lumbar segments of vertebrate column (L4-6) were used to study the potential functions of KNa channels. KNa channels were detected in ~80% membrane patches which displayed classical characterizations like large single channel conductance, subconductance states, and a block of single channel currents at positive potentials. KNa channels expressed distinct cytoplasmic Na+ and Cl– concentration-dependent activation in these classes of neurons. Moreover, we demonstrated the existence of Slack and Slick in DRG neurons by RT–PCR. Using tetrodotoxin (TTX, 100 nmol/L) to block Na+ influx, we found KNa currents were outward K+ currents which might contribute to repolarization of DRG action potential. With regards to replacement extracellular Na+ with Li+, we conclude that the outward KNa currents contribute to the depolarizing afterpotential (DAP) of rat medium diameter DRG neurons.
Materials and methods
DRG neuron isolation Three-to-five-week-old Wistar rats (male) were killed by decapitation. The lumbar segments of the vertebrate column were dissected and the lumbar L4, L5, and L6 DRG, together with the dorsal, ventral roots, and attached spinal nerves were taken out from the outside of the spinal column. These 6 DRG were transferred into iced Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island. NY, USA) 13.5 g/L, NaCl 2.15 g/L, HEPES 2.0 g/L pH 7.4, 320 mOsm) immediately. After the removal of attached nerves and surrounding connective tissues, DRG were minced with iridectomy scissors and incubated with enzymes, including 1 mL collagenase (type I; Sigma-Aldrich, St. Louis, MO, USA) 2 mg/mL, 1 mL trypsin (Type IX, Sigma) 0.5 mg/mL, and 50 μL DNase, 4 mg/mL [in calcium-free buffer with 4 mg/mL BSA(bovine serum albumin)] in a 37 ºC shaking bath (170 r/min) for 35-40 min with gently mechanical trituration every 10 min. The addition of 8 mL of pre-incubated DMEM [including 20% FBS (fetal bovine serum)] was used to stop the enzymatic digestion. The isolated neurons were plated on 0.5 mg/mL poly-lysine coated glass coverslips and maintained in a 37 ºC humidified incubator with 5% CO2 for at least 2 h before use. The medium neurons with a diameter of 25-35 μm were used in the experiments.
Electrophysiology For whole-cell clamp, the pipette solution contained the following (in mmol/L): 140 K-gluconate, 20 KCl, 10 HEPES, 5 EGTA, 2 MgATP, and 0.3 Na2GTP (pH 7.2 with KOH, and 300 mOsm). The external solution contained (in mmol/L): 145 NaCl, 2.5 KCl, 4 MgCl2, 1 EGTA, 10 HEPES, and 10 glucose (pH 7.3 with NaOH, and 310 mOsm). To make the Na+-free saline, 145 mmol/L NaCl was replaced with 145 mmol/L LiCl. For the inside-out recordings, the pipette extracellular solution contained (in mmol/L): 140 methanesulfonic acid, 150 KOH, 10 KCl, 10 HEPES, and 2 MgCl2 (pH 7.2 with methanesulfonic acid). Testing solutions bathing the cytoplasmic face of the patch membrane contained (in mmol/L): 100 KCl, 60 KOH, 60 methanesulfonic acid, 5 EGTA, and 10 HEPES; 0, 20, 40, and 80 mmol/L NaOH was added for different concentrations of the Na+i solution (pH 7.2 with methanesulfonic acid). For different concentrations of Cl–i, redundant Cl– was replaced with same quantity of methanesulfonic acid to make 10, 100, and 160 mmol/L Cl–. Osmolarity was measured by a vapor pressure osmometer (Wescor INC., Logan, Utah, USA) and adjusted to 300–310 mOsm (pipette solution) and 310–330 mOsm (extracellular solution). All experiments were performed at room temperature (22–25 ºC)[13].
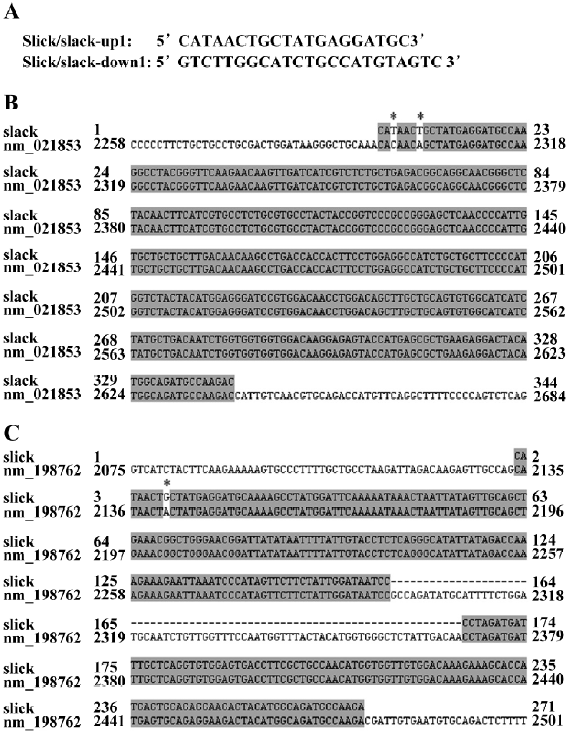
RT–PCR The RNeasy Mini Kit (QIAGEN, Valentia, CA, USA) was used to extract the total RNA from the rat DRG[14]. The cDNA of the Slick and Slack were amplified by RT–PCR with the Qiagen OneStep RT–PCR kit (QIAGEN, Valentia, CA, USA). Two primers for amplifying Slack and Slick (the upstream primer 5´-CATAACTGCTATGAGGATGC-3´ and the downstream primer 5´-GTCTTGGCATCTGCCATGTAGTC-3´) were used in the RT–PCR reaction. The RT–PCR products were extracted by QIAquick Gel Extraction Kit (QIAGEN, Valentia, CA, USA) and then ligated into a pCR2.1-TOPO vector (Invitrogen, Carlsbad, CA, USA) for the sequence analysis.
Data analysis Data were analyzed with Igor 5.03 (Wavemetries, Lake Oswego, OR, USA), Clampfit (Axon Instruments Inc., Foster City, CA, USA), and SigmaPlot (SPSS, Chicago, IL, USA). Unless stated otherwise, the data are presented as mean±SEM. Significance was tested by Student’s t-test, and differences in the mean values were considered significant at a probability of P<0.05.
The dose–response curve for the open probability (Po) of KNa was drawn according to the Hill equation Po=P(max)/(1+[EC50/[Na+]i]n), where P(max) is the maximum Po of the KNa currents, and [Na+]i is the concentration of cytoplasmic Na+. EC50 and n denote the Na+ concentration of the half-maximal effect and the Hill coefficient, respectively.
Results
KNa channels in DRG neurons In our experiments, KNa channels were present in approximately 80% in all inside-out patches. The representative single-channel currents were evoked by 80 mmol/L cytoplasmic Na+ (Na+i) at different holding potentials which contained 2 opened KNa channels (Figure 1A). The unitary chord conductance of the KNa channel was 201±3.8 pS (n=8) by fitting the current–voltage relationship curve through the line function (Figure 1B). Under this condition, KNa channels displayed linear open characterization from –100 to 0 mV. When the membrane potential was more positive than the potassium equilibrium potential, the single-channel currents exhibited inward rectification and opened in bursts (Figure 1A; +60 mV), during which they fluctuated between the fully open, closed, and the substates. This was proposed as the result of the Na+ block of outward KNa currents at positive potentials.
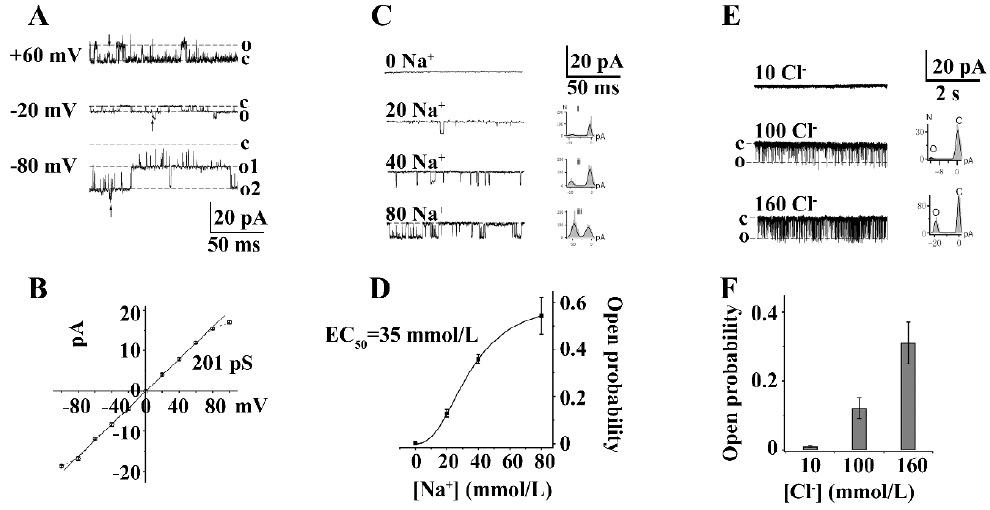
KNa channels exhibited different Na+ concentration-dependent activation in various neurons. The effect of different Na+i concentrations on the Po of KNa channels was studied in medium diameter DRG neuron cell bodies. Single-channel currents were not activated in the absence of Na+i, but could be gradually evoked in 20, 40, and 80 mmol/L Na+i (Figure 1C), although the activity of KNa channels was not completely open in 80 mmol/L Na+i (n=12). The best fit of the data using the Hill equation obtained the following parameters: Pmax (maximum Po) is 0.62, EC50 is 35 mmol/L, and n (Hill coefficient) is 2.4 (Figure 1D). One possible explanation for the steep relationship between Po and the Na+i concentration is that the binding of 2–3 Na+ was necessary to open a KNa channel. These results suggested that Slack KNa channels exist in medium diameter DRG neurons.
Slack and Slick KNa channels are reported to have overlapping distribution in the central neural system[6,15]. In order to understand the existence of Cl–-activated Slick channel in medium diameter rat DRG neurons, different concentrations of cytoplasmic Cl– (10, 100, and 160 mmol/L) were used to study the single-channel currents in an inside-out clamp (Figure 1E). The single channel conductance of Cl–-activated K+ channels is 182±1.2 pS at –100 mV with frequent subconductance currents. These Cl–-activated K+ channels exhibited a similar burst, open mode like cloned Slick channels in Chinese Hamster Ovary cells[3]. The Po of the Cl–-activated K+ channels at different cytoplasmic Cl– concentrations is shown in Figure 1F (n=6). The results suggested that Slick KNa channels may also exist in medium diameter DRG neuron cell bodies.
Both slack and slick exist in DRG neurons by RT–PCR Total RNA from the rat DRG was extracted and then used as the RT–PCR template. The RT–PCR products amplified by primers were 2 bands according to the DNA electrophoresis. One of the 2 bands was approximately 250 bp, and another was approximately 350 bp. The RT–PCR products were subcloned into a pCR2.1-TOPO vector for DNA sequencing (Figure 2A). The resulting sequences showed a very high homology to the rat potassium channel subunit (Slack; PubMed NM_021853; Figure 2B) and rat Na+- and Cl–-activated, ATP-sensitive potassium channel (Slick; PubMed NM_198762; Figure 2C). The results demonstrated that both Slack and Slick exist in rat DRG neurons.
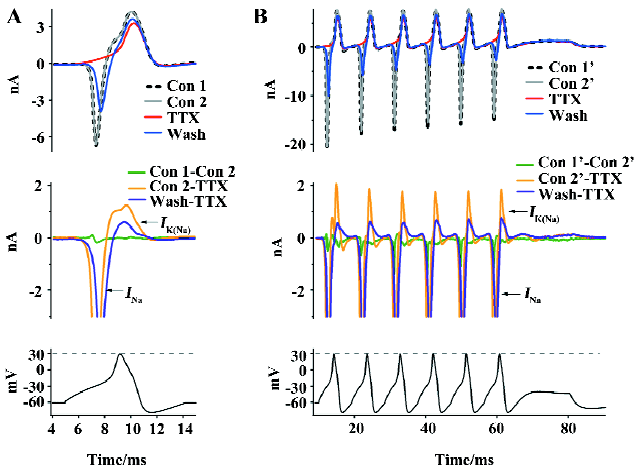
Na+ influx-dependent outward KNa currents It has been reported that Na+ influx through voltage-gated Na+ channels during a single action potential could produce a transient activation of KNa channels[8,9]. In order to study the physiological roles of KNa channels in medium diameter DRG neurons, we used a single action potential recorded from medium diameter DRG neurons in a current clamp to simulate the physiological response of KNa channels. In a free Ca2+ extracellular solution, the inward Na+ current (INa) and the following outward KNa current (IK(Na)) were evoked (Figure 3A). In order to divide the INa from other potential inward currents, we used 100 nmol/L TTX to block the inward INa (Figure 3A). TTX completely blocked the inward INa (Figure 3A; n=6). The single action potential peak amplitude was 30 mV (Figure 3A), which is a lot lower than the TTX-sensitive Na+ channels reverse potential (>50 mV). The following outward current was not INa, but a Na+ influx-activated outward current, which was believed to be the Na+-activated K+ current[16,17]. Rat DRG neurons usually have continuous action potentials firing. We created a train of action potentials as a continuous stimulation waveform using HEKA Pulse and Igor software in which each was the same as the single action potential in Figure 3A (Figure 3B). Similar results were recorded in medium diameter DRG neurons (Figure 3B; n=6). KNa channels currents were outward K+ currents under physiological action potential firing, which suggested that they play an important role in medium diameter DRG action potentials transferring.
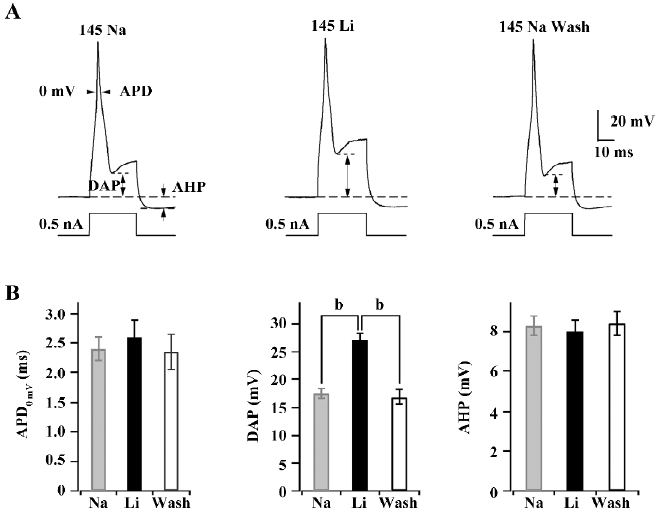
KNa channels contribute to DAP in medium diameter DRG neurons The replacement of extracellular Na+ with Li+ is usually performed to study the assumed contribution of KNa channels in the regulation of action potential in current clamps. Single action potentials were evoked by short (30 ms) current pulses of 0.5 nA. The shape of action potential (AP) waveforms was compared from a certain cell in 145 mmol/L Na+ or replacement by 145 mmol/L Li+ and washout with 145 mmol/L Na+ at intervals of 1–2 min, respectively (Figure 4A).The absolute value of DAP was increased from 17.5±0.8 mV to 27.1±1.2 mV after the replacement of extracellular Na+ with Li+, but action potential duration (APD) at 0 mV (APD0 mV) and after hyperpolarizing potential (AHP) were not affected (Figure 4B). Washing out with 145 mmol/L Na+ could recover the DAP of action potentials. The results indicated that KNa channels are required for action potential repolarization in medium diameter rat DRG neurons.
Discussion
DRG neurons transfer much complex sensory information like pain, temperature, proprioceptive, and tactile information[8,9]. Different sensory information might depend on different size DRG neurons. Sensory information transfer was thought to be coded on the DRG action potential firing frequency, amplitude, and firing pattern. The shape of neuron action potentials was dependent on the opening of various ion channels on the cell periphery. In this study, the results showed that the Slack and Slick Na+-activated K+ channels existed and contributed to action potential DAP in acutely isolated, medium diameter rat DRG neuron cell bodies. Native KNa channels had distinct properties similar with the cloned Slack and Slick channels, including a large single channel conductance (201 pS; Figure 1), sensitivity to intracellular Na+ and Cl– (Figure 1), multiple subconductance states, and a block of single channel currents at high positive potentials (Figure 1)[3,10]. RT–PCR demonstrated that both Slack and Slick KNa channels existed in the DRG neurons (Figure 2). This method has been successfully used to demonstrate that the β2-subunit, but not β3-subunit, induces the inactivation of calcium-activated potassium (BK) channel in small DRG neurons[14]. However, how KNa channels are opened under physiological conditions and whether KNa channels contribute to regulate the physiological functions in medium diameter rat DRG neurons is unknown. Using a real action potential stimulation waveform, whole-cell KNa currents appeared to be activated by Na+ influx through TTX-sensitive Na+ channels (Figure 3). The transient currents did not appear to be the results of the lack of space clamp because the neurons examined were small with very short processes and their series resistance was compensated. Additionally, Na+ current was blocked reversibly by TTX. In a current clamp, we demonstrated that the KNa channels were required for action potential DAP in medium diameter rat DRG neurons by replacing Na+ with Li+ (Figure 4). The effect of replacing Na+ on the DAP did not appear to be a result of an action of the Na+–Ca2+ exchanger or the Na+–H+ exchanger because of the intracellular solution containing EGTA or a high HEPES concentration.
Functions of KNa channels Over the past several years, many physiological functions of KNa channels have been proposed. One of the surprises is that such channels can act over a wide range of time scales to influence the action potential firing pattern of neurons. The physiological roles of KNa channels have been difficult to characterize because of the lack of specific KNa channel blockers. However, there have been several studies showing that KNa channels contribute to the regulatory neuronal activity and the action potential waveform to produce adaptation of firing rates and to set the resting membrane potential[5,16]. The kinetic properties of Slack channels suggest that they contribute to currents that develop slowly during maintained neuronal firing. Na+-dependent slow AHP lasting many seconds have been described in various neurons depending on Na+ influx and following repetitive neuronal firing[18,19]. KNa channels participate in the DAP following a single action potential in rat hippocampal CA1 pyramidal cells[20]. The size of the DAP was controlled by the activation of an opposing KNa conductance that was detected as early as 5–10 ms after a single action potential[20]. In this work, we found that the KNa channels were activated by a Na+ influx evoked by a single action potential in normal Ca2+ free extracellular saline in medium diameter DRG neurons. The activated KNa currents were outward following Na+ influxes. Using ionic replacement, we demonstrated that KNa channels were required for DAP, but not APD0 mV or AHP.
A recent study that found that the activity of Slack channels can be enhanced by estradiol raises the possibility that the activation of KNa channels contributes to estradiol-dependent neuroprotection in ischemia[21]. Although evidence of the possible role of KNa in pathologies is circumstantial, it raises that possibility that these channels could be therapeutically useful drug targets. Similarly, the existing function of Slack and Slick KNa channels in medium diameter rat DRG neurons may be useful in the research of therapeutic drugs for the treatment of pain.
References
- Kameyama M, Kakei M, Sato R, Shibasaki T, Matsuda H, Irisawa H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature 1984;309:354-6.
- Dryer SE. Na+-activated K+ channels: a new family of large-conductance ion channels. Trends Neurosci 1994;17:155-60.
- Bhattacharjee A, Joiner WJ, Wu M, Yang Y, Sigworth FJ, Kaczmarek LK. Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J Neurosci 2003;23:11681-91.
- Bischoff U, Vogel W, Safronov BV. Na+-activated K+ channels in small dorsal root ganglion neurons of rat. J Physiol 1998;510:743-54.
- Dale N. large A. sustained Na+- and voltage-dependent K+ current in spinal neurons of the frog embryo. J Physiol 1993;462:349-72.
- Egan TM, Dagan D, Kupper J, Levitan IB. Na+-activated K+ channels are widely distributed in rat CNS and in Xenopus oocytes. Brain Res 1992;584:319-21.
- Safronov BV, Vogel W. Properties and functions of Na+-activated K+ channels in the soma of rat motoneurones. J Physiol 1996;497:727-34.
- Bader CR, Bernheim L, Bertrand D. Sodium-activated potassium current in cultured avian neurons. Nature 1985;317:540-2.
- Dryer SE, Fujii JT, Martin AR. A. Na+-activated K+ current in cultured brain stem neurons from chicks. J Physiol 1989;410:283-96.
- Yuan A, Santi CM, Wei A, Wang ZW, Pollak K, Nonet M, et al. The sodium-activated potassium channel is encoded by a member of the Slo gene family. Neuron 2003;37:765-73.
- Yaksh TL, Hammond DL. Peripheral and central substrates involved in the rostrad transmission of nociceptive information. Pain 1982;13:1-85.
- Scroggs RS, Fox AP. Calcium current variation between acutely isolated adult rat dorsal root ganglion neurons of different size. J Physiol 1992;445:639-58.
- Gao SB, Wang CM, Chen XS, Yu WW, Mo BW, Li CH. Cerebrosides of baifuzi, a novel potential blocker of calcium-activated chloride channels in rat pulmonary artery smooth muscle cells. Cell Biol Int 2007;31:908-15.
- Li W, Gao SB, Lv CX, Wu Y, Guo ZH, Ding JP, et al. Characterization of voltage-and Ca2+-activated K+ channels in rat dorsal root ganglion neurons. J Cell Physiol 2007;212:348-57.
- Bhattacharjee A, Kaczmarek LK. For K+ channels, Na+ is the new Ca2+. Trends Neurosci 2005;28:422-8.
- Haimann C, Bernheim L, Bertrand D, Bader CR. Potassium current activated by intracellular sodium in quail trigeminal ganglion neurons. J Gen Physiol 1990;95:961-79.
- Hess D, Nanou E, Manira A, El . Characterization of Na+-activated K+ currents in larval lamprey spinal cord neurons. J Neurophysiol 2007;97:3484-93.
- Descalzo VF, Nowak LG, Brumberg JC, McCormick DA, Sanchez-Vives MV. Slow adaptation in fast-spiking neurons of visual cortex. J Neurophysiol 2005;93:1111-8.
- Franceschetti S, Lavazza T, Curia G, Aracri P, Panzica F, Sancini G, et al. Na+-activated K+ current contributes to postexcitatory hyperpolarization in neocortical intrinsically bursting neurons. J Neurophysiol 2003;89:2101-11.
- Liu X, Stan LL. Sodium-activated potassium conductance participates in the depolarizing afterpotential following a single action potential in rat hippocampal CA1 pyramidal cells. Brain Res 2004;1023:185-92.
- Zhang L, Sukhareva M, Barker JL, Maric D, Hao Y, Chang YH, et al. Direct binding of estradiol enhances Slack (sequence like a calcium-activated potassium channel) channels’ activity. Neuroscience 2005;131:275-82.
